Abstract
The protective effect of pear pomace water extract (PPWE) against hepatic lipid peroxidation was investigated in rats fed a 41% kcal fat diet containing 0.21% cholesterol (HFCD). For 5 weeks, 200 or 400 mg/kg of PPWE was administrated once daily via oral gavage. Body weights were lower in the PPWE-treated group than in the control group. Serum total antioxidant capacity increased, whereas hepatic thiobarbituric acid reactive substances significantly decreased after the administration of PPWE. PPWE recovered the HFCD-induced reduction of hepatic glutathione S-transferase and glutathione peroxidase activity. The serum alanine aminotransferase and aspartate aminotransferase activities significantly decreased on PPWE treatment. The present investigation suggests that PPWE represents a valuable natural antioxidant source for use in the health food industry.
Keywords: Antioxidative enzymes, High fat/cholesterol diet, Lipid peroxidation, Liver damage, Pear pomace
Introduction
Oxidative stress has been defined as a state of imbalance between the generation of reactive species and the activity of the antioxidative defense system [1]. Oxidative stress leads to various chronic health problems, such as cardiovascular diseases, diabetes, nonalcoholic fatty liver diseases, neurological disorders, inflammatory diseases, certain cancers, decreased immunity, and aging processes [2]. Excess levels of reactive oxygen species (ROS) and free radicals caused by oxidative stress may damage cells by directly attacking cellular DNA, proteins, and lipids or by initiating chain reactions [3]. A high-fat diet causes excessive energy intake and is reported to increase oxidative stress in various tissues of rodents [4]. Moreover, experimental studies show that a high-fat diet can be associated with increased hepatic oxidative stress in mammals [5].
The free radical scavenger system in the human body acts as a physiological defense system to protect tissues against ROS. This defense system is divided into a non-enzyme defense system and an enzyme defense system including superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, and glutathione S-transferase (GST) [4, 6–8].
Owing to the presence of bioactive compounds, pears possess anti-inflammatory, antitussive, diuretic, and antihyperglycemic activities [9]. Pear is a potential source of phenolic compounds, such as arbutin, chlorogenic acid, caffeic acid, and coumaric acid, which are antioxidants [10]. Pear pomace, the byproduct produced during industrial processing, comprises peels, pulps, stems, cores, and seeds [11]. Most of pear pomace is discarded, causing environmental pollution, although it has important dietary components including dietary fiber, polyphenols, and triterpenes. Pear peel has been reported to have a more positive influence on the plasma antioxidant capacity than pear pulp [12, 13]. However, these results are conflictive, particularly concerning the antioxidative capacity of pear peels in the rats fed additional cholesterol [12, 13]. Furthermore, the capacity of the pear to enhance biological defense systems, which are necessary to cope with excessive ROS production, and protect the liver has not been reported.
In the present study, we show that a pear pomace water extract (PPWE) suppresses hepatic lipid peroxidation and protects against liver damage via enhancement of hepatic antioxidative enzyme activity. On the basis of our results, we suggest that pear pomace may be used as an antioxidative agent to prevent oxidative stress-related chronic health problems.
Materials and methods
Preparation of pear pomace extracts
Pear pomace from Pyrus pyrifolia Niitaka was prepared using water extraction. Briefly, 100 g of pear pomace was soaked in 2 L water for 24 h at room temperature. The extract was filtered under reduced pressure and then evaporated and freeze-dried. The yield obtained was 32.6%.
Animals
Sprague–Dawley male rats (3 weeks old) were purchased from the Central Laboratory Animal Inc. (Seoul, Korea) and housed at the University of Mokpo National Animal Care Service facilities with a 12 h light/dark cycle. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Mokpo National (MNU-IACUC-2014-003). At 4 weeks of age, the rats were randomly assigned into four groups based on PPWE supplementation: normal diet control (ND-C) with vehicle (n = 8); high-fat/cholesterol diet control (41% kcal fat diet containing 0.21% cholesterol) (HFCD-C) with vehicle (n = 8); HFCD-C with 200 mg/kg body weight (BW) PPWE (PPWE 200) (n = 8); and HFCD-C with 400 mg/kg BW PPWE (PPWE 400) (n = 8). The ingredient compositions of the experimental diets are shown in Table 1. The PPWE was orally administered via oral gavage. Change in the BW of all rats was monitored weekly. After 5 weeks of treatment, rats from each group were killed by decapitation after fasting for 8 h.
Table 1.
Composition of diets
| Group | ||
|---|---|---|
| Normal | High-fat/Cholesterol | |
| Kcal% | ||
| Protein | 20 | 17 |
| Carbohydrate | 63 | 43 |
| Fat | 17 | 41 |
| Total | 100 | 100 |
| Ingredients (g/100 g diet) | ||
| Casein | 20.0 | 20.0 |
| l-Cysteine | 0.3 | 0.3 |
| Corn starch | 39.8 | 5.0 |
| Sucrose | 10.0 | 34.0 |
| Dextrin | 13.2 | 10.0 |
| Cellulose | 5.0 | 5.0 |
| Corn oil | 7.0 | 1.0 |
| Milk fat (anhydrous)a | – | 20.0 |
| Mineral Mix. | 3.5 | 3.5 |
| Vitamin Mix. | 1.0 | 1.0 |
| Choline barbiturate | 0.2 | 0.2 |
| Cholesterol | – | 0.15 |
| Ethoxyquin | – | 0.004 |
| Total | 100.0 | 100.154 |
The high-fat/cholesterol diet contains approximately 0.21% cholesterol
aAnhydrous milk fat typically contains approximately 0.3% cholesterol
Biochemical evaluation of serum
Blood samples were collected and centrifuged at 2000 g at 4 °C for 10 min. The supernatants were immediately stored at −70 °C for further use. Total antioxidant capacity (TAC) was measured using an ELISA kit (Cell Biolabs, Inc.) following the manufacture’s protocol. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and blood urea nitrogen (BUN) were determined using a Beckman automatic analyzer (Beckman, USA).
Hepatic thiobarbituric acid reactive substances (TBARS) content and antioxidant enzymes
The livers were excised immediately, rinsed with PBS, and then weighed. An appropriate portion of the liver was homogenized in an ice-cold Tris buffer (pH 7.4). The homogenates were centrifuged at 12,000×g for 20 min at 4 °C and then again at 105,000×g for 1 h at 4 °C. The supernatant (cytosol) was used for measuring the activities of GPx [Northwest Life Science Specialties (NWLSS) LLC, Vancouver, USA] and GST (Sigma-Aldrich, USA). TBARS contents (Cell Biolabs, Inc.) were measured in the microsomes. Protein content was measured using the Bradford protein assay (Sigma, St. Louis, MO, USA).
Statistical analysis
Statistics were analyzed using the SPSS program (Version 23, Chicago, USA). Results are expressed as the mean ± S.E. and comparisons are based on one-way analysis of variance followed by Duncan’s multiple range test. A p value of < 0.05 was considered statistically significant.
Results and discussion
Effect of the PPWE on body weight, food intake, energy intake, and food efficacy ratio
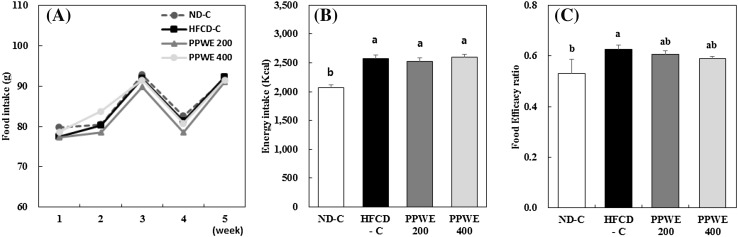
An HFCD results in excessive energy intake and increased oxidative stress [4, 12]. In the present study, the food intake of rats in all groups was the same [Fig. 1(A)]. However, energy intake was significantly higher in the rats fed the HFCD than in rats fed the ND [Fig. 1(B)]. The HFCD increased the food efficacy ratio (FER) compared to the ND intake [Fig. 1(C)]. FER in the rats treated with PPWE decreased compared to that in the rats fed the HFCD-C, although this difference was not statistically significant [Fig. 1(C)]. The HFCD tended to increase the body weight (BW) of rats after 5 weeks of intake compared to the ND intake. The BW of rats treated with PPWE 200 mg/kg/day significantly reduced in comparison with that of rats fed the HFCD-C. However, the decrease in the BW of rats treated with a higher dose of PPWE 400 mg/kg/day was not significantly different compared to that of the rats treated with the HFCD-C or ND-C (Table 2). Epidemiological studies have shown a relation between excessive fat accumulation and oxidative stress [14, 15]. Although the cause of this association remains uncertain, fat accumulation accompanied by the subsequent inflammatory responses has been considered as a source of oxidative stress [16]. In a previous study, we showed that PPWE inhibits fat accumulation by inhibiting adipogenesis and inducing apoptosis in 3T3-L1 cells [17]. Therefore, the inhibitory effect of the PPWE on fat accumulation may be associated with antioxidative capacity.
Fig. 1.
Effect of the pear pomace water extract (PPWE) on the food intake (A), energy intake (B), and food efficacy ratio (C) in rats. Mean values with the same letter are not significantly different by Duncan’s multiple range test (p < 0.05). ND-C, Normal diet + distilled water; HFCD-C, High-fat cholesterol diet + distilled water; PPWE 200, High-fat cholesterol diet + PPWE 200 mg/kg BW; PPWE 400, High-fat cholesterol diet + PPWE 400 mg/kg BW
Table 2.
Body weights
| Group | Initial | 1st week | 2nd week | 3rd week | 4th week | 5th week |
|---|---|---|---|---|---|---|
| ND-C | 110.5 ± 2.01 | 167.8 ± 1.35ab | 218.3 ± 1.75b | 274.8 ± 1.68ab | 322.7 ± 2.46ab | 360.1 ± 1.56ab |
| HFCD-C | 110.6 ± 1.89 | 169.6 ± 1.45a | 225.6 ± 2.48a | 281.7 ± 3.20a | 332.7 ± 4.35a | 369.6 ± 5.09a |
| PPWE 200 | 111.0 ± 1.75 | 165.0 ± 2.09ab | 219.7 ± 3.74ab | 275.3 ± 4.05ab | 319.5 ± 4.22b | 354.7 ± 7.30b |
| PPWE 400 | 111.1 ± 1.96 | 163.1 ± 1.73b | 219.1 ± 3.68ab | 277.1 ± 2.43ab | 322.3 ± 2.76ab | 360.0 ± 4.98ab |
Mean values with the same letter are not significantly different by Duncan’s multiple range test (p < 0.05)
ND-C, Normal diet + distilled water; HFCD-C, High-fat cholesterol diet + distilled water; PPWE 200, High-fat cholesterol diet + pear pomace water extract 200 mg/kg BW; PPWE 400, High-fat cholesterol diet + pear pomace water extract 400 mg/kg BW
Effect of the PPWE on serum TAC, hepatic TBARS, and antioxidative enzymes
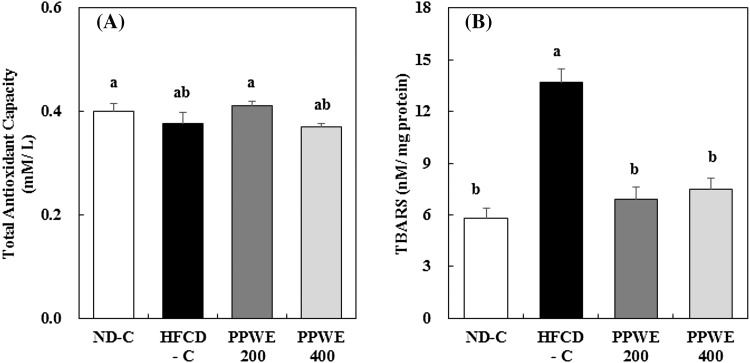
To show the antioxidative capacity of the PPWE against oxidative stress induced by high fat and cholesterol intake in vivo, we investigated the effect of the PPWE on the serum TAC and hepatic TBARS content and antioxidative enzyme activity in rats fed an HFCD. Serum TAC tended to decrease in the rats fed an HFCD; however, this was not significant. The PPWE at a dose of 200 mg/kg/day increased the TAC to the same level as that of rats in the ND-C [Fig. 2(A)]. Although no significant difference in serum TAC was found, we observed that the PPWE reduced the hepatic microsomal TBARS content, which was increased by the HFCD [Fig. 2(B)]. The lipid peroxidation assay was used to measure the amount of peroxide produced, which is the primary oxidation product during the initial stages of oxidation [18, 19]. Previous studies have shown that fruit peels possess a higher content of bioactive compounds, including polyphenols, thereby having higher antioxidative activity [20, 21]. Leontowicza et al. [13] reported that the antioxidative potential of the pear, determined by analyzing the DPPH and NO radical-scavenging activity, was significantly higher in the peels than in the pulp. Other research has shown that pear seeds contain the highest concentration of phenolic substances, followed by that in the peel and pulp [22]. Pear pomace used in the present study comprised peels, pulps, stems, cores, and seeds. Therefore, we presume that the bioactive compounds in peels and seeds might contribute to the decrease in the TBARS content of PPWE-treated rats.
Fig. 2.
Effect of the pear pomace water extract (PPWE) on the total antioxidant capacity (A) and thiobarbituric acid reactive substances (B) in rats. Mean values with the same letter are not significantly different by Duncan’s multiple range test (p < 0.05). ND-C, Normal diet + distilled water; HFCD-C, High-fat cholesterol diet + distilled water; PPWE 200, High-fat cholesterol diet + PPWE 200 mg/kg BW; PPWE 400, High-fat cholesterol diet + PPWE 400 mg/kg BW
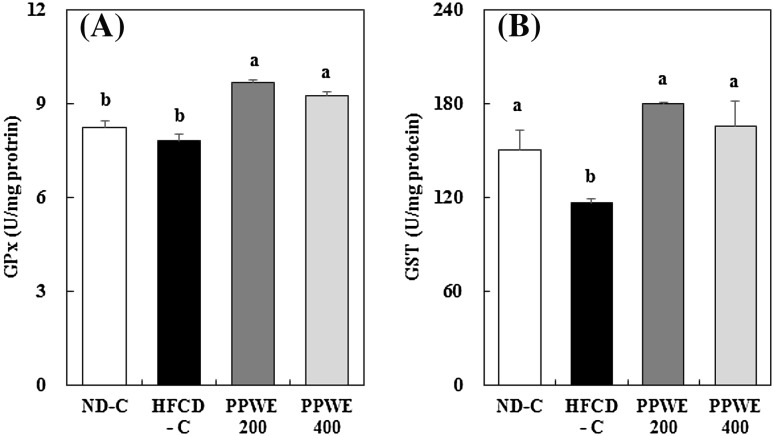
Biological defense system against ROS in cells cannot completely prevent the detrimental effects of excessive ROS [23]. Therefore, additional antioxidants, which can increase antioxidative enzyme activity or detoxify toxicants such as lipid byproducts and hydrogen peroxide, help cells to tackle excessive ROS production. GPx, in association with GST, plays an important role in detoxifying lipid byproducts and hydrogen peroxide derived in response to infection or through physiological metabolism [24]. We measured the hepatic activity of GPx and GST to verify that PPWE activates these enzymes, thereby reducing TBARS content. PPWE treatment increased GPx activity [Fig. 3(A)]. In addition, the HFCD reduced GST activity, and PPWE recovered the GST activity to the same level as that of rats fed the ND [Fig. 3(B)]. We did not observe dose-dependent increases in hepatic GPx and GST activities. GPx and GST are commonly regulated by transcription factor Nrf2, which is a member of the basic leucine zipper NF-E2 family. Nrf2 plays a crucial role in deactivating or eliminating ROS and carcinogens [25–28]. Meanwhile, caffeic acid and its ester chlorogenic acid, which are the most abundant polyphenols in pear, are reported to stimulate GST activities. Furthermore, this stimulation is caused by the interaction of Nrf2 and AREs [28]. Therefore, we can assume that polyphenols in pear, particularly caffeic and chlorogenic acids, up-regulate GPx and GST activities through the Nrf2 signaling pathway. Our results indicate that the PPWE-induced increase in hepatic GPx and GST activities might reduce hepatic TBARS.
Fig. 3.
Effect of the pear pomace water extract (PPWE) on the glutathione peroxidase (A) and glutathione S-transferase (B) in rats. Mean values with the same letter are not significantly different by Duncan’s multiple range test (p < 0.05). ND-C, Normal diet + distilled water; HFCD-C, High-fat cholesterol diet + distilled water; PPWE 200, High-fat cholesterol diet + PPWE 200 mg/kg BW; PPWE 400, High-fat cholesterol diet + PWE 400 mg/kg BW
Effect of the PPWE on serum AST, ALT, and BUN
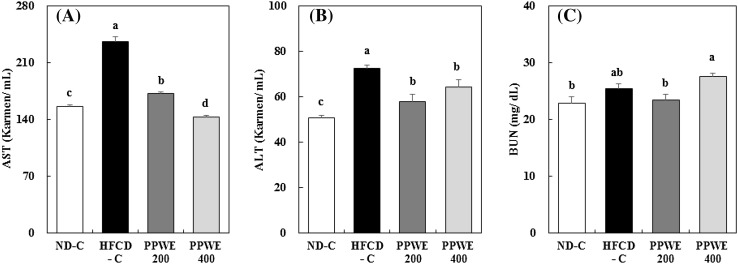
The increased content of TBARS in rats fed the HFCD indicates that ROS produced by HFCD-induced oxidative stress may damage hepatic cells. Increased hepatic lipid peroxidation in cells is accompanied by an increased lactate dehydrogenase release in the medium, indicating the oxidative induction of plasma membrane damage [29]. Therefore, we measured the ALT and AST activities and BUN content in the serum to reveal the protective effect of the PPWE against hepatic damage, which was via improved antioxidative capacity. ALT and AST has been suggested as useful predictors of liver pathology. Furthermore, they are used as markers for chronic hepatitis C [30]. It has been established that hepatic dysfunction in obesity is connected with oxidative stress and steatosis accompanying insulin resistance, resulting in nonalcoholic fatty liver disease (NAFLD). NAFLD extends from simple steatosis to inflammatory steatohepatitis and finally to possible long-term injury. ALT has also been applied as an indicator of NAFLD [30, 31]. As expected, the HFCD showed increases in ALT and AST compared to the ND. The PPWE significantly decreased ALT and AST activities in the rats fed an HFCD, [Fig. 4(A) (B)]. Therefore, we can suggest that PPWE protects the liver against fat accumulation and hepatic dysfunction. Meanwhile, the HFCD tended to increase serum BUN, and PPWE at 200 mg/kg/day decreased it to the same level as that of the ND. However, high-dose PPWE (400 mg/kg/day) increased serum BUN [Fig. 4(C)]. Our data indicate that the PPWE protects the liver against HFCD-induced hepatic lipid accumulation and damage. We can presume that the PPWE-induced increase in hepatic antioxidative enzyme activity contributes to the protection of the liver against damage caused by lipid peroxidation.
Fig. 4.
Effect of the pear pomace water extract (PPWE) on the aspartate aminotransferase (A), alanine aminotransferase (B), and blood urea nitrogen (C) in rats. Mean values with the same letter are not significantly different by Duncan’s multiple range test (p < 0.05). ND-C, Normal diet + distilled water; HFCD-C, High-fat cholesterol diet + distilled water; PPWE 200, High-fat cholesterol diet + PPWE 200 mg/kg BW; PPWE 400, High-fat cholesterol diet + PPWE 400 mg/kg BW
In the present study, we demonstrated the antioxidative capacity of PPWE in the rats fed an HFCD. PPWE inhibited fat accumulation in rats fed an HFCD. As expected, PPWE effectively reduced the hepatic microsomal TBARS that was increased by HFCD, suggesting that PPWE in liver has an antioxidant potential. In addition, the antioxidant potential of PPWE might be partially achieved through the increase in activities of hepatic antioxidative enzyme such as GPx and GST, which play an important role in detoxifying lipid byproducts. We also showed that PPWE had a protective effect against liver damage determined by ALT and AST. Therefore, PPWE possesses potential as a natural antioxidative agent and may be applicable in the health food industry.
Acknowledgements
This research was supported by the Bio-industry Technology Development Program by the, Ministry of Agriculture, Food and Rural Affairs (313019-03-3-HD030). The authors would like to thank Enaga (www.enago.co.kr) for the English language review.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kennedy G, Spence VA, McLaren M, Hill A, Underwood C, Belch JJF. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic. Biol. Med. 2005;39:584–589. doi: 10.1016/j.freeradbiomed.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 2.Aruoma O. Free radical, oxidative stress, and antioxidants in human health and disease. J. Am. Oil. Chem. Soc. 1998;75:199–212. doi: 10.1007/s11746-998-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ming M, Guanhua L, Zhanhai Y, Guang C, Xuan Z. Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 2009;113:872–877. doi: 10.1016/j.foodchem.2008.03.064. [DOI] [Google Scholar]
- 4.Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Shork KR, Schimke J, Barazzoni R, Sreekumaran NK. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. Am. J. Physiol. Endocrinol. Metab. 2002;282:E1055–E1061. doi: 10.1152/ajpendo.00554.2001. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim W, Lee US, Yeh CC, Szabo J, Bruckner G, Chow CK. Oxidative stress and antioxidant status in mouse liver: Effects of dietary lipid, vitamin E and iron. J. Nutr. 1997;127:1401–1406. doi: 10.1093/jn/127.7.1401. [DOI] [PubMed] [Google Scholar]
- 6.Prangthipa P, Surasianga R, Charoensiria R, Leardkamolkarnb V, Komindrc S, Yamborisuta U, Vanavichitd A, Kongkachuichaia R. Amelioration of hyperglycemia, hyperlipidemia, oxidative stress and inflammation in steptozotocin-induced diabetic rats fed a high fat diet by riceberry supplement. J. Funct. Foods. 2013;5:195–203. doi: 10.1016/j.jff.2012.10.005. [DOI] [Google Scholar]
- 7.Wang J, Cao Y, Wang C, Sun B. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohydr. Polym. 2011;86:1192–1197. doi: 10.1016/j.carbpol.2011.06.014. [DOI] [Google Scholar]
- 8.Xiao S, Xie G, Wang J, Hou X, Wang X, Wu W, Liu X. Chicoric acid prevents obesity by attenuating hepatic steatosis, inflammation and oxidative stress in high-fat diet-fed mice. Food Res. Int. 2013;54:345–353. doi: 10.1016/j.foodres.2013.07.033. [DOI] [Google Scholar]
- 9.Li X, Wang T, Zhou B, Gao W, Cao J, Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.) Food Chem. 2014;152:531–538. doi: 10.1016/j.foodchem.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Koo JH, Eun JB. Antioxidant activities of methanol extracts and phenolic compounds in Asian pear at different stages of maturity. Food Sci. Biotechnol. 2006;15:44–50. [Google Scholar]
- 11.Rabetafika HN, Bchir B, Blecker C, Paquot M, Wathelet B. Comparative study of alkaline extraction process of hemicelluloses from pear pomace. Biomass Bioenergy. 2014;61:254–264. doi: 10.1016/j.biombioe.2013.12.022. [DOI] [Google Scholar]
- 12.Leontowicz H, Gorinstein S, Lojekc A, Leontowicza M, Ciz M, Soliva-Fortunyg R, Park YS, Jung ST, Trakhtenberg S, Martin-Belloso O. Comparative content of some bioactive compounds in apples, peaches and pears and their influence on lipids and antioxidant capacity in rats. J. Nutri. Biochem. 2002;13:603–610. doi: 10.1016/S0955-2863(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 13.Leontowicza M, Gorinstein S, Leontowicz H, Krzeminski R, Lojek A, Katrich E, Ciz M, Martin-Belloso O, Soliva-Fortunyg R, Haruenkit R, Trakhtenberg S. Apple and pear peel and their influence on plasma lipid and antioxidant potentials in rats fed cholesterol-containing diets. J. Agric. Food Chem. 2003;51:5780–5785. doi: 10.1021/jf030137j. [DOI] [PubMed] [Google Scholar]
- 14.Keaney JF, Larson MG, Vasan RS, Wilson WF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 15.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J. Clin. Endocrinol. Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 16.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Inverst. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhyu J, Kim MS, You MK, Bang MA, Kim HA. Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Nutr. Res. Pract. 2014;8:336–339. doi: 10.4162/nrp.2014.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavino VC, Miller JS, Ilharebha SO, Milo GE, Cornwell DG. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue culture. J. Lipid Res. 1981;22:763–769. [PubMed] [Google Scholar]
- 19.Zhao JT, Ma DH, Luo M, Wang W, Zhao C, Zu YG. Fu Yj, Wink M. In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.] Food Res. Int. 2014;56:243–251. doi: 10.1016/j.foodres.2013.12.028. [DOI] [Google Scholar]
- 20.Bocco A, Cuvelier ME, Richard H, Berset C. Antioxidant activity and phenolic composition of citrus peel and seed extracts. J. Agric. Food Chem. 1998;46:2123–2129. doi: 10.1021/jf9709562. [DOI] [Google Scholar]
- 21.Ghasemi K, Chasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak. J. Pharm. Sci. 2009;22:277–281. [PubMed] [Google Scholar]
- 22.Kolniak-Ostek J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communus L) Food Chem. 2016;203:491–497. doi: 10.1016/j.foodchem.2016.02.103. [DOI] [PubMed] [Google Scholar]
- 23.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu CH, Tseng MC, Cheng W. Identification and cloning of the antioxidant enzyme, glutathione peroxidase, of white shrimp, Litopenaeus vannamei, and its expression following Vibrio alginolyticus infection. Fish Shellfish Immunol. 2007;23:34–45. doi: 10.1016/j.fsi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:312–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 26.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends. Mol. Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Katsuoka F, Motohashi H, Engel JD, Yamamoto M. Nrf2 transcriptionally activities the mafG gene through an antioxidant response element. J. Biol. Chem. 2005;280:4483–4490. doi: 10.1074/jbc.M411451200. [DOI] [PubMed] [Google Scholar]
- 28.Feng R, Lu Y, Bowman LL, Qian Y, Castranova V, Ding M. Inhibition of activator protein-1, NF-κB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005;280:27888–27895. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- 29.Nunzio ND, Valli V, Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fatty Acids. 2011;85:121–127. doi: 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in nonalcoholic fatty liver disease. J. Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Anderson FH, Zeng L, Rock NR, Yoshida EM. An assessment of the clinical utility of serum ALT and AST in chronic hepatitis C. Hepatol. Res. 2000;18:63–71. doi: 10.1016/S1386-6346(99)00085-6. [DOI] [PubMed] [Google Scholar]