Abstract
Bostrycin, a red antibacterial agent produced by Nigrospora sp. no. 407, is considered for meat processing. To optimize production, the culture conditions of submerged fermentation (SmF) and solid-state fermentation (SSF) were investigated. The optimal SmF conditions were a medium containing 1.0% cane molasses and incubation at 30 °C and 150 rpm for 6 days. In SSF, other than bostrycin, less pigment was produced and the optimal ratio of bagasse to water was 1:2 for 10 days. The production and recovery rate of bostrycin by SmF were 120 mg/L and 40%, respectively. Bostrycin exhibited thermostable, pH-dependent color change and dose-dependent antibacterial activity against Clostridium botulinum. Bostrycin-modified meat turned strong red for at least 24 h and could not be removed by washing; bostrycin maintained its antibacterial activity with a bacteriostasis rate of 91% on Staphylcoccus aureus. This is an easy and inexpensive means of acquiring bostrycin from molasses and sugarcane.
Keywords: Bostrycin, Agro-industrial residue, Solid-state fermentation, Reddening agent, Meat processing
Introduction
There is an increasing global trend toward the efficient utilization of agro-industrial residues as raw materials in bioprocesses for the production of bulk chemicals and value-added fine products. Cane molasses and sugarcane bagasse are two abundant by-products of the sugar industry. Cane molasses comprises 45% (w/v) total sugar, including 35% (w/v) sucrose and 10% (w/v) converted sugars (fructose and glucose) [1]. Therefore, it is considered to be the most economical source of carbon for microbial fermentation. In addition to high levels of sugar, cane molasses also contains protein, fat, minerals, vitamins and other beneficial compounds for microorganisms [1]. Sugarcane bagasse, the fibrous waste remaining after sugar extraction, is produced in large quantities by the sugar and alcohol industries. In general, 1 ton of sugarcane generates 280 kg of bagasse. Approximately 54 million tons of dry bagasse are produced annually worldwide [2, 3]. In addition to reducing capital costs, the efficient utilization of agro-industrial wastes reduces the environmental problems caused by improper disposal.
In developed countries, demands for safe and nutritious food products with attractive sensory characteristics are increasing [4, 5]. Consequently, several studies have used natural ingredients to replace synthetic chemicals for achieving these goals [6, 7]. Natural ingredients exhibiting functions in coloring and antimicrobial activity have been reported. For example, annatto extract has exhibited antimicrobial activities against strains of C. botulinum and C. perferingens [5, 8]. In addition, the extract of the flower of Rosa canina L. (dog rose) exhibited antibacterial activity [9] and other additives from polyphenol-rich fruits were reported to be effective against Clostridium [10]. However, few reports have discussed the antimicrobial activity of the microbe-derived red agent against C. botulinum.
Recently, we conducted a screening for antibiotic-producing microorganisms from soil. Bostrycin, a red agent, was purified and identified from Nigrospora sp. [11]. Bostrycin has been obtained from several fungi, including Bostryconema alpestre [12], Nigrospora oryzae [13], Arthrinium phaeospermum [14], Alternaria eichhorniae [15, 16], and Aspergillus sp. [17]. These studies mainly focused on the antibacterial and phytotoxic activities of bostrycin. Furthermore, few studies have examined bostrycin fermentation [18]. We demonstrated that bostrycin from Nigrospora sp. no. 407 exhibits antibacterial activity only against Gram-positive bacteria including C. botulinum [11]. However, the potential application of bostrycin in the prevention of C. botulinum contamination in the food industry has not been evaluated.
The red antibiotic bostrycin has a tetrahydroanthraquinone skeleton comprising 2 active carbonyl groups and can function as a coupling agent for preventing biomaterial-centered infection and protein immobilization. The carbonyl groups of bostrycin can directly and spontaneously react with the amino groups of proteins through the Maillard reaction. Consequently, bostrycin can be immobilized, exhibiting antibacterial properties on matrixes, such as a nonwoven PP fabric sample [11].
In this study, the optimal conditions for bostrycin production using cane molasses and sugarcane bagasse in submerged fermentation (SmF) and solid-state fermentation (SSF) were established. Several properties of purified bostrycin or bostrycin-modified meat were evaluated to estimate the potential of bostrycin for food processing.
Materials and methods
Materials
Freshly slaughtered pork was purchased from a local market in Kaohsiung, Taiwan. Sugarcane bagasse was obtained from a local sugarcane store. All other chemicals were of reagent grade or higher purity than it.
Microorganisms and culture conditions
A fungal strain no. 407 was isolated from the soil, which was identified as Nigrospora sp. and deposited at the Bioresource Collection and Research Center (BCRC; Hsinchu, Taiwan) of Taiwan under accession number BCRC 930134. The organism was maintained on YM agar slants (1% glucose, 0.5% peptone, 0.3% malt extract, and 0.3% yeast extract) at 30 °C, as described in a previous study [11].
For SmF, fungi from a slant culture were inoculated in 50 mL of basal medium (basal medium A: 0.5% peptone, 0.3% malt extract, and 0.3% yeast extract; basal medium B: 1% cane molasses) containing various carbon or nitrogen sources (Tables 1, 2) in a 250-mL flask. Fermentation was conducted at 30 °C for 6 days on a rotary shaker at 150 rpm. The culture broth was filtered through 4 or 5 layers of muslin cloth, and the filtrate was centrifuged at 12,000×g at 4 °C for 20 min. The cell-free supernatant was recognized as the crude antibiotic and used for subsequent assays.
Table 1.
The effect of various carbon sources on the bostrycin production of strain no. 407 in submerged culture
| Additive* | Absorbance (OD502 nm) | Antimicrobial activity (mm) |
|---|---|---|
| None | 0.96 ± 0.12a | 0.1 ± 0.1a |
| Fructose | 10.27 ± 0.64b | 10.2 ± 0.3b |
| Glucose | 8.47 ± 0.51c | 9.8 ± 0.3b |
| Lactose | 1.96 ± 0.22a | 4.5 ± 0.5 |
| Maltose | 5.47 ± 0.32d | 7.8 ± 0.6c |
| Molasses | 10.70 ± 0.28b | 10.7 ± 0.3b |
| Sucrose | 8.47 ± 0.35c | 9.9 ± 0.4b |
| Starch | 4.97 ± 0.31d | 7.9 ± 0.2c |
| Cellulose | 0.93 ± 0.16a | 0.1 ± 0.1a |
* The culture medium contained basal medium A and the listed carbon sources (1%). The data are shown as mean ± standard deviation (SD; n = 3)
a,b,c,dDifferent letters indicate significant differences (p < 0.05) in Scheffe post hoc tests following one-way ANOVAs. The means of any groups that do not share a letter are significantly different
Table 2.
The effect of various nitrogen sources on the bostrycin production of strain no. 407 in submerged culture
| Additive* | Absorbance (OD502 nm) | Antimicrobial activity (mm) |
|---|---|---|
| None | 10.51 ± 0.27a | 10.6 ± 0.3a |
| Ammonium sulfate | 0.25 ± 0.05b | 1.0 ± 0.1 |
| Urea | 0.29 ± 0.03b | 0.1 ± 0.1 |
| Casein | 5.77 ± 0.25c,d | 9.2 ± 0.2a,b |
| Malt extract | 6.13 ± 0.21c,d | 10.2 ± 0.2c |
| Peptone | 3.17 ± 0.15a | 8.9 ± 0.2a |
| Tryptone | 5.69 ± 0.16c | 9.8 ± 0.2b,c |
| Soy meal | 2.39 ± 0.09 | 8.0 ± 0.1 |
| Yeast extract | 6.32 ± 0.10d | 9.6 ± 0.3b,c |
* The culture medium contained basal medium B and the listed nitrogen sources (1%). The data are shown as mean ± SD (n = 3)
a,b,c,dDifferent letters indicate significant differences (p < 0.05) in Scheffe post hoc tests following one-way ANOVAs. The means of any groups that do not share a letter are significantly different
For SSF, strain no. 407 was inoculated from a plate culture into a solid-state medium comprising 5 g of available sources (rice bran, corn flour, soy meal, and sugarcane bagasse) and 10 mL of water in a 250-mL flask. After cultivation for 10 days at 30 °C, the entire culture was soaked in 50-mL water for 30 min and squeezed through a cloth. The aqueous extract was centrifuged to remove particles, and the supernatant was recognized as the crude antibiotic and used for subsequent assays. The recovery rate of bostrycin was estimated through its antimicrobial activity, as described in the following section. Alternatively, it was measured at 502 nm using a spectrophotometer, according to the visible maximal absorption peak λ max of bostrycin [14]. The differences among these treatments were analyzed through ANOVA with a post hoc Scheffe test.
Antimicrobial activity assay through an agar-diffusion assay
The antimicrobial activity of the antibiotic was assessed through an agar-diffusion assay, according to a modified version of a previously described method [19]. In brief, Staphylcoccus aureus BCRC 10780T culture (OD600 = 0.1, 105 colony-forming units) was mixed with 20 mL of a nutrient agar medium (Difco Laboratories, Detroit, MI). After the nutrient agar solidified, circular wells (diameter: 9 mm) were bored into the agar. Subsequently, 50 μL of diluted antibiotic sample (approximately 1–10 μg) was added to each well and the areas of microbial growth inhibition were measured after incubation at 35 °C for 16 h. The growth inhibition zones of S. aureus were observed as a clear zone around the well. The diameter of inhibition zone was calculated by subtracting the diameter of the well (9 mm) from the mean diameter of these clear zones. Antibacterial activity was determined by measuring the diameter of the inhibition zone, which indicated the extent of the inability of the test organism to survive in the presence of bostrycin.
The antibacterial activity against C. botulinum ATCC 17855 was assayed by the laboratories of the National Defense Medical Center (Taipei, Taiwan). Sterile paper discs (diameter: 6 mm) containing bostrycin (5 and 10 μg) were placed on an agar plate containing C. botulium, and the plate was left to incubate. The zone of inhibition was measured after anaerobic culture at 37 °C for 5 days.
Recovery and purification of bostrycin
Bostrycin was isolated according to a method described in a previous study [11]. The cell-free supernatant of SmF (1000 mL) was extracted twice using chloroform (1000 mL each time), and the combined extract was concentrated to near dryness under reduced pressure. The residue was then triturated using 750 mL of n-hexane. The extract was placed on a silica gel G60 column (3.8 cm × 30 cm) packed with 100-g silica gel (80–100 mesh, Merck Co.) and washed with 500 mL of n-hexane. The column was developed in a batchwise process using the following solvent sequence: 1000 mL of n-hexane–ethyl acetate (10:1), 1000 mL of n-hexane–ethyl acetate (10:2), and 1000 mL of n-hexane–ethyl acetate (10:3). Finally, the column was eluted using 1000 mL of a chloroform–methanol solvent (6:4). The active fractions were collected, washed, dried, and analyzed for purity.
Temperature stability of bostrycin
The effect of heat on the antibacterial activity was evaluated by treating the bostrycin at various temperatures (30–100 °C at intervals of 10 °C) for 60 min in a water bath. The remaining activity of the heat-treated sample was determined by conducting an antimicrobial activity assay. The original activity was defined as 100%.
Bostrycin modification on meat
Pork was cut into rectangular strips (approximately 2 cm × 1 cm × 0.5 cm), immersed in 70% alcohol for 30 s, and thoroughly washed with deionized water 5×. Bostrycin was immobilized on pork through the Maillard reaction by soaking the pork in a bostrycin solution (OD = 1–5) at 4 °C for 12 h. The meat samples were subsequently rinsed with deionized water for further use.
Antibacterial activity test of meat samples
The antibacterial activities of the meat samples were assessed using a shake-flask test and measured according to the ratio of bacteriostasis (R) to S. aureus [20]. R was calculated using the following equation:
where A and B are the mean numbers of bacterial colonies on the meat samples before and after the shake-flask test, respectively.
Results and discussion
Characteristics of strain no. 407
When Nigrospora sp. no. 407 cultured in YM agar plates at 30 °C, an extracellular red pigment appeared on Day 2, followed by the formation of white mycelium (data not shown). Over time, a heavy brown–red mycelial mat developed on the surface of the medium; red pigments had diffused into the agar.
Bostrycin production by Nigrospora sp. no. 407 in SmF
Effect of various carbon sources on bostrycin production
Various carbon sources at concentrations of 1% were added to the basal medium A to test their effects on bostrycin production (Table 1). Bostrycin production was higher than that of the control when cane molasses, fructose, glucose, maltose, and sucrose were added but not when cellulose was added. These results agree with those reported for the use of sucrose or glucose in N. oryzae [13], Alternaria eichhorniae [16], and N. sp [18] culture for bostrycin production. Furthermore, fructose and cane molasses are found to be two more suitable carbon sources (1.25-fold increase). In addition, the effects of fructose (0–3%) and cane molasses (0–5%) concentrations were evaluated. The optimal concentrations were 1.5 and 1.0% for fructose and cane molasses, respectively (data not shown). The inexpensive carbon source, cane molasses, is attractive despite having a slightly weaker effect than fructose.
As described in the Introduction section, cane molasses contains sucrose, fructose, and glucose. All these sugars can be efficiently utilized by Nigrospora sp. no. 407 for bostrycin production, as summarized in Table 1. Cane molasses therefore results in 1.2-fold higher bostrycin production than that of sucrose.
Effect of nitrogen sources on bostrycin production in SmF
Various organic and inorganic nitrogen sources tested as additives to the basal medium B (1% molasses) at a concentration of 1% substantially affected bostrycin production, as summarized in Table 2. Production significantly differed between absorbance with and without external nitrogen sources. The interference effects of external nitrogen sources indicate that cane molasses itself is apparently a sufficient supplier of nitrogenous compounds for bostrycin production. Overall, this evidence strongly suggested that cane molasses, a cheap and renewable material, is a suitable carbon and nitrogen source for bostrycin production in the food industry.
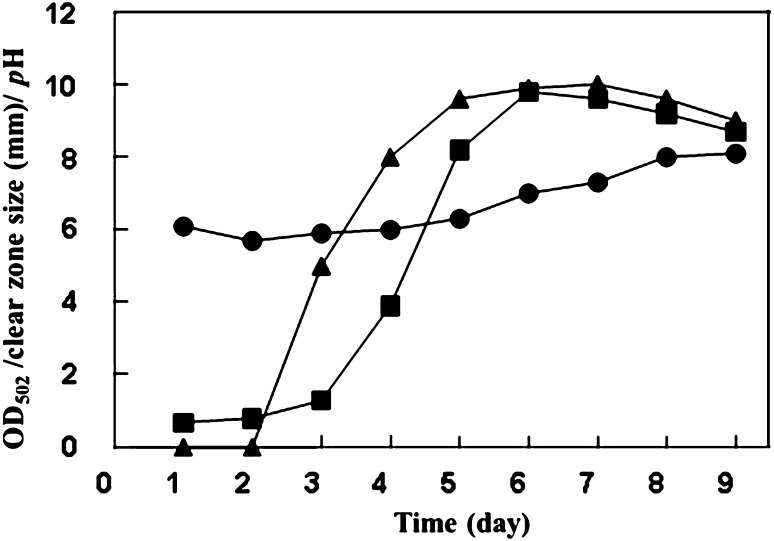
Time course of bostrycin production on SmF
The time course of bostrycin production with 1% cane molasses is shown in Fig. 1. Two measurements of bostrycin production, the clear zone size and the absorbance of OD502 nm, reached their maximum level on the early stationary phase after 6 days of cultivation. However, they were not directly proportionate. This phenomenon was caused by undesirable pigment contamination produced in the culture medium that could be separated from bostrycin through column chromatography in the purification step. Under these cultivation conditions, the antibacterial activity was approximately 10 mm/50 μL in the culture broth.
Fig. 1.
Time course of bostrycin production, inhibition activity, and changes in the pH profile of Nigrospora sp. strain no. 407 in submerged culture conducted with the optimal production medium containing 1.0% molasses. Bostrycin production was measured according to the OD502 (filled square), inhibition activity was measured according to the diameter of the clear zone in mm (filled triangle), and pH change during cultivation was profiled (filled circle)
Optimal conditions for SSF
In recent years, SSF has received more attention than SmF owing to its higher yields and more favorable product characteristics. Because of the utilization of inexpensive agro-industrial residues as raw materials, SSF has lower capital and operating costs than SmF. Based on the experimental data of SmF from this study, sugarcane bagasse is anticipated to be an alternative substrate for SSF to reduce costs and facilitate the scaling up of production.
Among several available sources of SSF (e.g., rice bran, corn flour, soy meal, and sugarcane bagasse), sugarcane bagasse was the only suitable medium for bostrycin production, with its output being approximately 10–40-fold higher than that of other media. When sugarcane bagasse is used as a substrate in SSF, the addition of other carbon sources (e.g., glucose, fructose, glycerol, starch, and cellulose) or nitrogen sources (e.g., peptone, yeast extract, malt extract, and urea) did not enhance bostrycin production. The original content of sugarcane bagasse is demonstrated to be the optimal carbon–nitrogen ratio for SSF. Our results also suggest that the optimal fermentation medium comprised water and sugarcane bagasse at a ratio of 2:1 (w/w), cultured at 30 °C for 10 days (data not shown).
Comparison of bostrycin production by the SmF and SSF systems
Even in the optimal medium, the antimicrobial activity of SSF is 30% lower than that of SmF. However, bostrycin was stringently produced from Nigrospora sp. no. 407 cultured in a 5-L jar fermenter. This is speculated to be the least favorable oxygen supply for growing aerobic Nigrospora sp. in a scaled-up liquid culture. Additionally, several phenomena, including the dramatically reduced absorbance of OD502 nm, lighter broth color, and less pigment observed in column chromatography, indicate that bostrycin was produced with less color contamination in SSF than in SmF. It might not be possible for Nigrospora sp. to produce other pigments in nutrient-deficient SSF. Nevertheless, agro-industrial residues can currently be utilized for bostrycin production, not only sugarcane bagasse in SSF but also cane molasses in SmF. These are promising alternatives for the industrial production of bostrycin by Nigrospora sp.
Purification and characteristics of bostrycin
Purification of bostrycin
A red pigment contributing to the antibacterial activity of the culture fluid was readily extracted into chloroform and subsequently isolated as a purified crystal compound. Red crystals (absolute weight: 120 mg) were obtained from 1 L. of SmF broth with 44% recovery (Table 3).
Table 3.
Purification of bostrycin from strain No. 407
| Step | Total volume (mL) | Total activity (mm) | Recovery rate (%)* |
|---|---|---|---|
| Supernatant | 1000 | 10,566 ± 404 | 100 |
| Chloroform extract (in hexane) | 750 | 8492 ± 118 | 80 ± 2 |
| Silica gel G60 | 500 | 4673 ± 316 | 44 ± 1 |
* The recovery rate for each step as compared to the crude extract. The data are shown as mean ± SD (n = 3)
Spectral changes of bostrycin according to pH
The visible absorbance spectra of purified bostrycin from 400 to 700 nm was scanned (data not shown). The original red pigment was red, dark red, and purple in acidic (pH 4.5), neutral (pH 7), and basic (pH 8.5) environments, respectively. The resonance structures of bostrycin changed under various pH conditions, showing different energy gaps between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO). This provides bostrycin with a pH indicator function for food processing. The analytical and spectral analyses of the crystal red compound were consistent with a previous description of bostrycin [21].
Thermal stability of bostrycin
The thermal stability of bostrycin was investigated by incubating it in methanol at various temperatures for 1 h. The high residual activities (>80%) illustrate that bostrycin was stable between 30 and 100 °C (data not shown). The heat resistance of bostrycin is practical for future applications in the food industry.
Antibacterial activity of bostrycin against C. botulinum
In our previous research [11], bostrycin exhibited potent antibacterial activity against a wide range of Gram-positive bacteria, including C. botulinum, a crucial foodborne bacterial pathogen. Herein, our results further demonstrated that it exhibits dose-dependent antibacterial activity against C. botulinum (data not shown). The cell wall of Gram-positive bacteria comprises a single, thick homogeneous layer of peptidoglycan that is located outside the plasma membrane and lacks a periplasmic space in contrast, the cell wall of Gram-negative bacteria contains a thin peptidoglycan layer covered with an outer membrane comprising lipopolysaccharide. Bostrycin directly acts on the nascent pentapeptide of the peptidoglycan of Gram-positive bacteria and blocks cell-wall biosynthesis to exert its antibacterial activity. The absence of this effect in Gram-negative bacteria may be due to the presence of the outer envelope that does not comprise peptidoglycans. Additional experimental data are required to support this speculation.
Bostrycin modification on meat
Effect of curing on meat color
Bostrycin contains two carbonyl functional groups that conjugate with the amine groups of proteins through the Maillard reaction. The color intensity of the bioconjugated matrix depends on the amount of bostrycin deposited on the surface. As shown in Fig. 2(A), the intensity of the meat’s redness was proportional to the increase in bostrycin concentration. Bostrycin deposition on meat was indicated by the change in the color of the surface to deep red.
Fig. 2.
Visual comparison of the meat samples treated with various concentrations of bostrycin at 0 h (A) and 24 h (B). (a) Untreated meat; (b–f) meat treated with increasing concentrations of bostrycin (OD502 nm = 1–5)
Even after 24-h treatment, the bostrycin-modified meat maintained its strong color compared with that of the blank test, which turned to pale white [Fig. 2(B)]. This is the first time a natural product from fungi has exhibited the ability to color meat as well as strong antibacterial activity against C. botulinum.
Antibacterial activity of the bostrycin-treated meat
The antibacterial activity of bostrycin-modified meat was determined using the shake-flask test. Untreated and bostrycin-treated meats were incubated in an oven at 30 °C for 12 h. As shown in Fig. 3, a clear bacteria-free solution formed on the bostrycin-modified meat, indicating that bostrycin inhibits bacterial growth and that no bacteria were present in the supernatant of the liquid culture. The bacteriostasis ratio against to S. aureus reached 91%. Bostrycin, which was bound to the surface of the meat samples, maintained its inhibitory effect on bacterial cell growth over time. The redness of the bostrycin-immobilized meat may therefore be an indicator of its antibacterial activity.
Fig. 3.

Antibacterial activity of bostrycin on the meat samples. Compared with the untreated meat sample (left), a clear bacteria-free solution was obtained from the bostrycin-treated meat sample (right) after 12-h incubation, indicating that bostrycin can inhibit the growth of S. aureus
Summary and conclusion
Bostrycin produced by Nigrospora sp. can be conveniently obtained from agro-industrial residues through both SSF and SmF. Its properties of long-lasting reddening and thermostable antibacterial activity make bostrycin a food additive as useful as nitrates and nitrites. The LD50 of bostrycin administered intraperitoneally to mice is 200 mg/kg [22]; this dose is thought to be similar to that of nitrate. Bostrycin has been shown to inhibit the growth of prostate cancer, gastric cancer, and lung cancer in vitro and is a potential anticancer drug [23–25]. Bostrycin can currently be produced in a cost-effective manner using agro-industrial residues. Therefore, it has the potential to be used in food processing as a safe color fixative and antibacterial agent. Further research is necessary to determine the optimal dosage of bostrycin to be used in a food matrix.
Acknowledgements
The authors appreciate the financial support provided by the National Science Council (Taipei, Taiwan) through the Grants NSC99-2221-E-390-014 and MOST 104-2633-B-390-001. In addition, the authors thank the laboratory staff of the Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan) and the Institute of Preventive Medicine, National Defense Medical Center (Taipei, Taiwan) for their assistance with the antibacterial assay of bostrycin. The authors would like to thank Enago (www.enago.co.kr) for the English language review.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Jiang L, Wang J, Jiang S, Wang X, Cen P, Xu Z. Butyric acid fermentation in a fibrous bed bioreactor with immobilized Colstridium tyrobutyricum from cane molasses. Bioresour. Technol. 2009;100:3403–3409. doi: 10.1016/j.biortech.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Rodrigues RCLB, Felipe MGA, Sil JBA, Vitolo M. Response surface methodology for xylitol production from sugarcane bagasse hemicellulosic hydrolyzate using controlled vacuum evaporation process variables. Process Biochem. 2003;38:1231–1237. doi: 10.1016/S0032-9592(02)00290-X. [DOI] [Google Scholar]
- 3.Rowell RM, Keany FM. Fiberboards made from acetylated bagasse fiber. Wood Fiber Sci. 1991;23:15–22. [Google Scholar]
- 4.Alves de Lima RO Azevedo L, Ribeiro LR, Salvadori DM. Study on the mutagenicity and antimutagenicity of a natural food colour (annatto) in mouse bone marrow cells. Food Chem. Toxicol. 2003;41:189–192. doi: 10.1016/S0278-6915(02)00208-9. [DOI] [PubMed] [Google Scholar]
- 5.Galindo-Cuspinera V, Westhoff DC, Rankin SA. Antimicrobial properties of commercial annatto extracts against selected pathogenic, lactic acid, and spoilage microorganisms. J. Food Protect. 2003;66:1074–1078. doi: 10.4315/0362-028X-66.6.1074. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Chin KB. Evaluation of sodium lactate as a replacement for conventional chemical preservatives in comminuted sausages inoculated with Listeria monocytogenes. Meat Sci. 2003;65:531–537. doi: 10.1016/S0309-1740(02)00245-0. [DOI] [PubMed] [Google Scholar]
- 7.Mor-Mur M, Yuste J. High pressure processing applied to cooked sausage manufacture: physical properties and sensory analysis. Meat Sci. 2003;65:1187–1191. doi: 10.1016/S0309-1740(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 8.Huhtanen C. Inhibition of Clostridium botulinum by spice extracts and aliphatic alcohols. J. Food Protect. 1980;43:195–196. doi: 10.4315/0362-028X-43.3.195. [DOI] [PubMed] [Google Scholar]
- 9.Kumarasamy Y, Cox P, Jaspars M, Rashid M, Sarker S. Bioactive flavonoid glycosides from the seeds of Rosa canina. Pharm. Biol. 2003;41:237–242. doi: 10.1076/phbi.41.4.237.15663. [DOI] [Google Scholar]
- 10.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics – a Finnish perspective. Mol. Nutr. Food Res. 2007;51:684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 11.Yang WJ, Yang CS, Huang CJ, Chen KS, Lin SF. Bostrycin, a novel coupling agent for protein immobilization and prevention of biomaterial-centered infection produced by Nigrospora sp. No. 407. Enzyme Microb. Technol. 2012;50:287–292. doi: 10.1016/j.enzmictec.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Noda T, Take T, Otani M, Miyauchi K, Watanabe T, Abe J. Structure of bostrycin. Tetrahedron Lett. 1968;9:6087–6090. doi: 10.1016/S0040-4039(00)70801-X. [DOI] [PubMed] [Google Scholar]
- 13.Furuya K, Shirasaka M. Antibiotics from fungi. IV. Production of rhodosporin (bostrycin) by Nigrospora oryzae. Sankyo Kenkyusho Nempo 21: 165–168. (1969).
- 14.Eijk GWV. Bostrycin, a tetrahydroanthraquinone pigment and some other metabolites from the fungus Arthrinium phaeospermum. Experimentia. 1975;31:783–784. doi: 10.1007/BF01938463. [DOI] [Google Scholar]
- 15.Charudattan R, Rao KV. Bostrycin and 4-deoxybostrycin: two nonspecific phytotoxins produced by Alternaria eichhorniae. Appl. Environ. Microbiol. 1982;43:846–849. doi: 10.1128/aem.43.4.846-849.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shabana YM, Elwakil MA, Charudattan R. Effect of nutrition and physical factors on mycelial growth and production of pigments and nonchromatic UV-absorbing compounds of Alternaria eichhorniae. J. Phytopathol. 2001;149:21–27. doi: 10.1046/j.1439-0434.2001.00564.x. [DOI] [Google Scholar]
- 17.Namikoshi M, Negishi R, Nagai H, Dmitrenok A, Kobayashi H. Three new chlorine containing antibiotics from a marine-derived fungus Aspergillus ostianus collected in Pohnpei. J. Antibiot. (Tokyo) 2003;56:755–761. doi: 10.7164/antibiotics.56.755. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Deng Z, Guo Z, Chen J, Wang J, Zou K. Anthraquinone Metabolites from Endophytic Nigrospora sp BM-2 and Optimization of Fermentation Medium for High Bostrycin Production. J. Pure Appl. Microbio. 2013;7:2489–2494. [Google Scholar]
- 19.Barry AI. Procedures and theoretical considerations for testing antimicrobial agents in agar media. In Antibiotics in Laboratory Medicine (Logan, V., ed.), William & Wilkins, Baltimore, pp. 10–16. (1980).
- 20.He MZ, Yan J, Gong Y. Anti-bacterial fabric and the evaluation of its property. Shanghai Textile Science & Technology. 2005;33:62–64. [Google Scholar]
- 21.Noda T, Take T, Watanabe T, Abe J. The structure of bostrycin. Tetrahedron. 1970;26:1339–1346. doi: 10.1016/S0040-4020(01)93004-2. [DOI] [PubMed] [Google Scholar]
- 22.Berdy J. CRC Handbook of antibiotic compounds. Boca Raton FL, CRC Press, pp. 231. (1980).
- 23.Chen CQ, Fang LK, Liu JW, Zhang JW, Yang GX. Yang W. Effects of marine fungal metabolites (1386A) from the South China Sea on proliferation, apoptosis and membrane potential of gastric cancer cell line MCG-803. Chin. J. Pathophysiol. 26: 1908–1912. (2010).
- 24.Chen WS, Hou JN, Guo YB, Yang HL, Xie CM, Lin YC. She ZG. Bostrycin inhibits proliferation of human lung carcinoma A549 cells via downregulation of the PI3 K/Akt pathway. J. Exp. Clin. Cancer Res. 2011;30:17. doi: 10.1186/1756-9966-30-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin W, Fang LK, Liu JW, Cheng WQ, Yun M, Yang HL. Inhibitory effects of marine fungal metabolites from the South China Sea on prostate cancer cell line DU-145. Int. J. Intern. Med. 2008;35:562–564. [Google Scholar]