Abstract
The effects of antioxidants on lifespan have been widely studied. Our previous study showed supplementation with N-acetyl-l-cysteine (NAC) extends the lifespan of Caenorhabditis elegans. Here we aimed to determine the lifespan-extending mechanism involved with NAC and the effect of NAC on Alzheimer’s disease (AD). NAC further increased the lifespan of age-1 and clk-1 mutants, which have increased lifespan owing to reduced insulin/IGF-1-like signaling and mitochondrial function, respectively. There was no additional lifespan extension in eat-2 background, a genetic model of dietary restriction (DR), by NAC. Gene knockdown experiments revealed that the effect of NAC is not dependent on SKN-1, a protein-sensing DR status, whereas DAF-16, a transcription factor regulating stress-responsive genes, is required for lifespan extension by NAC. NAC delayed paralysis caused by amyloid beta. Our results show that NAC mimics the effect of DR on lifespan, possibly through the induction of DAF-16 nuclear localization and may retard the incidence of AD.
Electronic supplementary material
The online version of this article (doi:10.1007/s10068-017-0079-1) contains supplementary material, which is available to authorized users.
Keywords: N-acetyl-l-cysteine, C. elegans, Dietary restriction, Lifespan, Amyloid beta
Introduction
Aging is one of the most complicated biological phenomena and is regulated by many genetic and environmental factors. Studies on the aging process are increasingly employing molecular genetic approaches to identify specific factors that influence the rate of aging. There are three most widely studied lifespan-extending processes in C. elegans, including reduced insulin/IGF-1-like signaling, reduced mitochondrial function, and dietary restriction (DR) [1–3]. The first gerontogene identified in C. elegans is age-1, which is an adaptor molecule that transfers the insulin/IGF-1-like signaling from a membrane receptor, DAF-2, to intracellular kinases, AKT-1/AKT-2 [1]. Reduced insulin/IGF-1-like signaling increases the lifespan of many species, including yeast, C. elegans, Drosophila melanogaster, and mice [2]. Genetic screening of long-lived mutants revealed that many genes involved in mitochondrial functions can modulate lifespan [3]. Another active research area in the field of aging is the search for novel environmental interventions that extend lifespan and retard age-related physiological changes. Until recently, the only intervention causing increased lifespan and reduced incidence and/or delayed onset of age-related pathologies in nearly every species tested is DR, also called caloric restriction (CR). The nematode C. elegans uses bacteria as a food source, and the reduced food intake significantly extends both mean and maximum lifespan [4]. Genetic mutants with a reduced pharyngeal pumping rate, eat-2 mutants, have a decreased food intake rate and show a longevity phenotype [5]. Mice fed a diet with a 40% reduction in calories exhibit a 43% extension in lifespan and reduced oxidative damage to mitochondrial proteins and DNA [6].
Although various scientific results have been reported to explain this phenomenon, the mechanisms by which DR extends lifespan are still unclear. In C. elegans, increased longevity by DR is mediated by activated protein kinase (AMPK) and dependent on DAF-16 [7]. The lifespan extension of the eat-2 mutant is suppressed in eat-2; sir-2.1 double mutants, suggesting dependence on SIR-2.1, a C. elegans homolog of the Sir-2 family of NAD+-dependent protein deacetylases [8]. PHA-4, a transcription factor required for the development of foregut, and SKN-1, a protein that regulates response to oxidative stress, are necessary for response to DR [9, 10]. A gene-expression profiling study identified two downstream targets of SKN-1, cup-4 and nlp-7, that are necessary for DR-induced longevity [11]. CUP-4 is required for colecomocyte endocytosis, and NLP-7 is a neuropeptide-like protein in C. elegans [11].
In addition to identifying the genetic factors mediating the response to DR, researchers have attempted to find compounds that can mimic the effects of DR on lifespan and age-related changes. Metformin, a well-known drug for type 2 diabetes, increases the lifespan of C. elegans in a manner that requires AMPK [12]. In mice, metformin increases both lifespan and healthspan, such as motility and insulin resistance, without a need for reduced food intake [13]. Resveratrol, a polyphenol compound found in red wine, extends the lifespan of yeast, C. elegans, and D. melanogaster [8, 14]. Recent studies show that treatment with polyamines increases the lifespan of various model organisms [15]. In mice, synthetic polyamines increase survival and retard age-related pathological changes while not affecting food intake [16]. In humans, centenarians have higher levels of polyamines in their blood compared to non-centerarians [17].
N-acetyl-l-cysteine (NAC) is a cysteine derivative with an acetyl group attached to nitrogen. NAC is an organo-sulfur compound found in Allium plants, such as garlic and onion [18]. NAC is known to have antioxidant and anti-cancer properties [19]. It can increase cellular levels of glutathione and thereby modulate the response to oxidative stress [20]. A previous study showed that dietary supplementation with NAC increases resistance to various environmental stressors, such as oxidative stress, heat stress, and ultraviolet irradiation [21]. In C. elegans, NAC significantly extends both mean and maximum lifespan and increases the number of progeny and the gravid period [21]. Expression of two longevity-related genes, hsp-16.2 and sod-3, is significantly induced by NAC treatment in C. elegans [21].
In the present study, we examined the effect of NAC on lifespan as a possible candidate for a DR mimetic. The genetic pathways involved in the action of NAC were studied. We also determined the effect of NAC on one of the most well-documented age-related neurodegenerative diseases, Alzheimer’s disease (AD), using a C. elegans model of AD. Our study can be used for the identification of a novel mimetic of DR and provide scientific background for the development of therapeutic treatments for age-related diseases.
Materials and methods
Worm stains and maintenance
The wild-type N2 strain and all long-lived mutant strains, age-1 (hx546), clk-1 (e2519), and eat-2 (ad465), were purchased from the C. elegans Genetics Center (CGC, Minneapolis/St. Paul, MN, USA). The green fluorescent protein (GFP)-expressing strain TJ356 carrying a transgene daf-16 fused to GFP (zls356 IV [daf-16p::daf-16a/b::GFP, rol-6]), and the transgenic strain CL4176-expressing muscle-specific human amyloid beta (Aβ)1-42 (dvls27 [myo-3/Aβ1-42/let UTR, rol-6]) were used for the DAF-16 localization assay and Aβ-induced toxicity assay, respectively. Worms were cultured on agar plates containing Nematode Growth Media (NGM) (1.7% agar, 2.5 mg/ml peptone, 25 mM NaCl, 50 mM KH2PO4 pH 6.0, 5 μg/ml cholesterol, 1 mM CaCl2, and 1 mM MgSO4) at 20 °C. Escherichia coli OP50 was used as the food source.
Lifespan assay
Five young adult (L4) worms were allowed to lay eggs on a fresh NGM plate at 20 °C. After 4 h, all adult worms were removed from the plate and the eggs were maintained at 20 °C for 3 days. Sixty age-synchronized worms were transferred to fresh NGM plates pretreated with 5-fluoro-2′-deoxyruridine to inhibit internal hatching. The live worms were transferred to fresh NGM plates every other day. The number of worms survived and dead was scored every day until all worms were dead. Worms lost during the assay or exhibiting internal hatching were excluded from the statistical analysis.
DR using bacterial dilution
Young adult age-synchronized worms (n = 60) were transferred to fresh NGM plates containing NAC (0 or 5 mM), 5-fluoro-2′-deoxyuridine (12.5 mg/l), and 500 μl of 1000 × Ampicillin. Lifespans were compared between control (E. coli, 5 × 109 cells/ml) and those subjected to DR (5 × 108 cells/ml). Next, 200 µl of a bacteria culture at each concentration was spotted on NGM plates.
Gene knockdown by RNAi
All RNAi clones studied were taken from the Ahringer RNAi library and verified by sequencing [22]. An empty vector (EV) bacterial clone was used as a negative control for gene knockdown by RNAi. Worms were fed RNAi from the larval stages. Five gravid young adult worms were transferred to a fresh NGM plate containing 100 µg/ml ampicillin, 12.5 µg/ml tetracycline, 0.4 mM isopropyl-β-D-thio-galactoside (IPTG, Sigma-Aldrich, St. Louis, MO, USA), and 0.5 mg/ml 5-fluoro-2′-deoxyuridine and spotted with culture fluid of RNAi bacteria clone expressing the necessary double-stranded RNA. All adult worms were removed after 4 h of egg-laying. After 3 days, sixty age-synchronized worms were picked and transferred to a fresh plate with double-stranded RNA-expressing bacteria (n = 60).
Subcellular localization of DAF-16
Age-synchronized TJ356 worms were exposed to 5 mM NAC at 20 °C for 7 and 9 days. Then, the worms were mounted on a glass slide coated with 2% agarose and anesthetized with 1 M sodium azide. After the slide was covered with a coverslip, the expression of DAF-16::GFP was observed using a confocal microscope (Olympus FV10i, Olympus, Tokyo, Japan). The worms were classified according to subcellular localization of DAF-16::GFP as cytosolic, nucleus, or intermediate (both cytosolic and nuclear).
Aβ-induced toxicity
The CL4176 strain has a human Aβ1-42 transgene, the expression of which is induced in muscles by heat shock and leads to rapid paralysis via aggregation of Aβ peptides [23]. Five L4/young adult CL4176 worms were transferred to a fresh NGM plate and were allowed to lay eggs for 5 h at 15 °C. After the five adult worms were removed, eggs were maintained at 15 °C for 5 days. Fifteen to thirty young-adult worms were transferred to a fresh NGM plate for 2 h at 15 °C to lay eggs. Then, the NGM plates were placed at 25 °C. From 24 hours after the temperature increase, paralyzed worms were scored every hour until all worms were paralyzed. A worm moving only its head or not responding to mechanical stimuli was considered to be paralyzed.
Statistical analysis
For statistical analysis of lifespan and paralysis assay, we employed the log-rank test [24]. The log-rank test is a non-parametric Mantel-Cox test widely used for statistical comparison between two survival curves. A p value lower than 0.05 was considered as statistically significant. For comparison of the subcellular localization of DAF-16 between untreated control and NAC-treated worms, we used the standard two-tailed Student’s t-test.
Results and discussions
The lifespan-extending effect of NAC overlaps with that of the eat-2 mutation
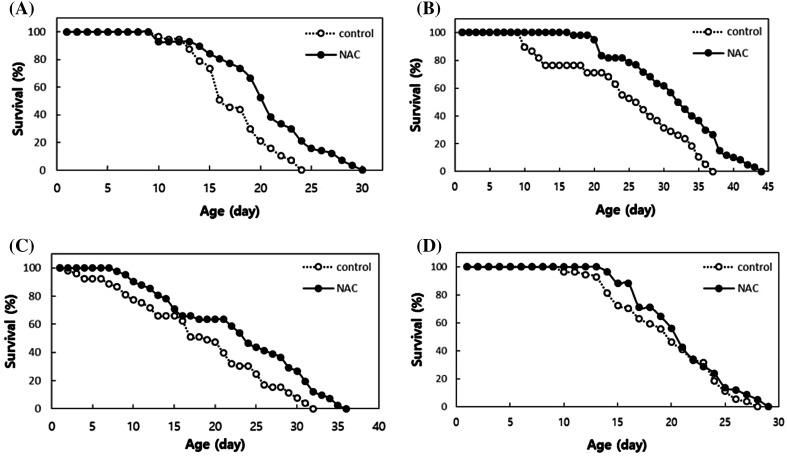
To determine which lifespan-extending process is involved with NAC among three known processes mentioned previously, we examined the effect of dietary supplementation with NAC on the lifespan of long-lived mutants representing each lifespan-extending process. The age-1 mutants have reduced insulin/IGF-1-like signaling and show the longevity phenotype [1]. The clk-1 mutants have reduced mitochondrial electron transport chain activity and live longer than wild-type N2 individuals [3]. The eat-2 mutants take in less food owing to the lower food intake rate and hence are widely used as a genetic model of DR [4]. As previously reported, NAC treatment extended the lifespan of wild-type N2 worms (Fig. 1A). The mean lifespan increased from 17.5 to 20.7 days when N2 worms were treated with NAC (p < 0.001). There was an additional lifespan extension in age-1 and clk-1 animals that were fed NAC (Fig. 1B, C). The mean lifespans of age-1 mutants were 24.8 and 31.8 days in untreated and NAC-treated worms, respectively (p = 0.002). NAC supplementation in clk-1 mutants increased the mean lifespan from 18.4 to 23.0 days (p < 0.001). The additional lifespan-extension in age-1 or clk-1 mutants with NAC suggests that the cellular lifespan-extending process involved with NAC may be independent of reduced insulin/IGF-1-like signaling or mitochondrial electron transport chain activity. Interestingly, supplementation with NAC failed to further extend the lifespan of eat-2 (p = 0.278) (Fig. 1D). Therefore, it is suggested that eat-2 mutation and NAC supplementation modulate the same lifespan-extending process in C. elegans. Independent replicative experiments also showed similar effects on the lifespan of long-lived mutants (Table S1). The lifespan-extending effect of NAC was first shown in the fruit fly, D. melanogaster [25]. In the present study, we showed for the first time that NAC-induced longevity mimics the effect of DR on lifespan. The effects of NAC specifically overlap with the lifespan-extending effects of the eat-2 mutation but not with age-1 or clk-1 mutations, which extend lifespan via reduced insulin/IGF-1-like signaling and mitochondrial electron transport chain activity, respectively [1, 26].
Fig. 1.
The effect of NAC on the lifespan of N2 and long-lived mutants. (A) Dietary supplementation with NAC significantly increases the lifespan of wild-type N2 (p < 0.05). (B) There is an additional lifespan extension by NAC in the long-lived age-1 mutants, in which lifespan increases owing to reduced insulin/IGF-1-like signaling. (C) The long lifespan of clk-1, conferred by reduced mitochondrial electron transport chain activity, is further extended by NAC treatment. (D) The lifespan of the genetic model of DR, eat-2, is not affected by NAC supplementation (p > 0.05)
NAC mimics the effect of DR on lifespan
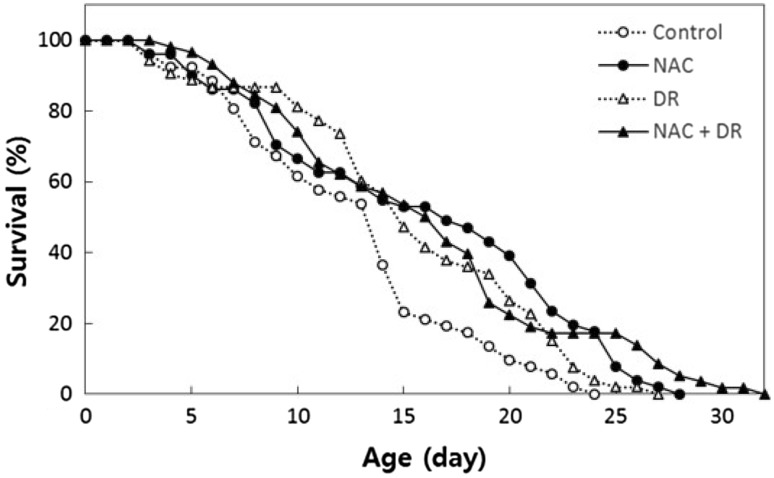
After finding that NAC supplementation has no effect on lifespan extension in eat-2 mutants, we examined the effect of NAC on the lifespan of animals with restricted food supply. Because C. elegans use E. coli as a food source, the most widely used DR method is to restrict the number of bacteria added to the culture plates. As the number of bacteria is reduced, lifespan significantly extends (Fig. 2). The mean lifespan of the control group, fed 5 × 109 bacteria/mL, was 12.7 days, whereas that of the DR group, fed 5 × 108 bacteria/ml, was 15.5 days (p = 0.041, 21.4% increase). Dietary supplementation with NAC in worms fed 5 × 109 bacteria/ml significantly extended the lifespan compared to untreated controls. The mean lifespan of the NAC-treated group was 16.0 days (p = 0.003, 26.0% increase). However, the lifespan of worms subject to both DR and NAC-treatment (mean lifespan, 16.2 days) was not significantly different from that of the worms treated either with DR alone or NAC alone (Fig. 2). Our results support our previous conclusion that the effects of NAC and DR overlap and suggest that dietary supplementation with NAC mimics DR-induced longevity in C. elegans. Independent repeated experiments also showed similar effects on lifespan (Table S2).
Fig. 2.
The overlapping effect of NAC and DR on lifespan extension. Dietary supplementation with NAC or DR using bacterial dilution significantly increases both mean and maximum lifespan in wild-type controls (n = 60). The control group is fed 5 × 109 cells/ml bacteria, and the DR group is fed 5 × 108 cells/ml bacteria. There was no additional increase in lifespan by the combined intervention of NAC and DR compared to the lifespan of worms treated with either NAC or DR alone (p > 0.05)
NAC is independent of SKN-1 and requires DAF-16
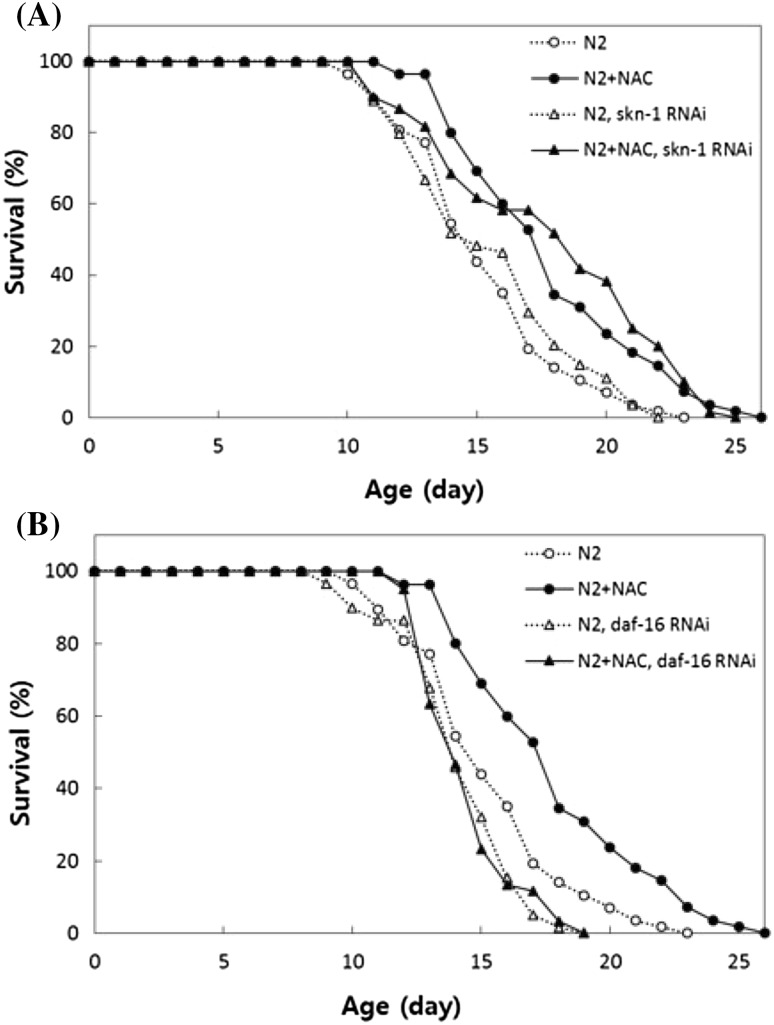
Next, we attempted to determine the role of NAC in DR-related longevity. Many genetic factors involved in DR-related longevity have been identified, including SKN-1, PHA-4, SIR-2, AMPK, and DAF-16 [7–11]. However, the exact cellular mechanisms involved with each factors have not been fully understood yet. We examined the effect of knocking down two genes, skn-1 and daf-16, which are required for lifespan extension by DR. SKN-1 is an worm ortholog of mammalian transcription factor Nrf-2 and expressed in two ASI neurons and the intestine [10]. A previous study found that activation of SKN-1 in neurons is required for DR-induced lifespan extension and suggested that neuronal SKN-1 senses DR and signals the rest of the body to respond to DR [10]. DAF-16 is an intracellular downstream target of insulin/IGF-1-like signaling and is known to modulate the response to various environmental stressors and play a role in DR-induced lifespan extension [27]. As observed in the current study, supplementation with NAC increased both mean and maximum lifespan significantly (Fig. 3). Longevity phenotype conferred by NAC was not inhibited by skn-1 knockdown. Treatment with NAC increased the lifespan of worms in which the expression of skn-1 was knocked down; the mean lifespans were 15.6 days in the untreated control and 17.9 days in the NAC-treated group (p = 0.002) (Fig. 3A). However, the lifespan-extending effect of NAC disappeared when daf-16 was suppressed. The lifespan was not significantly different between untreated and NAC-treated animals when the expression of daf-16 was blocked. The mean lifespans were 14.3 and 14.6 days in the untreated and NAC-treated worms, respectively (p = 0.869) (Fig. 3B). These data suggest that NAC works independently of SKN-1, presumably involved in other cellular signaling pathway, and requires DAF-16 for lifespan extension in C. elegans. AMPK, which senses cellular energy levels, mediates lifespan extension by DR, and its effect requires the activation of DAF-16 [6]. In the present study, we observed that the lifespan-extending effect of NAC was independent of insulin/IGF-1-like signaling, which also requires DAF-16. These findings suggest that DAF-16 is a common genetic factor involved in both DR response and insulin/IGF-1-like signaling, and NAC can activate DAF-16 particularly through DR response. The lifespan of C. elegans can also be increased by food deprivation or intermittent fasting, which require hsf-1, a transcription factor involved in heat shock response, and rheb-1, a low molecular weight GTPase, respectively [28, 29]. Therefore, it is clear that different methods of DR affect lifespan via different genetic pathways, some independent and others overlapping.
Fig. 3.
The effect of DR-related gene knockdown on NAC-induced longevity. (A) Knockdown of skn-1, encoding a transcription factor required for DR-induced longevity, has no effect on the lifespan extension induced by NAC supplementation (p > 0.05). (B) The lifespan-extending effect of NAC is completely abolished by RNAi of the daf-16 gene, which is one of the downstream targets of DR response in C. elegans (p < 0.05)
Nuclear localization of DAF-16 is accelerated by NAC
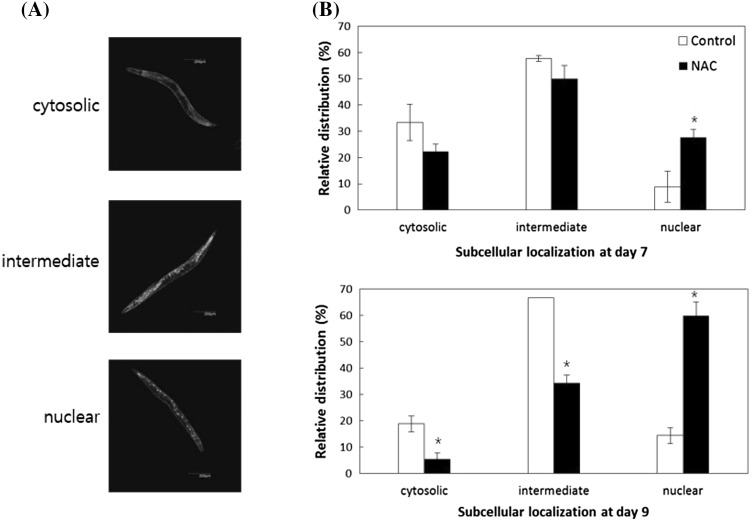
DAF-16-induced increased resistance to stressors and lifespan extension are achieved only when DAF-16 is localized to the nuclei [30]. Having found that DAF-16 is required for the increase in lifespan following NAC treatment, we examined whether NAC induces nuclear localization of DAF-16 in vivo. Subcellular localization of DAF-16 was determined using the TJ356 transgenic strain carrying the daf-16 transgene coupled to GFP. Worms were classified into three groups according to the subcellular distribution of GFP expression: worms in which fluorescence was seen throughout the body were counted as “cytosolic” and worms in which fluorescence was observed only in the nuclei were classified as “nuclear.” In “intermediate” worms, fluorescence was seen in both the cytosol and nuclei (Fig. 4A). Compared to untreated wild-type N2 worms, more nuclear localization of DAF-16 was observed in NAC-treated worms (Fig. 4B). In 7-day-old NAC-treated worms, 27.8 ± 2.94% (mean ± SEM) exhibited nuclear localization of DAF-16, whereas only 8.9 ± 5.89% showed DAF-16 nuclear translocation in the untreated controls (p = 0.045). In 9-day-old worms, the proportion of worms showing only cytosolic localization of DAF-16 decreased from 18.9 ± 2.94% to 5.6 ± 2.22% with NAC treatment (p = 0.022). Supplementation with NAC also significantly decreased the proportion of worms classified as “intermediate:” 66.7 ± 0.00% of wild-type control and 34.4 ± 2.94% of NAC-treated worms. The percentage of worms showing only nuclear-localized DAF-16 increased from 14.4 ± 2.94% to 60.0 ± 5.09% with NAC treatment. These results indicate that NAC possibly mimics DR-induced longevity through nuclear localization of DAF-16. Given the results presented herein, we suggest that NAC works upstream of DAF-16 and extends lifespan by stimulating nuclear localization of DAF-16. Future studies focusing on the identification of genetic pathways involved in NAC-induced longevity will broaden our understanding of the cellular mechanisms of lifespan extension via NAC and specify the molecular targets of NAC in vivo.
Fig. 4.
Modulation of subcellular localization of DAF-16 by NAC. (A) Subcellular localization of DAF-16 can be observed with the GFP transgene fused with the daf-16 gene. Worms are classified into three groups, “cytosolic,” “intermediate,” and “nuclear,” depending on the subcellular distribution of fluorescence. (B) In the worms pretreated with NAC, more GFP localizations into nuclei are observed compared to untreated control (n = 100). The error bar indicates standard error. Asterisk means the difference is statistically significant (p < 0.05)
NAC ameliorates Aβ-induced toxicity in an AD model
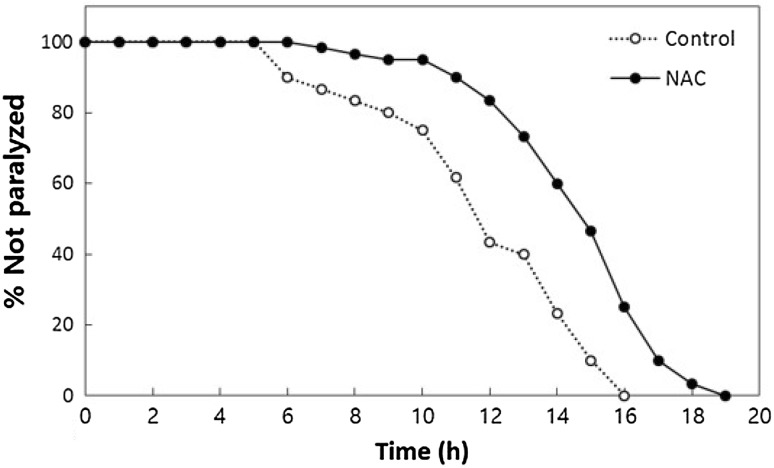
The probability of occurrance of many degenerative diseases increases exponentially with age. To investigate the effects of NAC on an age-related neurodegenerative disease, AD, we employed a C. elegans AD model. Worms pretreated with NAC showed significantly delayed Aβ-induced paralysis (Fig. 5). In the wild-type control group, 50% of worms were paralyzed 11.9 h after Aβ induction. However, 50% of NAC-treated worms were paralyzed after 14.8 h (24% increase, p < 0.001). Independent replicate experiments also showed a significant prevention of Aβ-induced toxicity by NAC treatment (data not shown). A previous study showed that DAF-16 is required for the inhibition of Aβ toxicity in this AD model [31]. Our results suggest that NAC has potential as a novel therapeutic treatment for AD. Previous studies show that some lifespan-extending compounds also have therapeutic effects on age-related diseases. The antioxidant chicoric acid reduces ROS accumulation and increases the lifespan of C. elegans [32]. Chicoric acid is reported to have strong anti-diabetic effects [32]. Another study found that a drug used to treat type 2 diabetes, metformin, extends the lifespan of C. elegans and mice [12, 13]. Treatment with metformin delays age-related reduction in mobility and accumulation of lipofuscin, a biomarker of aging [12]. Interestingly, the longevity phenotype conferred by metformin is independent of age-1 and requires AMPK and SKN-1, suggesting that metformin extends lifespan in a manner similar to DR and is not related to reduced insulin/IGF-1-like signaling [12]. Here, we found that NAC significantly decreases Aβ-induced toxicity in an AD model and mimics DR-induced lifespan extension. These data suggest that NAC is a promising candidate for a DR mimetic that extends lifespan and reduces Aβ-induced toxicity, which causes age-related AD. Further studies regarding the effect of NAC on aging in mammals and the identification of genetic pathways and target molecules involved in NAC-induced longevity should be conducted in the near future.
Fig. 5.
Inhibition of Aβ-induced toxicity by NAC. Age-synchronized worms harboring human Aβ transgene are pretreated with NAC (n = 60). Heat shock induces the expression of human Aβ transgene in muscle tissues and causes paralysis with time. Dietary supplementation with NAC significantly delays paralysis resulted from the induction of the human Aβ transgene (p < 0.05)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2015R1D1A1A01057435).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249:908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- 2.Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299:1342–1346. doi: 10.1126/science.1077991. [DOI] [PubMed] [Google Scholar]
- 3.Sedensky MM, Morgan PG. Mitochondrial respiration and reactive oxygen species in C. elegans. Exp. Gerontol. 2006;41:957–967. doi: 10.1016/j.exger.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 4.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 5.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994;74:121–133. doi: 10.1016/0047-6374(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 7.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 9.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447:550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 10.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 11.Park SK, Link CD, Johnson TE. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J. 2010;24:383–392. doi: 10.1096/fj.09-142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 15.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Fröhlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 16.Soda K, Dobashi Y, Kano Y, Tsujinaka S, Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009;44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Pucciarelli S, Moreschini B, Micozzi D, De Fronzo GS, Carpi FM, Polzonetti V, Vincenzetti S, Mignini F, Napolioni V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012;15:590–595. doi: 10.1089/rej.2012.1349. [DOI] [PubMed] [Google Scholar]
- 18.Yin MC, Hwang SW, Chan KC. Nonenzymatic antioxidant activity of four organosulfur compounds derived from garlic. J. Agric. Food Chem. 2002;50:6143–6147. doi: 10.1021/jf0204203. [DOI] [PubMed] [Google Scholar]
- 19.Yedjou CG, Tchounwou PB. N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health. 2007;4:132–137. doi: 10.3390/ijerph2007040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daraie B, Pourahmad J, Hamidi-Pour N, Hosseini MJ, Shaki F, Soleimani M. Uranyl acetate induces oxidative stress and mitochondrial membrane potential collapse in the human dermal fibroblast primary cells. Iran. J. Pharm. Res. 2012;11:495–501. [PMC free article] [PubMed] [Google Scholar]
- 21.Oh SI, Park JK, Park SK. Lifespan extension and increased resistance to environmental stressors by N-acetyl-l-cysteine in Caenorhabditis elegans. Clinics (Sao Paulo) 2015;70:380–386. doi: 10.6061/clinics/2015(05)13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 23.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J. Royal Stac. Soc. Ser. A. 1972;135:185–207. doi: 10.2307/2344317. [DOI] [Google Scholar]
- 25.Brack C, Bechter-Thüring E, Labuhn M. N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell. Mol. Life Sci. 1997;53:960–966. doi: 10.1007/PL00013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallo M, Park D, Riddle DL. Increased longevity of some C. elegans mitochondrial mutants explained by activation of an alternative energy-producing pathway. Mech. Ageing Dev. 2011;132:515–518. doi: 10.1016/j.mad.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 29.Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 31.Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat. Rev. Neurosci. 2008;9:759–767. doi: 10.1038/nrn2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, Casas F, Pessemesse L, Fouret G, Feillet-Coudray C, Cros G, Cabello G, Magous R, Wrutniak-Cabello C. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS One. 2013;8:e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.