Abstract
Although ginsenosides Rb1, Rb2, Rc, and Rd and ginseng extracts are shown to inhibit pancreatic lipase (PL) activity, the effects of the other ginsenosides, particularly the deglycosylated ones that are considered to show stronger biological activities than the glycosylated forms, are not clear. In this study, we observed the effects of various ginsenosides on PL activity. Results showed that the effects vary with each individual ginsenoside. Ginsenosides Rb1, Rd, Rg1, Rg3, and compound K significantly suppressed 43, 47, 75, and 55% of PL activity at the concentration of 100 μg/mL, respectively. Rg3 was discovered to be the most effective among various common ginsenosides, with a minimum effective concentration of 6.25 μg/mL. Ginsenosides F2 and Rf slightly enhanced PL activity. In addition, fermentation markedly enhanced the inhibitory effect of the ginseng root and ginseng berry, which might be attributed to the changes of ginsenoside profiles.
Keywords: Ginsenoside, Pancreatic lipase, Ginseng root, Ginseng berry, Fermentation
Introduction
Obesity is considered to increase the risk of various diseases, particularly heart disease, type 2 diabetes, and certain types of cancer and impair the quality of life [1–3]. Retarding the digestion and absorption of carbohydrates and fats in the gastrointestinal tract reduces energy harvest, which helps to prevent and treat obesity and diabetes. Numerous bioactive components from medicinal herbs have been reported to work as glucosidase inhibitors [4–6] or lipase inhibitors [7–9] and thereby prevent excess energy intake.
Ginseng is an ancient tonic reported to exert various physiological actions such as anti-cancer [10], anti-diabetes [11], and anti-inflammation [12]. In addition, it was demonstrated that ginseng showed an anti-obesity effect in murine fed a high-fat diet (HFD) [13]. Ginsenosides are the main components responsible for the biological activities of ginseng. Apart from ginseng root (GR), ginseng berry (GB), leaf, and stem were also indicated to contain ginsenosides [14, 15] and might show a distinct effect since they possess different ginsenoside profiles [16].
Pancreatic lipase (PL), released by the pancreas, is an enzyme that converts triglyceride into free fatty acids and glycerol before absorption in the digestive system [17]. Ginsenosides Rb1, Rb2, Rc, and Rd and ginseng extracts have been shown to suppress PL activity [18–21]. HFD-fed mice and rats supplemented with ginseng extracts also showed an increased amount of feces and fecal lipid content [22, 23], which indicated that ginseng or ginsenosides might prevent or treat obesity via increasing the excretion of fat via feces. However, the inhibitory effects on the PL activity of other ginsenosides, particularly the deglycosylated forms, have not yet been reported. The dammarane-type ginsenosides can be divided into two types: protopanaxadiol (PPD) and protopanaxatriol (PPT), according to the number of hydroxyl groups joined to sugar moieties by the dehydration reaction [24]. In addition, glycosylated forms of ginsenosides such as Rb1 and Re can be transformed to their deglycosylated forms such as compound K (cK) and Rh1, respectively by acid hydrolysis [25], enzymatic hydrolysis [26], or fermentation [15]. Moreover, deglycosylated ginsenosides are usually considered to show stronger biological activities than the primary forms [27]. In this study, we observed and compared the effects of various ginsenosides like the PPD-type Rb1, Rb2, Rc, Rd, Rg3, F2, Rh2, and cK and the PPT-type Re, Rf, Rg1, Rg2, and Rh1. The chemical structures of these ginsenosides are presented in Table 1. In addition, the effects of fermented ginseng root (FGR) and fermented ginseng berry (FGB) along with the unfermented ones are compared and analyzed since fermentation change the ginsenoside profiles.
Table 1.
Chemical structures of various ginsenosides
| R1 | R2 | R3 | |
|---|---|---|---|
| 20(S)-Protopanaxadiol-type | |||
| Rb1 | O-Glc(2-1)Glca | H | O-Glc(6-1)Glc |
| Rb2 | O-Glc(2-1)Glc | H | O-Glc(6-1)Arapb |
| Rc | O-Glc(2-1)Glc | H | O-Glc(6-1)Arap |
| Rd | O-Glc(2-1)Glc | H | O-Glc |
| Rg3 | O-Glc | H | OH |
| F2 | O-Glc | H | O-Glc |
| cK | OH | H | O-Glc |
| Rh2 | O-Glc | H | OH |
| 20(S)-Protopanaxatriol-type | |||
| Re | OH | O-Glc(2-1)Rhac | O-Glc |
| Rf | OH | O-Glc(2-1)Glc | OH |
| Rg1 | OH | O-Glc | O-Glc |
| Rg2 | OH | O-Glc(2-1)Rha | OH |
| Rh1 | OH | OH | O-Glc |
aβ-D-glucopyranosyl
bα-L-arabinopyranosyl
cα-L-rhamnopyranosyl
Materials and methods
Materials
Ginsenosides such as Rb1, Rb2, Rc, Rd, Rf, Rg1, and cK were purchased from Biotech (Nanjing, China). Ginsenosides Re, Rg2, Rg3, F2, Rh1, and Rh2 were obtained from Cogon Biotech (Chengdu, China). The NEFA assay kit was purchased from Wako (Osaka, Japan), whereas porcine pancreatic lipase (L3126), orlistat (O4139), and dimethylsulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). Finally, triolein was purchased from Avention (Incheon, Korea).
Fermentation of ginseng root and ginseng berry
GR and GB were fermented with mycotoxin non-producing Aspergillus niger and Aspergillus orzyae, respectively, based on methods presented in the previous study [15]. After fermentation, the culture broth was freeze-dried and extracted with water-saturated n-butanol at 80 °C. After filtration with Whatman No. 41 filter paper (Kent, UK), the filtrate was mixed with distilled water and stewed overnight. The upper phase was evaporated with a speed vacuum concentrator (Labogene, Denmark), and the residue was suspended in diethyl ether and extracted at 46 °C for 1 h to remove the fat-soluble impurities. After centrifuged at 8000 rpm for 10 min, the supernatant was discarded and the remaining solid content was used as samples for PL activity assay. The ginsenoside contents in each sample were determined by high performance liquid chromatography (HPLC) as described previously [15].
Activity assay of pancreatic lipase
Crude PL powder was suspended in sterilized distilled water (0.1 g/mL) and centrifuged at 12,000×g for 10 min. The supernatant was used as an enzyme solution while Tris–HCl buffer (0.1 M, pH = 8) was used as the assay buffer. Triolein was dissolved in acetone at a concentration of 5% and the mixture was used as a substrate solution. Various ginsenosides and the n-butanol fractions of GR, GB, FGR, and FGB were dissolved in DMSO. Afterward, 80 μL of the assay buffer, 5 μL of the enzyme solution, and 10 μL of various samples were added to a 96-well plate. The reaction was started by adding 5 μL of the substrate solution at 37 °C with 550 rpm shaking. After 30 min, the reaction was stopped by putting the plate on ice for 10 min. Then, 7 μL of the reaction mixture was transferred to another 96-well plate. The generated amount of oleic acid in the reaction mixture was determined using a NEFA assay kit according to the manufacture’s instruction.
Statistical analysis
Results were expressed as mean ± standard deviation. Differences were tested using one-way ANOVA and applying the least significant range tests. Statistical analyses used the SPSS statistical package (Chicago, IL). The significance level of the test results was set to p < 0.05.
Results and discussion
Effects of various ginsenosides on pancreatic lipase activity
PPD-type ginsenosides Rb1, Rb2, Rc, Rd, Rg3, F2, Rh1, and cK on PL activity were tested in vitro [Fig. 1(A)]. Results showed that ginsenosides Rb1, Rb2, Rc, Rd, Rg3, and cK have a tendency to inhibit PL activity. Among them, ginsenosides Rb1, Rd, Rg3, and cK significantly inhibited 43, 47, 75, and 55% of the PL activity, respectively, at the concentration of 100 μg/mL. Ginsenoside Rh2 at the tested concentration showed no effect, whereas ginsenoside F2 significantly increased 42% of the PL activity at the concentration of 200 μg/mL. Interestingly, ginsenoside Rg3 showed the most effective inhibitory effect, whereas its metabolite, ginsenoside Rh2, showed no effect. Overall, ginsenosides of deglycosylated form such as Rg3 and cK showed more effective inhibitory effect than the glycosylated forms of ginsenosides Rb1, Rb2, Rc, and Rd.
Fig. 1.
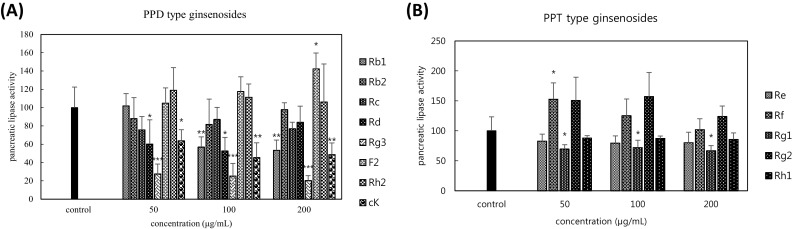
Effects of various PPD-type ginsenosides (A) and PPT-type ginsenosides (B) on the activity of pancreatic lipase. Pancreatic lipase activity = (Abs after treatment/Abs of control) × 100%. *p < 0.05, **p < 0.01, ***p < 0.001 versus control (n = 3)
With respect to PPT-type ginsenosides [Fig. 1(B)], ginsenosides Re and Rh1 showed no effect, whereas ginsenoside Rg1 significantly inhibited 30% of PL activity at the concentration of 50 μg/mL. Both ginsenosides Rf and Rg2 showed the tendency to increase PL activity, but only ginsenoside Rf significantly enhanced it.
Results show that the effects of ginsenosides on PL activity vary with each individual ginsenoside and deglycosylation might somehow enhance the effects. Overall, PPD-type ginsenosides showed more effective inhibitory effects on the PL activity compared with PPT-type ginsenosides. Since ginsenoside Rg3 was found to inhibit almost 70% of PL activity at the concentration of 50 μg/mL, we tested it again at much lower concentrations.
As shown in Fig. 2(A), the minimum effective concentration of ginsenoside Rg3 was 6.25 μg/mL, where about 40% of PL activity was inhibited. The half maximal inhibitory concentration (IC50) of ginsenoside Rg3 was between 6.25 and 12.5 μg/mL. Orlistat, an anti-obesity drug, was used as a positive control [Fig. 2(B)]. Orlistat also works as a lipase inhibitor. The mechanism of ginsenosides on PL activity might be different from orlistat, which inhibits PL activity by covalently binding to the serine residue of the active site [28]. It was supposed that ginsenosides might attach to the surface of triglyceride droplet and disturb the access of PL to the substrate [19].
Fig. 2.

Inhibitory effects of ginsenoside Rg3 (A) and orlistat (B) on the activity of pancreatic lipase. Pancreatic lipase activity = (Abs after treatment/Abs of control) × 100%. ***p < 0.001 versus control (n = 3)
Changes of ginsenoside profiles in ginseng root and ginseng berry after fermentation
GR and GB were fermented with mycotoxin-free Aspergillus niger and Aspergillus oryzae. The ginsenoside profiles of GR, GB, FGR, and FGB analyzed with HPLC [Fig. 3) were presented in Table 2. The primary ginsenosides were transformed to the deglycosylated forms. With respect to the root, PPD-type ginsenosides Rb1, Rb2, Rc, and Rd decreased from 93, 37, 25, and 50 mg/g, respectively, to 0. Ginsenoside Rg3 increased from 9 to 49 mg/g, cK increased from 0 to 90 mg/g, and Rh2 increased from 0 to 29 mg/g. As for the berry, PPT-type ginsenoside Re decreased from 212 to 0 mg/g, and Rg1 increased from 79 to 191 mg/g. From the structure, ginsenoside Re can be transformed to Rg1 after the rhamnose residue is removed.
Fig. 3.
HPLC chromatograms of ginsenosides detected from the extracts of ginseng berry (A), fermented ginseng berry (B), ginseng root (C) and fermented ginseng root (D)
Table 2.
Ginsenoside profiles of ginseng root, ginseng berry, fermented ginseng root, and fermented ginseng berry (mg/g)
| Rb1 | Rb2 | Rc | Rd | Re | Rf | Rg1 | Rg2 | Rg3 | F2 | Rh1 | Rh2 | cK | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GR | 93 | 37 | 25 | 50 | 71 | 43 | 68 | – | 9 | – | – | – | – |
| FGR | – | – | – | – | 30 | 25 | 46 | 19 | 49 | 14 | 27 | 29 | 90 |
| GB | 20 | 49 | 42 | 99 | 212 | – | 79 | 20 | – | – | – | – | – |
| FGB | 5 | 25 | 22 | 13 | – | – | 191 | 6 | 34 | 92 | 79 | – | 13 |
The contents of PPD-type ginsenosides such as Rb1, Rb2, Rc, Rd, Rg3, F2, Rh2, and cK and PPT-type ginsenosides such as Re, Rf, Rg1, Rg2, and Rh1 in n-butanol extracts were measured by HPLC (<1 mg/g)
GR ginseng root, FGR fermented ginseng root, GB ginseng berry, FGB fermented ginseng berry
As reported previously, ginsenoside Rb1 could be transformed to cK and ginsenoside Re could be transformed to Rh1 via Rg1 by glycosidases separated from microorganism [29]. Ginsenoside Rb1 is the most abundant ginsenoside in GR; however, after fermentation, the most abundant ginsenoside in FGR is ginsenoside cK. Similarly, ginsenoside Re is the most abundant ginsenoside in GB and ginsenoside Rg1 is the most abundant in FGB.
Effects of ginseng root and ginseng berry on pancreatic lipase activity
The effects of n-butanol fractions of GR, GB, FGR, and FGB on PL were also tested. In terms of the root, both GR and FGR significantly inhibited PL activity [Fig. 4(A)]. GR and FGR inhibited 52 and 86% of the PL activity at the concentration of 1 mg/mL, respectively. Fermentation enhanced the inhibitory effect of GR, which might be attributed to the increased contents of ginsenosides cK and Rg3.
Fig. 4.

Inhibitory effects of ginseng root (A) and ginseng berry (B) on the activity of pancreatic lipase. GR ginseng root, FGR fermented ginseng root, GB ginseng berry, FGB fermented ginseng berry. Pancreatic lipase activity = (Abs after treatment/Abs
With respect to the berry, GB showed no effect at the tested concentration, whereas FGB significantly inhibited 48% of the PL activity at the concentration of 0.25 mg/mL [Fig. 4(B)]. Fermentation dramatically enhanced the inhibitory effect of GB, which might be attributed to the increased contents of ginsenosides Rg1, Rg3, and cK.
At the concentration of 1 mg/mL, FGR inhibited nearly 90% of the PL activity, whereas FGB only inhibited about 60% PL activity. Because PPD-type ginsenosides are more effective than the PPT-type and FGR contains more PPD-type ginsenosides such as Rg3 and cK than FGB, FGR was more effective than FGB with regards to the inhibitory effect of PL activity.
A large number of research has indicated that ginseng or ginsenoside has anti-obesity effects on HFD-fed mice or rats [22, 30–32]. Ginsenoside Rb1 was reported to significantly ameliorate hepatic fat accumulation in HFD-induced obese rats [33]. Ginsenoside Re was reported to markedly lower blood glucose and triglyceride levels and protect against hepatic steatosis in HFD-fed mice [34]. cK was reported to possess hypoglycemic and insulin-sensitizing capabilities on type 2 diabetes induced by HFD and streptozotocin [35]. Ginsenoside Rh1 was reported to attenuate obesity by inhibiting adipocyte differentiation and inflammation [36].
Because dietary lipids are the major source of superfluous energy, the inhibition of PL activity and digestion of triglycerides in the intestine might be very important for the anti-obesity effect of ginseng products. The development of obesity is characterized by a long-term imbalance between energy intake and energy expenditure, and excess food intake is considered a primary cause [37]. Although several anti-obesity drugs, like orlistat and lorcaserin, are available to treat obesity, long-term use of these drugs is reported to cause many side effects and is invariably accompanied with weight regain after drug discontinuation [38, 39]. Complementary and alternative therapies, long used in the Eastern world, are currently receiving considerable attention and eliciting widespread interest worldwide. The inhibitory effect of ginsenosides on PL activity might be an important mechanism of the anti-obesity effect of ginseng and some ginsenosides.
In conclusion, the inhibitory effect on the PL activity of various common ginsenosides was evaluated to show that ginsenoside Rg3 was the most effective. Ginsenoside Rg3 can be generated from ginsenoside Rb1 or Rb2 by steaming, and red ginseng was shown to be rich in ginsenoside Rg3. In addition, the PPD-type ginsenosides are more effective than the PPT-type ginsenosides. Ginsenoside cK, a regular metabolite of the PPD-type ginsenosides and reputed to exert various biological activities [35, 40], was found to more remarkably suppress the PL activity than its precursors. Moreover, extracts of GR were shown to be more effective than that of GB, and fermentation efficiently enhanced the inhibitory effect of GR and GB on PL activity, which might be attributed to the increased contents of ginsenosides Rg1, cK, and Rg3.
Acknowledgements
This work was supported by the Cooperative Research Program for Agriculture Science and Technology Development, Rural Development Administration, Republic of Korea (Project No. PJ01123001) and the Promoting Regional Specialized Industry, the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT), Republic of Korea (Project No. R0004140).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Hauner H. Obesity and diabetes. Textbook of Diabetes, Fourth Edition, Wiley, Hoboken, NJ, 227–241 (2010)
- 2.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens. Res. 2010;33:386–393. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 3.Wolin KY, Carson K, Colditz GA. Obesity and cancer. The oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Önal S, Timur S, Okutucu B, Zihnioğlu F. Inhibition of α-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep. Biochem. Biotechnol. 2005;35:29–36. doi: 10.1081/PB-200041438. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wen S, Kota BP, Peng G, Li GQ, Yamahara J, Roufogalis BD. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005;99:239–244. doi: 10.1016/j.jep.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Fatmawati S, Kondo R, Shimizu K. Structure–activity relationships of lanostane-type triterpenoids from Ganoderma lingzhi as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2013;23:5900–5903. doi: 10.1016/j.bmcl.2013.08.084. [DOI] [PubMed] [Google Scholar]
- 7.Glisan SL, Grove KA, Yennawar NH, Lambert JD. Inhibition of pancreatic lipase by black tea theaflavins: Comparative enzymology and in silico modeling studies. Food Chem. 2017;216:296–300. doi: 10.1016/j.foodchem.2016.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchholz T, Melzig MF. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015;81:771–783. doi: 10.1055/s-0035-1565716. [DOI] [PubMed] [Google Scholar]
- 9.Badmaev V, Hatakeyama Y, Yamazaki N, Noro A, Mohamed F, Ho C-T, Pan M-H. Preclinical and clinical effects of Coleus forskohlii, Salacia reticulata and Sesamum indicum modifying pancreatic lipase inhibition in vitro and reducing total body fat. J. Funct. Foods. 2015;15:44–51. doi: 10.1016/j.jff.2015.03.007. [DOI] [Google Scholar]
- 10.Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat. Prod. Rep. 2015;32:256–272. doi: 10.1039/C4NP00080C. [DOI] [PubMed] [Google Scholar]
- 11.Yuan HD, Kim JT, Kim SH, Chung SH. Ginseng and Diabetes. J. Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-α-mediated inflammation: a mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J. Pharmacol. Sci. 2005;97:124–131. doi: 10.1254/jphs.FP0040184. [DOI] [PubMed] [Google Scholar]
- 14.Zhai L, Li Y, Wang W, Wang Y, Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Ahn HJ, Kim NY, Lee YN, Ji GE. Korean ginseng berry fermented by mycotoxin non-producing Aspergillus niger and Aspergillus oryzae: Ginsenoside analyses and anti-proliferative activities. Biol. Pharm. Bull. 2016;39:1461–1467. doi: 10.1248/bpb.b16-00239. [DOI] [PubMed] [Google Scholar]
- 16.Ko SK, Bae HM, Cho OS, Im BO, Chung SH, Lee BY. Analysis of ginsenoside composition of ginseng berry and seed. Food Sci. Biotechnol. 2008;17:1379–1382. [Google Scholar]
- 17.Chapus C, Rovery M, Sarda L, Verger R. Minireview on pancreatic lipase and colipase. Biochimie. 1988;70:1223–1233. doi: 10.1016/0300-9084(88)90188-5. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Zheng Y, Han L, Wang H, Saito M, Ling M, Kimura Y, Feng Y. Saponins (Ginsenosides) from stems and leaves of Panax quinquefolium prevented high-fat diet-induced obesity in mice. Phytomedicine. 2008;15:1140–1145. doi: 10.1016/j.phymed.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Zhang J, Liu W, Kimura Y, Zheng Y. Anti-obesity effects of protopanaxdiol types of ginsenosides isolated from the leaves of American ginseng (Panax quinquefolius L.) in mice fed with a high-fat diet. Fitoterapia. 2010;81:1079–1087. doi: 10.1016/j.fitote.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Karu N, Reifen R, Kerem Z. Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J. Agric. Food Chem. 2007;55:2824–2828. doi: 10.1021/jf0628025. [DOI] [PubMed] [Google Scholar]
- 21.Min S-W, Jung S-H, Cho K-H, Kim D-H. Antihyperlipidemic effects of red ginseng, crataegii fructus and their main constituents ginsenoside Rg3 and ursolic acid in mice. Biomol. Ther. 2008;16:364–369. doi: 10.4062/biomolther.2008.16.4.364. [DOI] [Google Scholar]
- 22.Lee MR, Kim BC, Kim R, Oh HI, Kim HK, Choi KJ, Sung CK. Anti-obesity effects of black ginseng extract in high fat diet-fed mice. J. Ginseng Res. 2013;37:308–314. doi: 10.5142/jgr.2013.37.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung S, Lee M-S, Shin Y, Kim C-T, Kim I-H, Kim YS, Kim Y. Anti-obesity and anti-inflammatory effects of high hydrostatic pressure extracts of ginseng in high-fat diet induced obese rats. J. Funct. Foods. 2014;10:169–177. doi: 10.1016/j.jff.2014.06.007. [DOI] [Google Scholar]
- 24.Park SU, Ahn D-J, Jeon H-J, Kwon TR, Lim H-S, Choi B-S, Baek K-H, Bae H. Increase in the contents of ginsenosides in raw ginseng roots in response to exposure to 450 and 470 nm light from light-emitting diodes. J. Ginseng Res. 2012;36:198–204. doi: 10.5142/jgr.2012.36.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhongyang F, Xie Z. Degradation of ginsenosides under mild acidic conditions monitored by high performance liquid chromatography. J. Dalian Univer. 1997;4:019. [Google Scholar]
- 26.Ko S-R, Choi K-J, Uchida K, Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1, and F1 from protopanaxatriol-type ginseng saponin mixture. Planta Med. 2003;69:285–286. doi: 10.1055/s-2003-38476. [DOI] [PubMed] [Google Scholar]
- 27.Choo M-K, Park E-K, Han MJ, Kim D-H. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 28.Guerciolini R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 1997;21:S12–S23. [PubMed] [Google Scholar]
- 29.Chi H, Ji GE. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol. Lett. 2005;27:765–771. doi: 10.1007/s10529-005-5632-y. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Li W, Li X, Zhang M, Chen L, Zheng Y-n, Sun G-z, Ruan C-c. Antidiabetic effects of malonyl ginsenosides from Panax ginseng on type 2 diabetic rats induced by high-fat diet and streptozotocin. J. Ethnopharmacol. 2013;145:233–240. doi: 10.1016/j.jep.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 31.Yuan HD, Quan HY, Jung M-S, Kim S-J, Huang B, Kim D-Y, Chung S-H. Anti-diabetic effect of pectinase-processed ginseng radix (GINST) in high fat diet-fed ICR mice. J. Ginseng Res. 2011;35:308–314. doi: 10.5142/jgr.2011.35.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollah ML, Cheon Y-P, In J-G, Yang D-C, Kim Y-C, Song J-C, Kim K-S. Inhibitory effects of cultivated wild ginseng on the differentiation of 3T3-L1 pre-adipocytes. J. Ginseng Res. 2011;35:45–51. doi: 10.5142/jgr.2011.35.1.045. [DOI] [Google Scholar]
- 33.Shen L, Xiong Y, Wang DQ, Howles P, Basford JE, Wang J, Xiong YQ, Hui DY, Woods SC, Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J. Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan HY, Yuan HD, Jung MS, Ko SK, Park YG, Chung SH. Ginsenoside Re lowers blood glucose and lipid levels via activation of AMP-activated protein kinase in HepG2 cells and high-fat diet fed mice. Int. J. Mol. Med. 2012;29:73. doi: 10.3892/ijmm.2011.805. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Zhang M, Gu J, Meng Z-j, Zhao L-C, Zheng Y-n, Chen L, Yang G-L. Hypoglycemic effect of protopanaxadiol-type ginsenosides and compound K on Type 2 diabetes mice induced by high-fat diet combining with streptozotocin via suppression of hepatic gluconeogenesis. Fitoterapia. 2012;83:192–198. doi: 10.1016/j.fitote.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Gu W, Kim KA, Kim DH. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol. Pharm. Bull. 2013;36:102–107. doi: 10.1248/bpb.b12-00558. [DOI] [PubMed] [Google Scholar]
- 37.Bojanowska E, Ciosek J. Can we selectively reduce appetite for energy-dense foods? An overview of pharmacological strategies for modification of food preference behavior. Curr. Neuropharmacol. 2016;14:118–142. doi: 10.2174/1570159X14666151109103147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfe SM. When EMA and FDA decisions conflict: Differences in patients or in regulation. BMJ. 2013;347:f5140. doi: 10.1136/bmj.f5140. [DOI] [PubMed] [Google Scholar]
- 39.Wood S. Diet Drug Orlistat Linked to Kidney, Pancreas Injuries Medscape Apr 14, 2011
- 40.Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, Kim HS, Kim DH, Hyun JW. Effect of compound K, a metabolite of ginseng saponin, combined with γ-ray radiation in human lung cancer cells in vitro and in vivo. J. Agric. Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]



