Abstract
Since time immemorial, turmeric has been widely marketed and consumed as dietary supplement due to its diverse medicinal properties. Curcuminoids—comprising a mixture of curcumin (CUR), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC)—are the prime bioactive constituents of turmeric. However, the usage of curcuminoids is limited by their chemical instability. The lack of information on comparative stability profiles of curcuminoids (in pure and mixture form) prompted us to study how pure curcuminoids and their mixtures behave under different stress degradation conditions. The order of stability of curcuminoids when exposed to acidic, alkaline, and oxidative degradation was found to be as follows: BDMC > DMC > CUR. While the pure and mixture forms of curcuminoids were stable against heat, they completely degraded upon exposure to sunlight. The degradation extent of curcuminoids (in mixture form) was substantially less as compared to their pure form; therefore, this suggested the synergistic stabilizing influence of DMC and BDMC in the curcuminoids’ mixture.
Keywords: Bisdemethoxycurcumin, Curcumin, Demethoxycurcumin, Stability, Stress degradation
Introduction
Turmeric is a most commonly used spice derived from the dried rhizomes of Curcuma longa, a perennial herb belonging to the Zingiberaceae family. In the literature, turmeric has been widely acknowledged for various pharmacological activities such as anti-inflammatory, anti-oxidant, antimicrobial, anticancer, hepatoprotective, and antidiabetic [1, 2]. The bioactivities of turmeric extract have been attributed to the presence of three phenolic compounds namely; curcumin (CUR), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC). These phenolic compounds are collectively known as curcuminoids or a curcuminoid mixture. In general, it is believed that CUR, one of the most extensively studied curcuminoids, is responsible for the therapeutic success of turmeric in a wide range of disorders [3]. However, the latest literature has reported DMC and BDMC as having similar or higher biological activities compared to the CUR. Furthermore, it is well acknowledged that DMC and BDMC have synergistically enhanced the bioactivities of CUR when they are the part of curcuminoid mixture [4].
Despite of their wide range of bioactivities, the use of curcuminoids has been limited by their stability concerns. Curcuminoids undergo degradation by hydrolysis (acidic or alkaline), oxidation and photodegradation [5–7]. The degradation of CUR via a spontaneous autoxidation process at physiological pH yields bicyclopentadione as the major product, while the minor products include; vanillin, ferulic acid, and feruloyl methane [8, 9]. In addition to this, a study has shown that curcuminoids are sensitive to light; therefore, they are completely decolorized upon exposure to ultraviolet and visible light. In this study, vanillin, vanillic acid, ferulic acid, ferulic aldehyde, p-hydroxy benzaldehyde, and p-hydroxybenzoic acid were identified as the photodegradation products of curcuminoids [7].
A recent pharmacological study on curcuminoids confirmed that the degradation products of CUR are the main bioactive molecules involved in the execution of biological effects [10, 11]. As an additive in many different types of processed food products, curcuminoids are exposed to various processing conditions including different temperatures and pH. This certainly affects the stability and biological activities of curcuminoids [12]. Hence, it is of utmost importance to understand degradation behavior of curcuminoids by exposing them to different stress degradation conditions.
The majority of earlier reported analytical methods have mainly focused on the quantification of curcuminoids rather than their stability profiling [13, 14]. To date, the stability profiling of curcuminoids has largely been based on high performance liquid chromatography (HPLC) by using commercial grade curcumin, which is a mixture of CUR, (75–80%), DMC (15–20%), and BDMC (3–5%) and in some cases with other drugs [15, 16]. Since curcuminoids differ in their chemical structure and have distinct activities, it is possible to assume that their relative stabilities can vary under different conditions. The stability studies on CUR, DMC, and BDMC are crucial for understanding the implications of the degradation products on human health. Furthermore, the stability studies may provide valuable information, relating to the extent to which these bioactive curcuminoids can retain their health promoting effects. Despite the importance of the information that could be gained from stability profiling compounds, there has been limited research on the stability of CUR, DMC, and BDMC. Therefore, we were prompted by this realization to study mechanistic insights on how these bioactive curcuminoids (CUR, DMC, BDMC) behave under different stress degradation conditions (i.e., acidic, alkaline, oxidative, photolytic, and thermal degradation). We herein report the systematic stability studies of pure and mixture form of curcuminoids by using a RP-HPLC method with emphasis to understand the influence of structural features on degradation pattern.
Materials and methods
Chemicals
Standards of CUR (purity 99.33%), DMC (purity 97.50%), and BDMC (purity 99.36%) were obtained as gift samples from Sami Labs Ltd. (Bangalore, India). The curcuminoid mixture (95.14% total curcuminoid content) was also obtained as gift sample from Kancor Ingredients Ltd (Kerala, India). HPLC grade acetonitrile and formic acid were purchased from Fisher Scientific (Mumbai, India). Purified water was prepared by using Millipore Direct-Q® 3 water purification system (Molsheim, France).
Instrumentation and chromatographic conditions
The HPLC system consisted of Shimadzu LC-20AD prominence system (Kyoto, Japan) equipped with a LC-20AD pump, DGU-20A5 degassing unit, SPD-M20A diode array detector, SIL-20AC HT auto sampler, CBM-20A communication bus module and CTO-10AS VP column oven. Data acquisition and analysis was performed by using a Shimadzu LC solution (version 1.25) software program. The chromatographic separation was achieved using Luna C18(2) column (150 × 4.6 mm i.d., 5 µm particle size, Phenomenex Inc., CA, USA) equipped with a Phenomenex guard column. The mobile phase consisting of acetonitrile: 0.1% formic acid (50:50 v/v), pumped at a flow rate of 0.8 mL/min. The mobile phase was filtered through 0.45 µm Millex HV® hydrophilic PVDF membrane filters (Millipore, Bedford, USA) and degassed before use. The column temperature was maintained at 40 °C and injection volume was 10 µL. CUR, DMC, and BDMC were detected at 425 nm and their degradation products were detected at 280 nm. The total chromatographic run time was set to 12 min.
Preparation of standard solutions
Stock solutions were freshly prepared in methanol and stored at 4 °C before HPLC analysis. The concentrations of individual CUR, DMC, BDMC, and curcuminoid mixture were 100, 240, 50, and 100 µg/mL respectively. All the prepared solutions were stored in amber colored volumetric flasks to protect from light.
Method validation
To ensure the validity of the developed RP-HPLC method, validation parameters such as system suitability, linearity, precision, accuracy, limit of detection (LOD), limit of quantification (LOQ), and robustness was performed in accordance with the International Conference on Harmonization (ICH) guidelines [17].
Forced degradation studies
Forced degradation studies of CUR, DMC, BDMC, and curcuminoid mixture were carried out as per ICH recommended stress conditions such as acidic, basic, oxidative, thermal, and photolytic conditions [18]. For each degradation study, four samples were prepared as follows: first was the blank solution stored under normal conditions, second was the blank subjected to degradation in the similar manner as the drug solution, third was the zero time sample containing the drug stored under normal conditions and fourth was the drug solution subjected to degradation.
Acid degradation
The acid degradation studies were carried out by transferring 1 mL of stock solution of CUR, DMC, BDMC, and curcuminoid mixture to 10 mL amber colored volumetric flask which contains 1 mL of 1 N hydrochloric acid (HCl) solution. The flasks were sealed and solution was heated at 80 °C for 2 h. The resultant solutions were cooled to room temperature, neutralized to pH 7 with 1 N sodium hydroxide (NaOH) solution. Finally the volume was adjusted to 10 mL with methanol, filtered through 0.2 µm syringe filters before injecting into HPLC system for further analysis.
Base degradation
The base degradation studies were carried out by taking 1 mL of stock solution of CUR, DMC, BDMC, and curcuminoid mixture in 10 mL amber colored volumetric flask to which 1 mL of 1 N NaOH solution was added. The flasks were wrapped and solution was heated at 80 °C for 2 h and cooled. The resultant solutions were then neutralized with 1 N HCl solution and the volume was diluted to 10 mL with methanol. Subsequently, the solutions were filtered with 0.2 µm syringe filters prior to their injection into HPLC system.
Oxidative degradation
The hydrogen peroxide (H2O2) induced oxidative degradation studies were performed by transferring 1 mL of stock solution of CUR, DMC, BDMC, and curcuminoid mixture to 10 mL amber colored volumetric flask which contains 1 mL of 30% H2O2 solution. The flasks were sealed and solution was heated at 80 °C for 2 h and cooled. The resultant solutions were adjusted to 10 mL with methanol, filtered through 0.2 µm syringe filters before injecting into HPLC system for further analysis.
Thermal degradation
Thermal degradation studies were carried out by transferring 1 mL of stock solutions of CUR, DMC, BDMC, and curcuminoid mixture to 10 mL amber colored volumetric flask to which 2 mL of methanol was added. The flasks were sealed and the solutions were heated at 80 °C for 2 h. Thereafter solutions were cooled; volume was made up with methanol, filtered through 0.2 µm syringe filters before injecting into HPLC system for further analysis.
Photodegradation
For photodegradation studies, 1 mL of methanolic stock solution of CUR, DMC, BDMC, and curcuminoid mixture were diluted to 10 mL with methanol and transferred into 10 mL transparent volumetric flasks. The flasks were sealed and exposed to direct sunlight for a time period of 6 h. Finally the solutions were filtered through 0.2 µm syringe filters before injecting into HPLC system for further analysis.
Statistical analysis
All the forced degradation studies were carried out in triplicates and data were expressed as mean ± SD. Microsoft excel was used to calculate mean, standard deviation, % relative standard deviation (%RSD), slope and correlation coefficient of the experimental data.
Results and discussion
Curcumin is a biphenolic compound having two hydroxyl-methoxy substituted aromatic rings connected to one another via a seven-carbon spacer that contains two α, β–unsaturated carbonyl (or diketo) groups [21]. However, the number of methoxy groups on phenyl rings in DMC and BDMC differ with CUR. DMC contains one methoxy group and BDMC is devoid of the methoxy group. This difference in chemical structure of these curcuminoids is not only responsible for their distinct bioactivities, but also their varied chemical stabilities [4, 19].
An efficient RP-HPLC method was successfully developed for the determination of stabilities of pure CUR, DMC, BDMC, and their mixtures under different stress degradation conditions. The developed method was validated according to the ICH guidelines and all the validation parameters were found to be within the acceptance criteria (Table 1). Under these conditions, sharp and symmetrical peaks with tailing factor less than 2% were obtained for CUR, DMC, and BDMC. Moreover, the mixture of the three curcuminoids was separated with good resolution. This developed RP-HPLC method was further able to separate both the curcuminoids and their degradation products. The chromatograms of pure CUR, DMC, BDMC, and their mixture at 425 and 280 nm are shown in Fig. 1. The retention times were around 7.34, 8.18, and 9.12 min for pure samples of BDMC, DMC, and CUR respectively. Almost similar retention times were also observed for these compounds in a curcuminoids mixture.
Table 1.
Summary of validation parameters for the developed HPLC method
| Parameter | CUR | DMC | BDMC | Acceptance criteria |
|---|---|---|---|---|
| Retention time (tR, min) | 9.12 | 8.18 | 7.34 | – |
| Theoretical plates (N) | 12,497 | 11,700 | 11,133 | N > 2000 |
| Tailing factor (T) | 1.06 | 1.09 | 1.10 | T < 2.0 |
| Linearity range (µg mL−1) | 2–12 | 4–24 | 0.5–3 | – |
| Correlation coefficient | 0.9997 | 0.9996 | 0.9999 | ≥0.990 |
| Slope | 101,689 | 95,773 | 120,134 | – |
| Intercept | −52,674 | −66,144 | −6386 | – |
| LOD (µg mL−1) | 0.22 | 0.57 | 0.05 | – |
| LOQ (µg mL−1) | 0.66 | 1.73 | 0.16 | – |
| Intraday precision (RSD%) | 0.13–0.22 | 1.24–1.39 | 0.83–1.12 | %RSD < 2.0 |
| Inter day precision (RSD%) | 0.24–0.39 | 0.70–1.38 | 0.75–1.10 | %RSD < 2.0 |
| Accuracy (% recovery) | 99.06–101.74 | 101.50–101.83 | 100.83–101.98 | 98.0–102.0% |
| Robustness | Complies | Complies | Complies | %RSD < 2.0 |
Fig. 1.
HPLC chromatogram of pure CUR, DMC, BDMC, and curcuminoids mixture at a wave length of (A) 425 nm and (B) 280 nm
Forced degradation studies
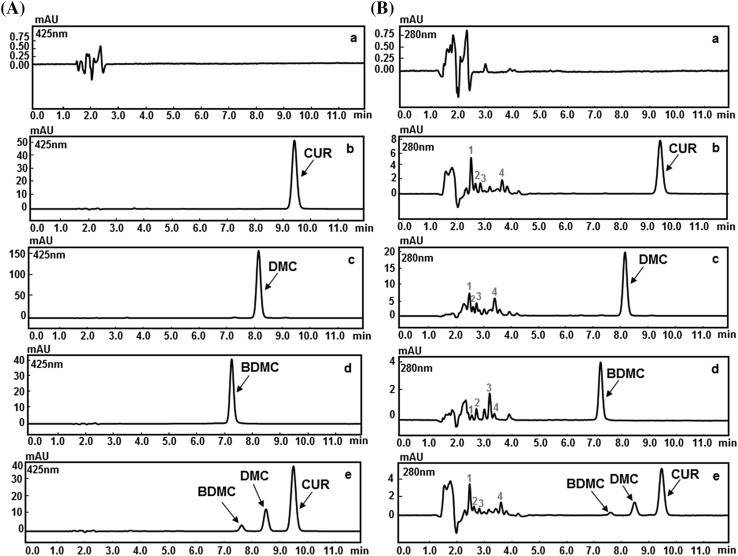
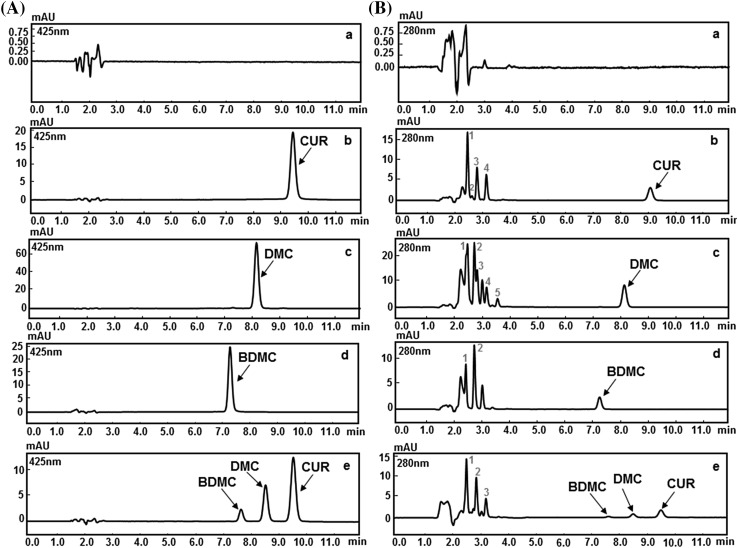
The chromatograms of the samples subjected to various forced degradation conditions such as acid, alkali, oxidation, heat and direct sunlight are presented in Figs. 2, 3, 4, 5, and 6 respectively. These chromatograms show resolved peaks for pure CUR, DMC, and BDMC as well as curcuminoids in their mixture along with some peaks of degradation products at different retention times. To identify the peaks due to degradation products, the chromatograms of the degraded samples were compared with the similarly treated blank. The number of degradation products with their retention times and the percent degradation of pure CUR, DMC, and BDMC as well as their mixture is highlighted in Table 2.
Fig. 2.
HPLC chromatograms of (a) blank (b) CUR (c) DMC (d) BDMC, and (e) curcuminoids mixture subjected to acidic degradation using 1 N HCl at 80 °C for 2 h at a wavelength of (A) 425 nm and (B) 280 nm. 1, 2, 3, and 4 represents degradation peaks
Fig. 3.
HPLC chromatograms of (a) blank (b) CUR (c) DMC (d) BDMC and (e) curcuminoids mixture subjected to base degradation using 1 N NaOH at 80 °C for 2 h at a wavelength of (A) 425 nm and (B) 280 nm. 1, 2, 3, 4 and 5 represents degradation peaks
Fig. 4.
HPLC chromatograms of (a) blank, (b) CUR, (c) DMC, (d) BDMC and (e) curcuminoids mixture subjected to oxidative degradation using 30% H2O2 at 80 °C for 2 h at a wavelength of (A) 425 nm and (B) 280 nm. 1, 2, 3, and 4 represents degradation peaks
Fig. 5.
HPLC chromatograms of (a) blank, (b) CUR, (c) DMC, (d) BDMC and (e) curcuminoids mixture subjected to thermal degradation by heating at 80 °C for 2 h at a wavelength of (A) 425 nm and (B) 280 nm
Fig. 6.
HPLC chromatograms of (a) blank, (b) CUR, (c) DMC, (d) BDMC and (e) curcuminoids mixture subjected to photodegradation by exposing to sunlight for 6 h t at a wavelength of (A) 425 nm and (B) 280 nm. 1, 2, and 3 represents degradation peaks
Table 2.
The number of degradation products, retention times, and the percentage degradation of both pure and mixture forms of curcuminoids subjected for forced degradation conditions
| Stress condition | Retention time of degradation products in mina | % Degradation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CUR | DMC | BDMC | Mixture | CUR | DMC | BDMC | Mixture | |||
| CUR | DMC | BDMC | ||||||||
| 1 N HCl, 80 °C, 2 h | 2.59 (1) 2.75 (2) 2.93 (3) 3.72 (4) |
2.44 (1) 2.68 (2) 2.81 (3) 3.47 (4) |
2.63 (1) 2.81 (2) 3.29 (3) 3.45 (4) |
2.59 (1) 2.74 (2) 2.93 (3) 3.72 (4) |
44.39 ± 0.92 | 36.84 ± 0.20 | 38.75 ± 1.66 | 37.52 ± 0.36 | 21.19 ± 0.38 | 20.18 ± 0.39 |
| 1 N NaOH, 80 °C, 2 h | 2.56 (1) 2.72 (2) 2.91 (3) 3.25 (4) |
2.56 (1) 2.81 (2) 2.91 (3) 3.25 (4) 3.64 (5) |
2.50 (1) 2.81 (2) |
2.58 (1) 2.93 (2) 3.28 (3) |
78.57 ± 0.04 | 70.96 ± 0.16 | 64.35 ± 0.05 | 78.81 ± 0.07 | 61.91 ± 0.03 | 50.00 ± 0.64 |
| 30% H2O2, 80 °C, 2 h | 2.58 (1) 2.99 (2) 3.46 (3) 3.71 (4) |
2.57 (1) 2.81 (2) 3.29 (3) 3.47 (4) |
2.81 (1) 3.28 (2) |
2.58 (1) 2.93 (2) 3.76 (3) |
89.12 ± 0.03 | 68.52 ± 0.10 | 64.69 ± 0.07 | 79.82 ± 0.02 | 65.86 ± 0.06 | 49.92 ± 0.09 |
| Thermal 80 °C, 2 h | Absent | Absent | Absent | Absent | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Sunlight 6 h | 2.70 (1) 2.91 (2) |
2.81 (1) 2.91 (2) 4.50 (3) |
2.63 (1) 2.81 (2) 5.27 (3) |
2.82 (1) 2.92 (2) |
100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
Values are mean ± standard deviation (SD)
aThe numbers in brackets (1, 2, 3, 4, and 5) denotes the degradation products
Acid degradation
The chromatograms of the acid degraded samples of CUR, DMC, BDMC, and the mixture along with peaks of their degradation products are shown in Fig. 2. The pure samples of CUR, DMC, and BDMC displayed percentage degradations that were around 44.39, 38.75, and 36.84, respectively. This was in contrast to the curcuminoids in the mixture that showed 37.52, 21.19, and 20.18 percentage degradations, respectively. Furthermore, it was observed that, under acidic conditions, pure CUR undergoes a slightly higher degradation when compared to DMC and BDMC. Interestingly, we noticed that there is a decrease in the percentage degradation of curcuminoids when they are in a mixture in comparison to their respective pure samples. By comparing the percentage degradations, it can be suggested that DMC and BDMC have a stabilizing effect in curcuminoids mixture. The extent of degradation of curcuminoids was also influenced by temperature. Congruous to previous reports [20], in this study, the percentage degradation (in acid) of pure CUR was found to be 44.39% after 120 min of incubation at 80 °C.
The pH of the medium is one of major factors that govern the stability of curcuminoids. The curcuminoids exhibit pH dependent keto-enol tautomerism. In acidic and neutral medium (i.e., pH 3–7) the keto form dominates and makes the curcuminoids an extraordinary potent H-atom donor. While in alkaline medium (i.e., pH > 8) the enol form predominates with electron donating property. In solvated state, at pH < 1, curcuminoids acquire fully conjugated protonated form (H4A+), whereas, at acidic conditions (1 ≤ pH ≤ 7) they are in the neutral form (H3A). As pH is traversed through alkaline end (pH > 9), curcuminoids were in fully deprotonated form (A3−) to generate highly negatively charged ions which degrade more rapidly. In general, the extent of degradation of curcuminoids depends upon the level of deprotonation and the destruction of the conjugated diene system [21, 22]. In this study, curcuminoids were in protonated state during acid degradation studies (1 N HCl, pH < 1), while they became deprotonated in basic degradation conditions (1 N NaOH, pH > 13). Hence, curcuminoids degradation is slow in acidic conditions and faster at basic conditions.
Base degradation
The base degraded chromatograms of pure CUR, DMC, BDMC, and their curcuminoids mixture are shown in Fig. 3. The percentage degradation of pure CUR (78.57%) was almost similar to that of CUR in the mixture (78.81%). However, we have witnessed a sharp decrease in the percentage degradation of DMC (61.91%) and BDMC (50.0%) in mixture when compared to DMC (70.96%) and BDMC (64.35%) in their respective pure forms. From these results, it is evident that in both pure and mixture forms, BDMC was more resistant to alkaline degradation than CUR and DMC.
The enhanced alkaline degradation of CUR and DMC could be attributed to the presence of one or more methoxy groups [6]. The reminiscent mechanism and the role of methoxy groups in alkaline degradation can be explained as follows. The alkaline degradation of curcuminoids is initiated by the deprotonation of one of the three hydroxyl groups followed by their auto-oxidation, thus yielding the degradation products [23]. The curcuminoids exhibit almost same extent of deprotonation which is largely due to hydroxyl groups on the phenyl ring. However, degree of auto-oxidation may vary depending upon the number of methoxy groups on phenyl ring. The phenyl methoxy group not only opens the epoxide but also found to be decisive for the ability of CUR, DMC, and BDMC to undergo oxidative transformation [19]. Several previously reported studies revealed that hydrogen bonding of o-methoxy phenolic functionality was responsible for the structure, configuration, and its ability to undergo auto-oxidation. The electron releasing behavior of methoxy group facilitates the easy transfer of electrons (hydrogen abstraction) from phenolate anion due to formation of an intramolecular hydrogen bond between o-methoxy group and phenolic hydrogen. Therefore, theoretically the auto-oxidation is faster for CUR (two methoxy groups), slower for DMC (one methoxy group) while BDMC resistant to auto-oxidize due to lack of methoxy groups [24]. Oxidative transformation of BDMC requires catalysis by horseradish peroxide and H2O2 or potassium ferricyanide. Interestingly, we observed that there was a moderate degradation for pure BDMC, which could be attributed due to higher temperature (80 °C for 120 min) employed in this study. However, the percentage degradation of DMC and BDMC in the mixture was considerably decreased as compared to their pure forms. From these findings, it is clear that the presence of DMC and BDMC in curcuminoids mixture might have reduced their percentage degradation by their synergistic stabilizing effect [19].
Oxidative degradation
The hydrogen peroxide (H2O2) induced oxidative degraded chromatograms of pure CUR, DMC, BDMC, and their curcuminoids mixtures are presented in Fig. 4. The percentage degradation of CUR in mixture (79.82%) was moderately decreased as compared to its pure form (89.12%). A similar trend was also observed for DMC in mixture (65.86%) in comparison with its pure form (68.52%). However, there was a significant change in percentage degradation when pure BDMC was compared to its mixture form. These results revealed that out of three curcuminoids tested, BDMC (both pure and mixture) exhibited more stability for H2O2 induced degradation. The degradation pattern of curcuminoids (both pure and mixture form) in oxidative (H2O2) degradation studies was found to be dependent on their anti-oxidant nature. Particularly, the anti-oxidant property and hydrogen donation ability of curcuminoids may be due to the presence of bulky alkyl group at ortho position with respect to phenolic hydroxyl group [25]. The hydrogen donation in curcuminoids occurs at three sites viz., one active methylene group and two phenolic –OH groups ortho to electron releasing –OCH3 group. As compared to CUR, DMC possess less potential for proton donation because it has only one phenolic –OH group ortho to electron donating –OCH3 group. The proton donation ability of BDMC is least due to the lack of –OCH3 groups [26]. Moreover, Masuda et al. have also reported the detailed mechanistic oxidative degradation of curcuminoids which proceeds via involvement of resonance stabilized radicals. Accordingly, the intermediate radical formed in case of BDMC degradation was found to be more stable due to the absence of both –OCH3 groups [27]. Therefore, among the curcuminoids BDMC resist the degradation while CUR readily undergoes oxidative degradation. These observations were in agreement with our experimental results. Furthermore, in the mixture form of curcuminoids, degradation of CUR was partially counteracted by the collaborative stabilizing effect of DMC and BDMC.
Thermal degradation
The pure samples of CUR, DMC, BDMC, and their curcuminoids mixture were subjected to thermal degradation and the resultant chromatograms are presented in Fig. 5. Interestingly, we observed the absence of degradation peaks or no change in the peak area accounting for CUR, DMC, and BDMC, which clearly suggest that both pure and mixture forms of curcuminoids were thermally stable. Generally, curcuminoids were reported to be stable to heating. However, the degree of their degradation depends upon the temperature and duration of heating. Several literature reports indicated that CUR and curcuminoids mixture could tolerate temperatures ranging from 80 to 85 °C [15, 20]. Nevertheless, the β-diketone linkage in curcuminoids has been found to be more vulnerable for thermal degradation at temperatures beyond 100 °C [28]. In our work, both pure and mixture forms of curcuminoids were stable for thermal degradation at 80 °C for 2 h.
Photo degradation
The pure samples of CUR, DMC, BDMC, and their curcuminoids mixture were exposed to photo degradation (direct sunlight for 6 h), and the respective chromatograms are presented in Fig. 6. The results reveal the disappearance of the peaks corresponding to individual and mixture form of curcuminoids in their respective chromatograms confirming susceptible character of curcuminoids toward light. This evidently suggests that the presence or absence of phenolic (OH) or methoxy (OCH3) groups on phenyl ring does not have significant influence in degradation. However, the photo degradation mainly proceeds through the cleavage β-diketone linkage, which eventually gets fragmented into smaller phenolic compounds as depicted in Fig. 7 [21].
Fig. 7.
Photodegradation of curcuminoids in methanol
In conclusion, the research findings reported herein are the first ever attempt to understand the relative degradation behavior of pure curcuminoids in comparison with their curcuminoid mixture under different forced degradation conditions. Our research findings suggest that the degradation pattern among the curcuminoids (both pure and mixture form) varies with conditions used in forced degradation studies. It was observed that in both cases of curcuminoids, CUR was vulnerable for degradation (least stable) while BDMC was most resistant to degradation (more stable). The order of stability of curcuminoids to acidic, alkaline, and oxidative degradation was found to be as follows: BDMC > DMC > CUR. Both pure and mixture forms of curcuminoid were stable against heat while they completely degraded upon exposure to sunlight. Besides, from this study it is evident that the presence of DMC and BDMC are responsible for enhanced stability of curcuminoid mixture as compared to their pure forms. The precise synergistic stabilizing mechanism of these curcuminoids with more emphasis on the role of structural elements (diketone moiety, methoxy groups, and hydroxyl groups) of the curcuminoids, which are responsible for distinct degradation behavior, warrants further investigations.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ishita C, Khaushik B. Turmeric and curcumin: biological actions and medical applications. Curr. Sci. 2004;87:44–50. [Google Scholar]
- 2.Singh P, Pandey K, Rizvi S. Curcumin: the yellow molecule with pleiotropic biological effects. Lett. Drug Des. Discov. 2015;13:170–177. doi: 10.2174/1570180812666150630184101. [DOI] [Google Scholar]
- 3.Ahmed T, Gilani AH. Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother Res. 2014;28:517–525. doi: 10.1002/ptr.5030. [DOI] [PubMed] [Google Scholar]
- 4.Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and mother nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Tonnesen HH, Karlsen J. Studies on curcumin and curcuminoids V. Alkaline degradation of curcumin. Z. Lebensm. Unters. Forsch. 1985;180:132–134. doi: 10.1007/BF01042637. [DOI] [PubMed] [Google Scholar]
- 6.Price LC, Buescher RW. Kinetics of alkaline degradation of the food pigments curcumin and curcuminoids. J. Food Sci. 1997;62:267–269. doi: 10.1111/j.1365-2621.1997.tb03982.x. [DOI] [Google Scholar]
- 7.Khurana A, Ho CT. High performance liquid chromatographic analysis of curcuminoids and their photo-oxidative decomposition compounds in Curcuma longa L. J. Liq. Chromatogr. 1988;11:2295–2304. doi: 10.1080/01483918808067200. [DOI] [Google Scholar]
- 8.Gordon ON, Luis PB, Sintim HO, Schneider C. Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J. Biol. Chem. 2015;290:4817–4828. doi: 10.1074/jbc.M114.618785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–1876. doi: 10.1016/S0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Liu C-C, An C-Y, Ji H-F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016;6:20872. doi: 10.1038/srep20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankun J, Wyganowska-Swiatkowska M, Dettlaff K, Jelinska A, Surdacka A, Watrobska-Swietlikowska D, Skrzypczak-Jankun E. Determining whether curcumin degradation/condensation is actually bioactivation (Review) Int. J. Mol. Med. 2016;37:1151–1158. doi: 10.3892/ijmm.2016.2524. [DOI] [PubMed] [Google Scholar]
- 12.Lee BH, Kim D, Kang S, Kim MR, Hong J. Changes in the chemical stability and antioxidant activities of curcuminoids under various processing conditions. Korean J. Food Sci. Technol. 2010;42:97–102. [Google Scholar]
- 13.Jadhav BK, Mahadik KR, Paradkar AR. Development and validation of improved reversed phase-HPLC method for simultaneous determination of curcumin, demethoxycurcumin and bis-demethoxycurcumin. Chromatographia. 2007;65:483–488. doi: 10.1365/s10337-006-0164-8. [DOI] [Google Scholar]
- 14.Wichitnithad W, Jongaroonngamsang N, Pummangura S, Rojsitthisak PA. Simple isocratic HPLC method for the simultaneous determination of curcuminoids in commercial turmeric extracts. Phytochem. Anal. 2009;20:314–319. doi: 10.1002/pca.1129. [DOI] [PubMed] [Google Scholar]
- 15.Gugulothu DB, Fernandes CB, Patravale VB. A versatile high performance liquid chromatography method for simultaneous determination of three curcuminoids in pharmaceutical dosage forms. Pharm. Anal. Acta. 2012;3:1–7. [Google Scholar]
- 16.Korany MA, Haggag, RS, Ragab MAA, Elmallah OA. A validated stability-indicating HPLC method for simultaneous determination of silymarin and curcumin in various dosage forms. Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2013.06.021
- 17.ICH Q2(R1). Validation of analytical procedures: text and methodology. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. (2005)
- 18.ICH Stability Testing of new drug substances and products Q1A(R2) International Conference on Harmonization. IFPMA, Geneva. (2003)
- 19.Gordon ON, Luis PB, Ashley RE, Osheroff N, Schneider C. Oxidative transformation of demethoxy- and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase IIα. Chem. Res. Toxicol. 2015;28:989–996. doi: 10.1021/acs.chemrestox.5b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dandekar PP, Patravale VB. Development and validation of a stability-indicating LC method for curcumin. Chromatographia. 2009;69:871–877. doi: 10.1365/s10337-009-0995-1. [DOI] [Google Scholar]
- 21.Lee W-H, Loo C-Y, Bebawy M, Luk F, Mason RS, Rohanizadeh R. Curcumin and its derivatives: their application in neuropharmacology and neuroscience in the twenty-first century. Curr. Neuropharmacol. 2013;11:338–378. doi: 10.2174/1570159X11311040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung MHM, Colangelo H, Kee TW. Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis. Langmuir. 2008;24:5672–5675. doi: 10.1021/la800780w. [DOI] [PubMed] [Google Scholar]
- 23.Naksuriya O, van Steenbergen MJ, Torano JS, Okonogi S, Hennink WE. A kinetic degradation study of curcumin in its free form and loaded in polymeric micelles. AAPS J. 2016;18:777–787. doi: 10.1208/s12248-015-9863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider C, Gordon ON, Edwards RL, Luis PB. Degradation of curcumin: from mechanism to biological implications. J. Agric. Food Chem. 2015;63:7606–7614. doi: 10.1021/acs.jafc.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MA, Shahidi F. Effects of natural and synthetic antioxidants on the oxidative stbility of borage and evening primrose triacylglycerols. Food Chem. 2001;75:431–437. doi: 10.1016/S0308-8146(01)00232-1. [DOI] [Google Scholar]
- 26.Rege S, Momin S, Wadekar S, Pratap A, Bhowmick D. Effect of demethoxycurcumin and bisdemethoxycurcumin on antioxidant activity of curcumin in refined sunflower oil. J. Food Process. Preserv. 2014;38:296–303. doi: 10.1111/j.1745-4549.2012.00777.x. [DOI] [Google Scholar]
- 27.Masuda T, Hidaka K, Shinohara A, Maekawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999;47:71–77. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- 28.Suresh D, Gurudutt KN, Srinivasan K. Degradation of bioactive spice compound: curcumin during domestic cooking. Eur. Food Res. Technol. 2009;228:807–812. doi: 10.1007/s00217-008-0993-9. [DOI] [Google Scholar]