Abstract
Introduction
Placental transfer of amino acids via amino acid transporters is essential for fetal growth. Little is known about the epigenetic regulation of amino acid transporters in placenta. This study investigates the DNA methylation status of amino acid transporters and their expression across gestation in human placenta.
Methods
BeWo cells were treated with 5-aza-2′-deoxycytidine to inhibit methylation and assess the effects on amino acid transporter gene expression. The DNA methylation levels of amino acid transporter genes in human placenta were determined across gestation using DNA methylation array data. Placental amino acid transporter gene expression across gestation was also analysed using data from publically available Gene Expression Omnibus data sets. The expression levels of these transporters at term were established using RNA sequencing data.
Results
Inhibition of DNA methylation in BeWo cells demonstrated that expression of specific amino acid transporters can be inversely associated with DNA methylation. Amino acid transporters expressed in term placenta generally showed low levels of promoter DNA methylation. Transporters with little or no expression in term placenta tended to be more highly methylated at gene promoter regions. The transporter genes SLC1A2, SLC1A3, SLC1A4, SLC7A5, SLC7A11 and SLC7A10 had significant changes in enhancer DNA methylation across gestation, as well as gene expression changes across gestation.
Conclusion
This study implicates DNA methylation in the regulation of amino acid transporter gene expression. However, in human placenta, DNA methylation of these genes remains low across gestation and does not always play an obvious role in regulating gene expression, despite clear evidence for differential expression as gestation proceeds.
Keywords: Placenta, amino acid transporter, methylation, gestation
Introduction
The placenta selectively mediates transfer of nutrients to the fetus, which is vital for optimal fetal development. Impaired placental amino acid transport results in reduced fetal growth [1, 2], which is associated with increased risk of adulthood disease [3]. Regulation of amino acid transporters occurs in response to short-term maternal stimuli [4], but could also occur across longer timescales. Epigenetic modification provides a potential mechanism for regulation of placental amino acid transporters, mediating early environmental influences across gestation [5]. The placenta has a unique DNA methylation profile with large regions of hypomethylation, containing developmental and tissue specific genes, interspersed with regions of high methylation [6]. The extent of epigenetic regulation by DNA methylation of functionally related genes such as amino acid transporters is unclear.
Accumulative, uniporter and facilitated amino acid transporters act in a coordinated way to transfer amino acids across the microvillous and basal plasma membrane (BM) of the placental syncytiotrophoblast [7, 8]. Placental amino acid metabolism also contributes to the rate of amino acid transfer [9, 10]. There are multiple classes of amino acid transporters, all of which are members of the SLC (solute carrier) gene series as outlined in Table 1 [11]. Accumulative transporters from the SLC1 (EAAT) [12], SLC7 (CAT) [15] and SLC38 (SNAT) [13, 14] families transport maternal amino acids into the syncytiotrophoblast. Amino acids are exchanged between the intracellular and extracellular pools by antiporters from the SLC1 (ASC) and SLC7 (LAT, y+LAT) families [16]. SLC16A10 (TAT1), SLC43A1 (LAT3) and SLC43A2 (LAT4) encode the BM facilitated transporters that mediate net amino acid transfer to the fetus, with SLC16A10 expression correlating with fetal and neonatal growth [7, 17].
Table 1.
Placental amino acid transporter gene expression change across gestation and summary of methylation changes
| Amino acid transporter | 1st trimester - term expression | 2nd trimester - term expression | Methylation change 1st trimester - term | Cell meth/ex relation | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Protein | Adj p val | logFC | Adj p val | logFC | Expression Change |
27K array | 450K array | |
| SLC1A1 | EAAT3 | 0.398 | -0.174 | 0.086 | -0.207 | no | no | up | same |
| SLC1A2 | EAAT2 | 0.001* | -0.548 | 0.306 | -0.029 | down | no | up | opposite |
| SLC1A3 | EAAT1 | 0.450 | -0.033 | 0.002* | -0.108 | down | up | up | opposite |
| SLC1A4 | ASCT1 | 0.069 | -0.096 | 0.029* | -0.055 | down | no | up | opposite |
| SLC1A5 | ASCT2 | 0.590 | -0.028 | 0.488 | -0.036 | no | no | up | opposite |
| SLC1A6 | EAAT4 | 0.502 | -0.040 | 0.996 | 0.000 | no | no | no | same |
| SLC1A7 | EAAT5 | 0.096 | -0.079 | 0.549 | 0.010 | no | up | up | opposite |
| SLC3A1 | rBAT | 0.205 | -0.540 | 0.297 | -0.043 | no | down | down | - |
| SLC3A2 | 4F2hc | 0.153 | 0.082 | 0.190 | 0.071 | no | up | no | opposite |
| SLC7A1 | CAT1 | 0.055* | -0.134 | 0.165 | 0.053 | down | down | down | opposite |
| SLC7A5 | LAT1 | 0.542 | 0.046 | 0.031* | 0.107 | up | up | up/down | opposite |
| SLC7A6 | y+LAT2 | 0.537 | 0.017 | 0.436 | -0.020 | no | no | no | opposite |
| SLC7A7 | y+LAT1 | 0.605 | 0.031 | 0.021* | -0.060 | down | - | up | none |
| SLC7A8 | LAT2 | 0.311 | 0.117 | 0.278 | 0.045 | no | no | up/down | opposite |
| SLC7A9 | b(0,+)AT1 | 0.135 | -0.083 | 0.865 | 0.009 | no | up | up | none |
| SLC7A10 | asc | 0.931 | -0.008 | 0.031* | 0.077 | up | down | up | opposite |
| SLC7A11 | xCT | 0.032* | -0.166 | 0.595 | 0.020 | down | down | up | opposite |
| SLC16A10 | TAT1 | 0.415 | 0.048 | 0.221 | -0.047 | no | no | no | none |
| SLC38A1 | SNAT1 | 0.092 | 0.077 | 0.124 | 0.055 | no | up | up/down | opposite |
| SLC38A2 | SNAT2 | 0.025* | 0.080 | 0.009* | 0.079 | up | no | up | none |
| SLC38A3 | SNAT3 | 0.852 | -0.013 | 0.159 | 0.067 | no | no | up | - |
| SLC38A4 | SNAT4 | 0.286 | -0.060 | 0.232 | 0.032 | no | no | up | same |
| SLC38A6 | SNAT6 | 0.088 | -0.073 | 0.008* | -0.19 | down | no | no | - |
| SLC43A1 | LAT3 | 0.983 | -0.002 | 0.107 | 0.044 | no | no | up | opposite |
| SLC43A2 | LAT4 | 0.154 | 0.051 | - | - | no | no | down | opposite |
Placental gene expression array data from first trimester (n = 4) compared to term (n = 4) and early second trimester (n = 14) compared to term (n = 9) samples. Data downloaded from GEO series GSE5999 and GSE9984. *p < 0.05; methylation (meth), expression (ex).Shading indicates transporters thought to have low expression in placenta. Bold indicates transporters that the data supports a role for methylation in the regulation of gene expression.
Expression and activity of specific placental amino acid transporters increase across gestation [18, 19], to help meet increasing fetal demand as gestation progresses [4, 20]. Maternal factors such as diet, smoking and vitamin D levels can alter placental amino acid transporter gene expression [17, 21], potentially via DNA methylation. Indeed, maternal nutrition [22], obesity [23], stress [24], toxin exposure [25] or smoking [26] during pregnancy, influence placental DNA methylation.
DNA methylation could mediate the placental adaptations to early exposures and increased fetal demand for nutrients across gestation [27]. Placentas from both small and large for gestational age babies have altered methylation profiles for specific genes [28, 29]. In addition, global methylation in human placenta is reduced with gestational diabetes or preeclampsia and increased with maternal obesity [30, 31]. The early environment influences placental glucose transport, potentially by epigenetic regulation of human placental glucose transporters. Indeed, associations between DNA methylation and glucose transporter expression have previously been demonstrated across gestation [32–34].
Taken together these studies suggest that DNA methylation may have a role in regulating placental nutrient transport. We therefore investigated whether this epigenetic mechanism is involved in regulating amino acid transporters in the human placenta.
Methods
Amino acid transporter DNA methylation in BeWo and HEK293 cells
Cell culture
BeWo human choriocarcinoma cells from the HPA Culture Collections (Salisbury, UK) or Human Embryonic Kidney 293 cells (HEK293) were cultured in DMEM/Ham’s F12 (1:1) media (with HEPES and l-glutamine, with phenol red) supplemented with 500 iu/ml penicillin, 500 iu/ml streptomycin, 1 mM glutamine and 10% fetal bovine serum (FBS; All Lonza). Cells were cultured in 32 mm wells at a density of 2.5 x 105 at 37°C in a humidified incubator (5% CO2 in air). At 24 h cells were incubated with or without 7.5 μM 5-aza-2′-deoxycytidine (5-AZA-dC; Sigma), a concentration previously shown to be effective in BeWo cells [35]. At 48 h RNA was extracted using RNAzol (Sigma-Aldrich, UK) with centrifugation and isopropanol precipitation. Each treatment was carried out in triplicate in three independent experiments.
Quantitative reverse transcription PCR (qRT-PCR)
RNA was quantified by UV absorption (NanoDrop 1000, Thermo Scientific, UK) and RNA integrity was confirmed by agarose gel electrophoresis. Total RNA (0.2 μg) was reverse transcribed into cDNA and gene expression was measured using qRT-PCR with a Roche Light-Cycler-480. Oligonucleotide probes were supplied by Roche (Universal Probe Library (UPL); Roche, UK) and primers supplied by Eurogentec (Seraing, Belgium): primer and probe details are listed in Supplementary Table I. For UPL probes cycle parameters were 95°C for 10 min; 45 cycles of 95°C for 10 s, 60°C for 30 s; then 72 °C for 1 s. For Perfect Probes the cycle parameters were 95°C for 10 min; 50 cycles of 95°C for 15 s, 50°C for 30 s and 72°C for 15 s. Intra-assay CV’s for each gene were 5-8%.
The geNorm™ human Housekeeping Gene Selection Kit (Perfect Probe, Primer Design Limited, UK) was used to select reference genes for normalization of the qPCR data. Reference gene stability differed between BeWo, HEK293 and placenta, with UBC and GAPDH being the most stable genes common to all tissues (Supplementary Figure 1). GAPDH was affected by 5-AZA-dC treatment and was expressed at a higher level in the cell lines compared to placenta. UBC was selected as the reference gene for this study as it did not change expression with 5-AZA-dC treatment and showed similar expression levels in all three tissues (Supplementary Figure 1).
Data analysis
mRNA levels are presented relative to UBC. Summary data are presented as mean (SEM). Data were log transformed if not normally distributed. Normally distributed data were analysed with a t-test and non-normally distributed data were analysed with a Mann-Whitney U test using SPSS® Statistics (Version 20, IBM, USA). A significant difference was accepted at p < 0.05. Data were converted to z-scores and a heatmap plotted using R version 3.3.2.
Human placental RNA sequencing
Placental samples
The study was conducted according to the guidelines in the Declaration of Helsinki, and the Southampton and South West Hampshire Research Ethics Committee approved all procedures. Written informed consent was obtained from all participating women. Placentas (n = 6) were collected from term pregnancies within 30 min of delivery. Villous samples (30 mg) were snap frozen in liquid nitrogen and stored at -80°C.
RNA Sequencing
RNA was extracted from placental samples using the miRNeasy mini kit with the RNase-free DNase Set (Qiagen, UK) according to manufacturer’s instructions. RNA was quantified by UV absorption (NanoDrop 1000, Thermo Scientific, UK). RNA quality was assessed with an RNA 2100 Bioanalyser (Agilent, USA) and accepted if the RNA integrity number (RIN) was above 6.0.
RNA samples (450 ng) were converted into cDNA libraries using the Illumina TruSeq Stranded mRNA sample preparation kit. Stranded RNA sequencing was carried out by Expression Analysis (Durham, USA) using HiSeq 2x50bp paired-end sequencing on an Illumina platform.
Data analysis
After sequencing, the analysis was performed by Expression Analysis using their in-house developed RNA-Seq bioinformatics pipeline (version 9) which uses a variety of internally developed and open source programs (https://expressionanalysis.github.io/ea-utils/). Across all samples, the median number of reads was 25.6 million following Quality Control analysis using the Expression Analysis/Quintiles in-house developed tool: fastq-mcf3. Adjusted sequencing information was aligned against the human transcriptome to identify the genes within each sample using the aligner STAR version 2.4 which is specifically designed for RNA-Seq. Counts (number of reads overlapping each gene) are expressed as Fragments Per Kilobase of exon per Million reads (FPKMs). The counts of fragments were normalized by dividing by the total length of all exons in the gene (or transcript); per kilobase of exon. This value was normalized against the library size; per million read means. Data was expressed as mean log2 FPKMs.
Amino acid transporter gene DNA methylation analysis in human placentas
Illumina Infinium HumanMethylation27 BeadChip (HM27) array data
Genome-scale DNA methylation analysis of 18 first trimester (elective abortion) and 14 third trimester normal placental villous tissue samples was performed using the HM27 array. Data was uploaded to the Gene Expression Omnibus (GEO), accession number GSE31781 [36]. We extracted amino acid transporter gene data from this DNA methylation data. The output presents methylation levels as beta values from 0 (unmethylated) to 1 (completely methylated). Data were expressed as changes in methylation at term compared to first trimester by t-test. P values were adjusted for false discovery rate and a significant difference accepted at p < 0.05.
Illumina Infinium HumanMethylation450 BeadChip (HM450) array data
Illumina Infinium HM450 array DNA methylation data from 5 first trimester and 10 third trimester chromosomally normal placental villous tissue samples was downloaded from GEO series GSE49343 [37]. We extracted data related to amino acid transporter genes from the DNA methylation data. Methylation levels are presented as beta values from 0 (unmethylated) to 1 (completely methylated). For each transporter, mean methylation values were calculated for CpGs grouped by region: 5’ promoter CpG island, shore or shelf, gene body or enhancers determined by the ENCODE Consortium using informatics [38]. Data were expressed as changes in methylation at term compared to first trimester and analysed by t-test with Bonferroni correction (116 tests): a significant difference accepted at p < 4.3 x 10-4.
Gene expression array data analysis in human placentas
Gene expression data from first trimester (≥ 12 weeks, n = 4) compared to term placenta (37-40 weeks, n = 4) and early second trimester (14-19 weeks, n = 14) compared to term (37-40 weeks, n = 9) placental samples were downloaded from GEO series GSE5999 [39] and GSE9984 [40] (Affymetrix Human Genome U133A). Data were compared between groups using the interactive web tool GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r). GEO2R performs comparisons on original submitter-supplied processed data tables using the GEOquery and limma (Linear Models for Microarray Analysis) R packages from the Bioconductor project. Data were normalized and expressed as log2 fold changes in gene expression at term compared to first or early second trimester. P values were adjusted for false discovery rate and a significant difference accepted at p < 0.05.
Results
This study aimed to investigate the DNA methylation and expression of 25 amino acid transporter genes (Table 1) in human placenta as follows. Accumulative transporter genes: SLC1A1, SLC1A2, SLC1A3, SLC1A6, and SLC1A7 (EAATs); SLC7A1 (CAT1); and SLC38A1, SLC38A2, SLC38A3, SLC38A4 and SLC38A6 (SNATs). Antiporter genes: SLC1A4 and SLC1A5 (ASCs); SLC7A5 and SLC7A8 (LATs); SLC7A6 and SLC7A7 (y+LATs); SLC7A9, SLC7A10 and SLC7A11; and the auxiliary proteins SLC3A1, and SLC3A2. Facilitated transporter genes: SLC16A10, SLC43A1 and SLC43A2.
Disruption of DNA methylation influences amino acid transporter gene expression
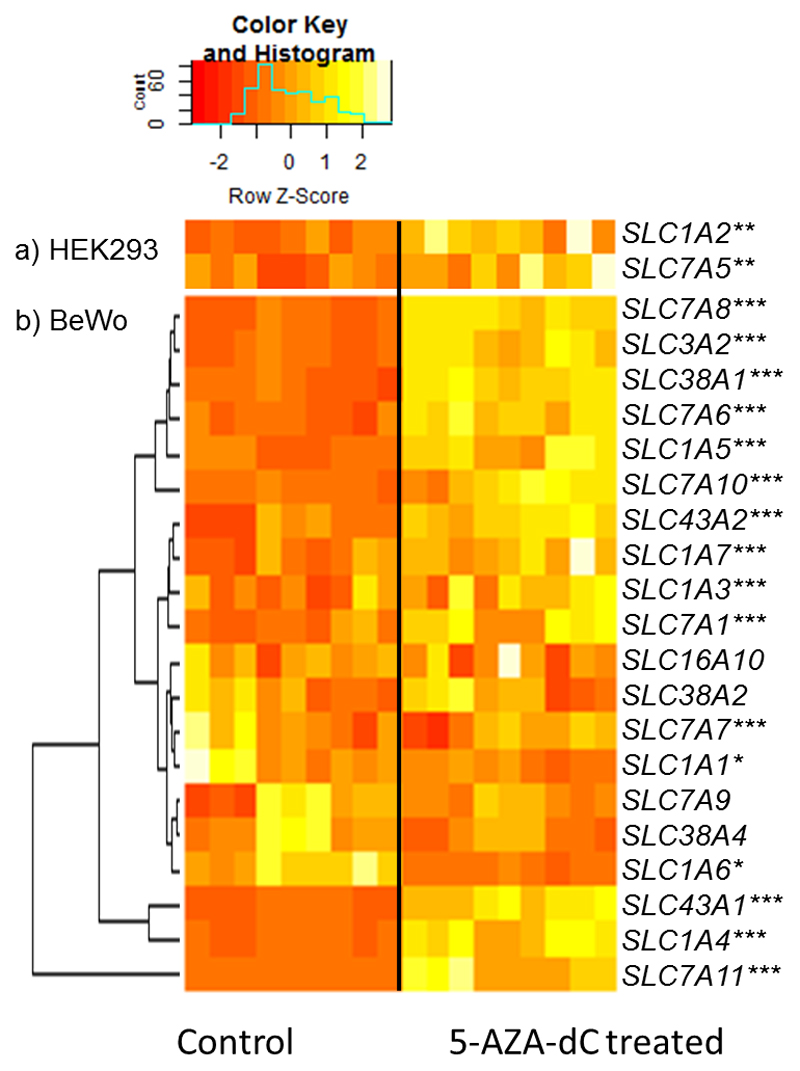
Cell culture experiments were used to assess whether DNA methylation influences amino acid transporter gene expression. Of the 25 targeted amino acid transporter genes; SLC3A1, SLC38A3 and SLC38A6 were not measured. The trophoblast derived BeWo cells were incubated with 5-AZA-dC which inhibits DNA methyltransferase activity thus disrupting global DNA methylation levels. 5-AZA-dC treatment increased mRNA expression levels of SLC7A1, SLC7A6, SLC1A4, SLC1A5, SLC43A1, SLC43A2, SLC7A10, SLC3A2, SLC7A8, SLC7A11, SLC1A3, SLC1A7 and SLC38A1 (Figure 1). Conversely, expression of SLC1A1 and SLC1A6 was reduced following 5-AZA-dC treatment in BeWo cells (Figure 1). SLC16A10, SLC7A9, SLC38A2, SLC38A4 and SLC7A7 gene expression was not altered by 5-AZA-dC treatment (Figure 1). SLC1A2 and SLC7A5 mRNA expression could not be detected in BeWo cells that had not been 5-AZA-dC treated (but was detectable following 5-AZA-dC treatment). HEK293 cells did show expression of SLC1A2 and SLC7A5 that had not been 5-AZA-dC treated. HEK293 cells were therefore used to investigate the expression of these two transporters and showed upregulation following 5-AZA-dC treatment (Figure 1).
Figure 1.
Heatmap representing amino acid transporter gene expression with and without 5-AZA-dC treatment in a) HEK293 cells and b) BeWo cells. Data presented as z-scores of relative mRNA expression clustered by expression pattern. n = 9 for both control and 5-AZA-dC treated cells. *p < 0.05, **p < 0.01, ***p < 0.001; treated group compared to control group. Data is for BeWo cell expression of all transporters except SLC1A2 and SLC7A5 whose mRNA expression could not be detected in BeWo cells that had not been 5-AZA-dC treated (was detectable following 5-AZA-dC treatment). The expression of these two transporters was measured in HEK293 cells.
Placental amino acid transporter genes show altered DNA methylation across gestation
HM27 array data
We examined the DNA methylation levels of 24 of the 25 targeted amino acid transporters in first trimester and term placenta using HM27 array data as SLC7A7 was not included in the array. Two probes targeted the promoter region of each gene except for SLC1A5 and SLC1A3 that were targeted by single probes. 39 out of the 46 probes targeted CpGs within CpG islands (Supplementary Table 2).
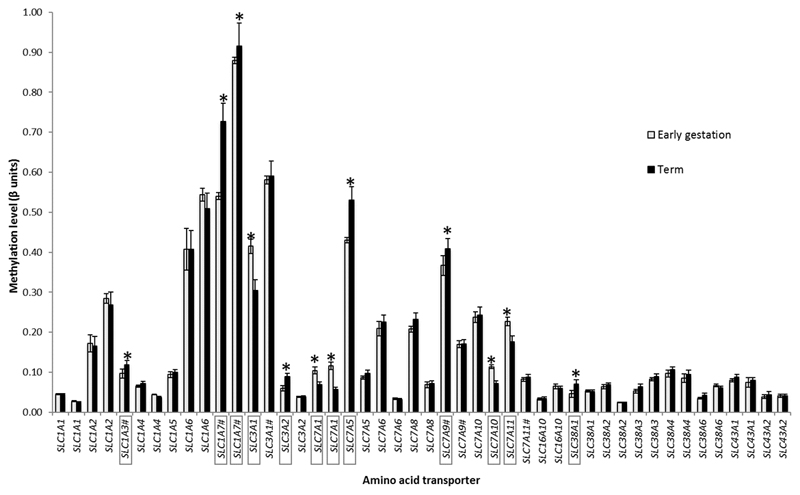
12 out of 46 CpG sites measured showed significantly altered methylation across gestation. There was increased DNA methylation across gestation at individual CpGs (CpG 227 ± 70 base pairs from transcription start site (TSS), Supplementary Table 2) in the following amino acid transporter genes: SLC1A3, SLC1A7, SLC38A1, SLC7A5, SLC3A2 and SLC7A9 (Figure 2). There was decreased DNA methylation across gestation at specific sites (CpG 533 ± 102 base pairs from transcription start site (TSS), Supplementary Table 2) in the following amino acid transporter genes: SLC7A1, SLC7A10, SLC3A1 and SLC7A11 (Figure 2).
Figure 2. Amino acid transporter gene DNA methylation across gestation.
DNA methylation levels of 24 amino acid transporters in first trimester and term placenta measured using an HM27 array. Two probes targeted CpGs in the promoter region of each gene except for SLC1A5 and SLC1A3 that were targeted by single probes; # indicates the CpG was not within a CpG island. Data presented as mean ± SEM. * p < 0.05, significant difference in DNA methylation of transporter gene (in box) between early (n = 18) and term (n = 14) placentas.
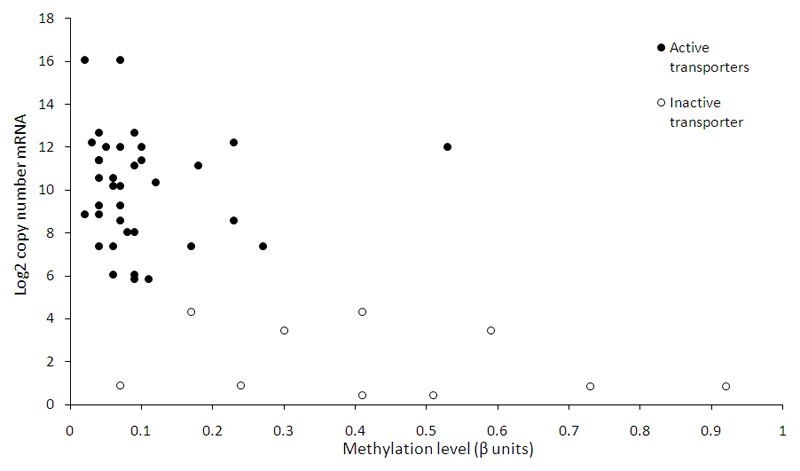
The 19 amino acid transporters studied that are known to be active in placenta [7] showed relatively low levels of promoter methylation (mean β-value 0.10) and high mRNA levels at term as measured by RNA sequencing (Figure 3). For 17 of these transporter genes the CpG sites measured were within CpG islands (Supplementary Table 2). The 5 amino acid transporters not known to be active in placenta (with lower mRNA levels) had relatively higher levels of promoter methylation (mean β-value 0.48) compared to active transporters p < 0.001, t-test (Figure 3). For 3 of these transporter genes the CpG sites measured were within CpG islands (Supplementary Table 2).
Figure 3.
Term placenta amino acid transporter gene expression measured by RNA Sequencing vs. promoter DNA methylation. Each point is the mean value for a specific amino acid transporter type. Solid circles represent transporters expressed and active in human placenta; open circles represents transporters not thought to be active in term human placenta. (Two points per transporter as two probes per promoter in the methylation array). Copy number = Fragments Per Kilobase of exon per Million reads (FPKM); RNA-sequencing at 19 million reads.
HM450 array data
To examine the DNA methylation levels of the 25 amino acid transporters in more detail within first trimester and term placenta we used publically available data from an HM450 methylation array. This array provides a much greater coverage of the genome in terms of CpG location, as well as covering 43 out of the 46 sites covered by the HM27 array. The number of probes targeting each transporter gene ranged from 6 to 46 and covered CpGs within the 5’ promoter CpG island, shore or shelf, gene body or ENCODE predicted enhancer elements.
At predicted enhancer sites DNA methylation across gestation was significantly increased in the amino acid transporter genes SLC1A2, SLC1A3, SLC1A7, SLC38A3, SLC38A4, SLC7A7, and SLC7A1; decreased in SLC43A2 and both increased and decreased in enhancers of SLC7A5 and SLC7A8 (Table 2). CpGs in shore and shelf regions had increased DNA methylation across gestation in the amino acid transporter genes SLC1A1, SLC1A4, SLC38A2, SLC43A1 and SLC7A10 (Table 2). The promoter regions of SLC1A5 and SLC7A1 had increased methylation across gestation, whereas SLC38A1 had both increased and decreased methylation in promoter regions (Table 2). CpG islands in the gene body of SLC7A9 showed increased methylation and that of SLC7A1 showed decreased methylation across gestation (Table 1).
Table 2. Placental HM450 array DNA methylation data for amino acid transporter genes across gestation (1st trimester–term).
| Amino acid transporter | Mean 1st trimester methylation (beta units) | Mean term methylation (beta units) | Change (dB) | p value | Regulatory Feature Group | Relation to Island | Gene region | |
|---|---|---|---|---|---|---|---|---|
| Gene | Protein | |||||||
| SLC1A1 | EAAT3 | 0.08 | 0.08 | -0.004 | 7.5E-01 | Promoter | Island | 1stExon |
| SLC1A1 | 0.24 | 0.16 | -0.080 | 1.9E-03 | Promoter | N_Shore | Body | |
| SLC1A1 | 0.63 | 0.84 | 0.207 | 2.8E-08* | S_Shore | Body | ||
| SLC1A2 | EAAT2 | 0.16 | 0.25 | 0.090 | 1.0E-03 | Island | 5'UTR;1stExon | |
| SLC1A2 | 0.22 | 0.29 | 0.076 | 1.3E-03 | N_Shore | Body | ||
| SLC1A2 | 0.62 | 0.76 | 0.143 | 7.6E-05* | Enhancer | Body | ||
| SLC1A2 | 0.71 | 0.90 | 0.185 | 2.4E-04* | Enhancer | 3'UTR | ||
| SLC1A3 | EAAT1 | 0.08 | 0.08 | 0.004 | 6.1E-01 | 1stExon;5'UTR | ||
| SLC1A3 | 0.65 | 0.75 | 0.105 | 4.1E-07* | Enhancer | Body | ||
| SLC1A4 | ASCT1 | 0.06 | 0.07 | 0.003 | 6.4E-01 | Promoter | Island | 1stExon;5'UTR |
| SLC1A4 | 0.59 | 0.71 | 0.127 | 5.1E-04 | Promoter | S_Shore | Body;5'UTR | |
| SLC1A4 | 0.29 | 0.43 | 0.137 | 5.0E-06* | N_Shore | TSS1500 | ||
| SLC1A4 | 0.81 | 0.80 | -0.009 | 5.0E-01 | Body;Body | |||
| SLC1A5 | ASCT2 | 0.07 | 0.07 | 0.002 | 6.2E-01 | Promoter | Island | 1stExon;5'UTR |
| SLC1A5 | 0.09 | 0.09 | 0.003 | 5.3E-01 | Promoter | S_Shore | TSS1500 | |
| SLC1A5 | 0.08 | 0.16 | 0.086 | 1.0E-05* | Promoter | N_Shelf | 1stExon;5'UTR | |
| SLC1A5 | 0.13 | 0.14 | 0.013 | 2.3E-01 | Promoter | N_Shore | TSS1500;Body | |
| SLC1A5 | 0.59 | 0.53 | -0.053 | 7.8E-04 | N_Shore | TSS1500;Body | ||
| SLC1A5 | 0.91 | 0.89 | -0.023 | 5.8E-02 | Body | |||
| SLC1A6 | EAAT4 | 0.47 | 0.45 | -0.017 | 4.0E-01 | 1stExon | ||
| SLC1A6 | 0.59 | 0.51 | -0.083 | 1.7E-02 | Body | |||
| SLC1A7 | EAAT5 | 0.72 | 0.81 | 0.091 | 2.1E-04* | 1stExon;5'UTR | ||
| SLC1A7 | 0.60 | 0.67 | 0.070 | 2.7E-04* | Island | Body | ||
| SLC1A7 | 0.83 | 0.84 | 0.010 | 6.7E-01 | S_Shore | Body | ||
| SLC1A7 | 0.82 | 0.85 | 0.022 | 7.5E-02 | N_Shore | Body | ||
| SLC1A7 | 0.63 | 0.74 | 0.117 | 7.9E-07* | Enhancer | Body | ||
| SLC3A1 | rBAT | 0.63 | 0.53 | -0.105 | 6.3E-03 | 1stExon | ||
| SLC3A1 | 0.05 | 0.06 | 0.014 | 7.5E-03 | Enhancer | Body | ||
| SLC3A1 | 0.54 | 0.59 | 0.045 | 7.8E-02 | Body | |||
| SLC3A2 | 4F2hc | 0.07 | 0.07 | 0.005 | 8.1E-02 | Promoter | Body | |
| SLC3A2 | 0.77 | 0.76 | -0.005 | 6.7E-01 | Promoter | N_Shore | Body | |
| SLC3A2 | 0.89 | 0.86 | -0.036 | 5.8E-04 | Enhancer | Body | ||
| SLC3A2 | 0.09 | 0.05 | -0.037 | 7.9E-03 | Body | |||
| SLC7A1 | CAT1 | 0.09 | 0.09 | -0.003 | 8.5E-01 | Promoter | Island | 5'UTR;1stExon |
| SLC7A1 | 0.65 | 0.71 | 0.061 | 8.1E-03 | Enhancer | 5'UTR | ||
| SLC7A1 | 0.69 | 0.71 | 0.025 | 3.8E-01 | Promoter | N_Shore | 5'UTR | |
| SLC7A1 | 0.43 | 0.40 | -0.026 | 2.8E-01 | S_Shelf | Body | ||
| SLC7A1 | 0.60 | 0.54 | -0.067 | 3.4E-05* | Island | Body | ||
| SLC7A1 | 0.68 | 0.71 | 0.035 | 1.2E-01 | Body | |||
| SLC7A1 | 0.68 | 0.65 | -0.030 | 2.2E-01 | Island | 3'UTR | ||
| SLC7A1 | 0.61 | 0.71 | 0.099 | 1.9E-03 | N_Shelf | 3'UTR | ||
| SLC7A1 | 0.05 | 0.08 | 0.030 | 2.4E-02 | N_Shore | 3'UTR | ||
| SLC7A5 | LAT1 | 0.07 | 0.11 | 0.042 | 1.7E-03 | Promoter | Island | 1stExon |
| SLC7A5 | 0.14 | 0.17 | 0.030 | 1.3E-02 | Enhancer | Body | ||
| SLC7A5 | 0.72 | 0.74 | 0.026 | 2.4E-01 | Island | Body | ||
| SLC7A5 | 0.45 | 0.54 | 0.088 | 1.6E-04* | Enhancer | S_Shore | TSS1500 | |
| SLC7A5 | 0.23 | 0.30 | 0.074 | 2.3E-03 | N_Shore | Body | ||
| SLC7A5 | 0.59 | 0.70 | 0.112 | 1.0E-04* | N_Shelf | Body | ||
| SLC7A5 | 0.80 | 0.72 | -0.080 | 1.2E-04* | Body | |||
| SLC7A5 | 0.88 | 0.93 | 0.054 | 1.6E-03 | Enhancer | 3'UTR | ||
| SLC7A5 | 0.88 | 0.88 | 0.001 | 8.6E-01 | 3'UTR | |||
| SLC7A6 | y+LAT2 | 0.06 | 0.06 | 0.001 | 8.2E-01 | Promoter | Island | 5'UTR |
| SLC7A6 | 0.11 | 0.11 | 0.001 | 9.2E-01 | Promoter | S_Shore | 5'UTR | |
| SLC7A6 | 0.92 | 0.90 | -0.017 | 4.7E-01 | S_Shelf | 5'UTR | ||
| SLC7A6 | 0.92 | 0.90 | -0.011 | 2.2E-01 | Promoter | N_Shore | TSS1500 | |
| SLC7A7 | y+LAT1 | 0.35 | 0.34 | -0.012 | 5.5E-01 | 5'UTR;1stExon | ||
| SLC7A7 | 0.19 | 0.26 | 0.069 | 5.9E-03 | TSS1500;5'UTR | |||
| SLC7A7 | 0.85 | 0.81 | -0.039 | 4.9E-02 | TSS1500;5'UTR | |||
| SLC7A7 | 0.12 | 0.13 | 0.013 | 5.7E-01 | N_Shelf | 1stExon;5'UTR | ||
| SLC7A7 | 0.20 | 0.19 | -0.006 | 7.7E-01 | N_Shore | TSS200 | ||
| SLC7A7 | 0.32 | 0.42 | 0.099 | 6.1E-03 | Enhancer | N_Shore | TSS1500 | |
| SLC7A7 | 0.58 | 0.54 | -0.045 | 3.5E-01 | Body | |||
| SLC7A7 | 0.38 | 0.49 | 0.109 | 1.2E-04* | Enhancer | Body | ||
| SLC7A8 | LAT2 | 0.08 | 0.08 | -0.001 | 9.3E-01 | 5'UTR;1stExon | ||
| SLC7A8 | 0.74 | 0.68 | -0.060 | 2.6E-04* | Enhancer | 5'UTR;1stExon | ||
| SLC7A8 | 0.72 | 0.69 | -0.033 | 3.4E-03 | Enhancer | Body;TSS1500 | ||
| SLC7A8 | 0.63 | 0.72 | 0.090 | 2.6E-07* | Enhancer | Body | ||
| SLC7A8 | 0.89 | 0.91 | 0.021 | 1.3E-01 | 3'UTR | |||
| SLC7A9 | b(0,+)AT1 | 0.40 | 0.46 | 0.062 | 1.7E-02 | 1stExon;5'UTR | ||
| SLC7A9 | 0.73 | 0.82 | 0.096 | 1.4E-04* | Island | Body;Body | ||
| SLC7A9 | 0.80 | 0.81 | 0.012 | 5.4E-01 | N_Shore | Body;Body | ||
| SLC7A9 | 0.82 | 0.85 | 0.023 | 7.3E-02 | S_Shore | Body;Body | ||
| SLC7A10 | asc | 0.10 | 0.09 | -0.008 | 5.1E-01 | Island | TSS1500 | |
| SLC7A10 | 0.42 | 0.48 | 0.064 | 7.3E-05* | S_Shelf | Body | ||
| SLC7A10 | 0.42 | 0.53 | 0.110 | 4.3E-04* | N_Shelf | Body | ||
| SLC7A10 | 0.55 | 0.50 | -0.045 | 5.6E-02 | N_Shore | Body | ||
| SLC7A10 | 0.60 | 0.70 | 0.103 | 1.9E-03 | S_Shore | 3'UTR;3'UTR | ||
| SLC7A11 | xCT | 0.07 | 0.08 | 0.005 | 5.3E-01 | 1stExon;5'UTR | ||
| SLC7A11 | 0.10 | 0.11 | 0.013 | 2.3E-01 | Body | |||
| SLC7A11 | 0.78 | 0.74 | -0.043 | 5.4E-04 | Enhancer | Body | ||
| SLC7A11 | 0.80 | 0.83 | 0.037 | 5.3E-05* | Enhancer | Body | ||
| SLC7A11 | 0.91 | 0.91 | 0.004 | 7.7E-01 | Enhancer | TSS1500 | ||
| SLC16A10 | TAT1 | 0.06 | 0.07 | 0.009 | 1.1E-01 | Promoter | Island | |
| SLC16A10 | 0.81 | 0.81 | 0.005 | 8.0E-01 | S_Shelf | Body | ||
| SLC16A10 | 0.35 | 0.38 | 0.032 | 3.4E-02 | Enhancer | Body | ||
| SLC38A1 | SNAT1 | 0.09 | 0.10 | 0.003 | 7.9E-01 | Promoter | Island | 5'UTR |
| SLC38A1 | 0.36 | 0.56 | 0.205 | 3.7E-08* | Promoter | S_Shore | TSS1500 | |
| SLC38A1 | 0.44 | 0.77 | 0.336 | 1.8E-07* | N_Shelf | 5'UTR | ||
| SLC38A1 | 0.18 | 0.21 | 0.025 | 2.0E-01 | Promoter | N_Shore | 5'UTR | |
| SLC38A1 | 0.90 | 0.88 | -0.016 | 1.8E-02 | Body | |||
| SLC38A1 | 0.93 | 0.90 | -0.027 | 5.1E-05* | Enhancer | 3'UTR | ||
| SLC38A2 | SNAT2 | 0.07 | 0.07 | 0.003 | 6.9E-01 | Promoter | Island | 5'UTR |
| SLC38A2 | 0.41 | 0.40 | -0.014 | 2.8E-01 | Promoter | S_Shore | TSS1500 | |
| SLC38A2 | 0.66 | 0.79 | 0.128 | 5.2E-05* | S_Shore | TSS1500 | ||
| SLC38A2 | 0.91 | 0.85 | -0.053 | 9.0E-03 | Promoter | N_Shelf | Body | |
| SLC38A2 | 0.07 | 0.07 | 0.005 | 5.5E-01 | Promoter | N_Shore | Body | |
| SLC38A2 | 0.93 | 0.93 | -0.001 | 9.6E-01 | 3'UTR | |||
| SLC38A3 | SNAT3 | 0.08 | 0.08 | 0.008 | 1.3E-01 | Island | 5'UTR | |
| SLC38A3 | 0.77 | 0.86 | 0.089 | 1.1E-03 | S_Shelf | 5'UTR | ||
| SLC38A3 | 0.51 | 0.75 | 0.235 | 8.0E-06* | Enhancer | N_Shore | TSS1500 | |
| SLC38A4 | SNAT4 | 0.10 | 0.11 | 0.011 | 8.6E-02 | 1stExon;5'UTR | ||
| SLC38A4 | 0.63 | 0.81 | 0.188 | 4.1E-06* | Enhancer | 5'UTR | ||
| SLC38A4 | 0.89 | 0.89 | 0.001 | 9.6E-01 | Enhancer | Body | ||
| SLC38A4 | 0.16 | 0.25 | 0.099 | 2.2E-04* | Enhancer | Body | ||
| SLC38A4 | 0.91 | 0.89 | -0.022 | 8.4E-02 | N_Shelf | TSS1500 | ||
| SLC38A6 | SNAT6 | 0.06 | 0.06 | 0.002 | 6.2E-01 | Unclassified | Island | 5'UTR |
| SLC38A6 | 0.90 | 0.92 | 0.014 | 6.4E-02 | Enhancer | Body | ||
| SLC43A1 | LAT3 | 0.12 | 0.11 | -0.005 | 4.0E-01 | Promoter | Island | 1stExon;5'UTR |
| SLC43A1 | 0.25 | 0.34 | 0.086 | 1.7E-03 | Enhancer | Body | ||
| SLC43A1 | 0.70 | 0.82 | 0.118 | 5.6E-06* | S_Shelf | Body | ||
| SLC43A1 | 0.07 | 0.08 | 0.012 | 3.3E-02 | S_Shore | Body | ||
| SLC43A1 | 0.53 | 0.59 | 0.062 | 7.4E-03 | N_Shore | Body | ||
| SLC43A2 | LAT4 | 0.04 | 0.04 | 0.038 | 3.8E-02 | Promoter | 5'UTR | |
| SLC43A2 | 0.63 | 0.52 | -0.110 | 3.3E-08* | Enhancer | S_Shore | Body | |
| SLC43A2 | 0.59 | 0.58 | -0.013 | 3.0E-01 | N_Shore | Body | ||
| SLC43A2 | 0.75 | 0.74 | -0.013 | 3.5E-01 | Body | |||
| SLC43A2 | 0.59 | 0.38 | -0.208 | 1.4E-07* | Enhancer | Island | 3'UTR | |
DNA methylation change across gestation. Bold indicates significance, * p < 4.3E-04.
Placental amino acid transporter genes show altered expression across gestation
We examined the gene expression of the 25 selected placental amino acid transporters (Table 1) across gestation using publically available gene expression array data from first trimester, early second trimester and term placentas. The transporters SLC7A7, SLC38A6, SLC1A2, SLC1A3, SLC1A4 and SLC7A11 showed decreased gene expression across gestation, whereas the transporters SLC7A5, SLC38A2 and SLC7A10 showed increased gene expression across gestation. Of these transporters, SLC1A2, SLC1A3, SLC1A4, SLC7A11, SLC7A5, and SLC38A2 were observed to have altered methylation across gestation in the separate HM450 array cohort.
Discussion
This study investigated DNA methylation of amino acid transporter genes in the placenta. In a trophoblast derived cell line, we observed changes to the expression of specific amino acid transporters following inhibition of DNA methylation. However, it is unclear whether these observations are due to direct effects on DNA methylation within the transporter genes themselves or methylation of indirect regulatory factors. Cohort data revealed that promoter DNA methylation of the placental amino acid transporter genes remains low across gestation and does not always play an obvious role in regulating gene expression. For specific transporter genes there were changes in DNA methylation across gestation and these methylation changes were located in enhancer regions. Analysis of publically available gene expression array data from human placenta showed expression changes across gestation for these genes also. Together these data suggest that DNA methylation may play a role in the regulation of amino acid transporter gene expression.
The findings of this study support a role for DNA methylation in the maintenance of long-term gene silencing [41] to determine whether a transporter is capable of being active in the placenta, rather than a mechanism for more subtle regulation of gene activity levels over time. This may act as a protective ‘buffer’ against potentially harmful methylation changes induced by exposure to adverse maternal environments and therefore be a protective mechanism for the fetus. For example, the amino acid transporters whose expression is absent or low in term placenta (as measured by RNA sequencing) were more highly methylated in term placenta at the promoter sites investigated using the HM27 methylation array. The amino acid transporter genes SLC1A6, SLC1A7 and SLC7A9 whose expression in human placenta is low [15, 42] tended to have consistently high levels of methylation across gestation. Indeed, inhibition of DNA methylation by 5-AZA-dC increased the expression of both SLC1A6 and SLC1A7, supporting a role for methylation in controlling the expression of these genes.
Most of the amino acid transporters with higher expression in the placenta showed low levels of methylation at term at the promoter sites investigated using the HM27 methylation array. The majority of CpG dense promoters when assessed genome-wide remain unmethylated which is permissive of gene expression activity, along with the histone mark H3K4me3; however additional epigenetic marks such as H3K27ac in these regions are more indicative of gene transcription [43, 44]. Methylation may therefore play a role in determining whether a transporter is capable of being activated or permanently inactivated within human placenta, but not directly influence the precise expression level. However, there are likely to be more CpG sites involved in regulating the expression of these genes and potentially multiple start sites for transcription that could be invoked. DNA methylation is just part of the epigenetic machinery and there may also be other epigenetic processes involved [41].
The amino acid transporters SLC1A2 SLC1A3, SLC1A4, SLC7A5, SLC7A11 and SLC7A10 showed changes in DNA methylation across gestation. These transporters also showed changes in gene expression across gestation in publically available gene expression array data and an inverse relationship between methylation and expression in the BeWo cell culture experiments. SLC1A3 showed the same methylation changes in both DNA methylation arrays. These data suggest that the expression of only a small number of amino acid transporters in the human placenta may be mediated by DNA methylation in the predicted enhancer regions covered on the array; although the effect sizes were small and direct functional testing is required. This is in contrast to placental glucose transporters that have variable methylation levels, which are associated with changes in gene expression across gestation [34]. A limitation to this study is that RNA sequencing data was only available in term placentas and not across gestation, whereas array data was available for early gestation and term placentas to allow comparison. While there was little evidence for direct epigenetic regulation of amino acid transporter expression overall, the relatively small percentage of CpG sites covered by the array mean that evidence for epigenetic control at other sites may not have been observed.
The limited relationships between DNA methylation and amino acid transporter gene expression are likely to be indirect via coordinated higher-level control in a more dynamic state than would normally be associated with CpG island-containing promoter methylation, as measured by the 27K array [8]. Although those modifications that can be identified within CpG islands are strongly functionally supported to impact on gene activity [45]. CpG island shore locations (2k either side of the island) are seen to be the most dynamic with respect to their methylation state and significant with respect to their functional impact on expression [46]. Indeed, the associations we did observe tended to be in CpGs within these shore regions and enhancer regions covered by the 450K array but not covered on the 27k array platform. The location of the CpGs analysed within each gene may also be a limitation to this study as only limited information on the methylation status of the regions could be gained. Although there are many cases whereby nearby CpGs regions are co-ordinately regulated. The fact that the relationships across gestation for methylation and expression were observed in array data from unrelated individuals and that we did not have details of the maternal environment, diet or anthropometric profile for the array samples were limitations of this study as we could not incorporate this into the analysis.
In these studies whole placental tissue was used for methylation and gene expression measures, however the cell heterogeneity within the placenta may influence these measures. We used BeWo cells for the DNA methylation experiments, which may have a different phenotype from term placenta as they originate from choriocarcinoma derived from invasive trophoblast. This model does however allow us to clearly see the effects of DNA methylation on gene expression in a trophoblast derived cell line.
Supplementary Material
In summary, our findings do not support a major role for DNA methylation within gene regulatory sites in directly influencing temporal differential expression of amino acid transporter genes in human placenta throughout gestation. This supports the idea that DNA methylation is involved in the long-term/robust repression of particular genes, whilst the hypomethylated state indicates a permissive promoter that is able to be expressed when required.
Funding
CS was supported by the Gerald Kerkut Charitable Trust
Footnotes
Authors' Roles
BN, KL, NC, RS, RL, JC: participation in study design. CS, BN, RS, JC: participation in study execution and analysis. CS, BN, KL, CB, NH, CC, RS, RL, JC: participation in manuscript drafting and critical discussion.
Conflict of Interest
None to declare
References
- [1].Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, Marconi AM, Pardi G, Sibley CP. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr Res. 1997;42(4):514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- [2].Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23(5):392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- [3].Lewis RM, Cleal JK, Hanson MA. Review: Placenta, evolution and lifelong health. Placenta. 2012;33(Suppl):S28–S32. doi: 10.1016/j.placenta.2011.12.003. [DOI] [PubMed] [Google Scholar]
- [4].Rosario FJ, Dimasuay KG, Kanai Y, Powell TL, Jansson T. Regulation of amino acid transporter trafficking by mTORC1 in primary human trophoblast cells is mediated by the ubiquitin ligase Nedd4-2. Clin Sci (Lond) 2016;130(7):499–512. doi: 10.1042/CS20150554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. 2014;94(4):1027–1076. doi: 10.1152/physrev.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schroeder DI, Blair JD, Lott P, Yu HO, Hong D, Crary F, Ashwood P, Walker C, Korf I, Robinson WP, LaSalle JM. The human placenta methylome. Proc Natl Acad Sci U S A. 2013;110(15):6037–42. doi: 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cleal JK, Glazier JD, Ntani G, Crozier SR, Day PE, Harvey NC, Robinson SM, Cooper C, Godfrey KM, Hanson MA, Lewis RM. Facilitated transporters mediate net efflux of amino acids to the fetus across the basal membrane of the placental syncytiotrophoblast. J Physiol. 2011;589(Pt 4):987–997. doi: 10.1113/jphysiol.2010.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Panitchob N, Widdows KL, Crocker IP, Johnstone ED, Please CP, Sibley CP, Glazier JD, Lewis RM, Sengers BG. Computational modelling of placental amino acid transfer as an integrated system. Biochim Biophys Acta. 2016;1858(7 Pt A):1451–1461. doi: 10.1016/j.bbamem.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Day PE, Cleal JK, Lofthouse EM, Goss V, Koster G, Postle A, Jackson JM, Hanson MA, Jackson AA, Lewis RM. Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta. 2013;34(12):1223–1231. doi: 10.1016/j.placenta.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lofthouse EM, Perazzolo S, Brooks S, Crocker IP, Glazier JD, Johnstone ED, Panitchob N, Sibley CP, Widdows KL, Sengers BG, Lewis RM. Phenylalanine transfer across the isolated perfused human placenta: an experimental and modeling investigation. Am J Physiol Regul Integr Comp Physiol. 2016;310(9):R828–R836. doi: 10.1152/ajpregu.00405.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hediger MA, Clemencon B, Burrier RE, Bruford EA. The ABCs of membrane transporters in health and disease (SLC series): introduction. Molecular aspects of medicine. 2013;34(2–3):95–107. doi: 10.1016/j.mam.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Noorlander CW, de Graan PN, Nikkels PG, Schrama LH, Visser GH. Distribution of glutamate transporters in the human placenta. Placenta. 2004;25(6):489–495. doi: 10.1016/j.placenta.2003.10.018. [DOI] [PubMed] [Google Scholar]
- [13].Desforges M, Greenwood SL, Glazier JD, Westwood M, Sibley CP. The contribution of SNAT1 to system A amino acid transporter activity in human placental trophoblast. Biochem Biophys Res Commun. 2010;398(1):130–134. doi: 10.1016/j.bbrc.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Desforges M, Mynett KJ, Jones RL, Greenwood SL, Westwood M, Sibley CP, Glazier JD. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J Physiol. 2009;587(Pt 1):61–72. doi: 10.1113/jphysiol.2008.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ayuk PT, Sibley CP, Donnai P, D'Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol. 2000;278(6):C1162–C1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- [16].Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol. 2008;20(4):419–426. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- [17].Day PE, Ntani G, Crozier SR, Mahon PA, Inskip HM, Cooper C, Harvey NC, Godfrey KM, Hanson MA, Lewis RM, Cleal JK. Maternal Factors Are Associated with the Expression of Placental Genes Involved in Amino Acid Metabolism and Transport. PLoS One. 2015;10(12):e0143653. doi: 10.1371/journal.pone.0143653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Loubiere LS, Vasilopoulou E, Bulmer JN, Taylor PM, Stieger B, Verrey F, McCabe CJ, Franklyn JA, Kilby MD, Chan SY. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta. 2010;31(4):295–304. doi: 10.1016/j.placenta.2010.01.013. [DOI] [PubMed] [Google Scholar]
- [19].Mahendran D, Donnai P, Glazier JD, D'Souza SW, Boyd RD, Sibley CP. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr Res. 1993;34(5):661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- [20].Chen YY, Rosario FJ, Shehab MA, Powell TL, Gupta MB, Jansson T. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin Sci (Lond) 2015;129(12):1131–1141. doi: 10.1042/CS20150511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cleal JK, Day PE, Simner CL, Barton SJ, Mahon PA, Inskip HM, Godfrey KM, Hanson MA, Cooper C, Lewis RM, Harvey NC. Placental amino acid transport may be regulated by maternal vitamin D and vitamin D-binding protein: results from the Southampton Women's Survey. Br J Nutr. 2015;113(12):1903–1910. doi: 10.1017/S0007114515001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoyo C, Fortner K, Murtha AP, Schildkraut JM, Soubry A, Demark-Wahnefried W, Jirtle RL, Kurtzberg J, Forman MR, Overcash F, Huang Z, et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control. 2012;23(4):635–645. doi: 10.1007/s10552-012-9932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- [25].Green BB, Karagas MR, Punshon T, Jackson BP, Robbins DJ, Houseman EA, Marsit CJ. Epigenome-Wide Assessment of DNA Methylation in the Placenta and Arsenic Exposure in the New Hampshire Birth Cohort Study (USA) Environmental health perspectives. 2016;124(8):1253–60. doi: 10.1289/ehp.1510437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59(10):1481–1490. doi: 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lewis RM, Demmelmair H, Gaillard R, Godfrey KM, Hauguel-de MS, Huppertz B, Larque E, Saffery R, Symonds ME, Desoye G. The placental exposome: placental determinants of fetal adiposity and postnatal body composition. Ann Nutr Metab. 2013;63(3):208–215. doi: 10.1159/000355222. [DOI] [PubMed] [Google Scholar]
- [28].Banister CE, Koestler DC, Maccani MA, Padbury JF, Houseman EA, Marsit CJ. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics. 2011;6(7):920–927. doi: 10.4161/epi.6.7.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Gagne LA, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6(5):566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].El Hajj N, Pliushch G, Schneider E, Dittrich M, Muller T, Korenkov M, Aretz M, Zechner U, Lehnen H, Haaf T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62(4):1320–1328. doi: 10.2337/db12-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nomura Y, Lambertini L, Rialdi A, Lee M, Mystal EY, Grabie M, Manaster I, Huynh N, Finik J, Davey M, Davey K, et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci. 2014;21(1):131–137. doi: 10.1177/1933719113492206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jansson T, Ekstrand Y, Wennergren M, Powell TL. Placental glucose transport in gestational diabetes mellitus. Am J Obstet Gynecol. 2001;184(2):111–116. doi: 10.1067/mob.2001.108075. [DOI] [PubMed] [Google Scholar]
- [33].Jansson T, Wennergren M, Powell TL. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am J Obstet Gynecol. 1999;180(1 Pt 1):163–168. doi: 10.1016/s0002-9378(99)70169-9. [DOI] [PubMed] [Google Scholar]
- [34].Novakovic B, Gordon L, Robinson WP, Desoye G, Saffery R. Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J Nutr Biochem. 2013;24(1):282–288. doi: 10.1016/j.jnutbio.2012.06.006. [DOI] [PubMed] [Google Scholar]
- [35].Wang Y, Pringle KG, Chen YX, Zakar T, Lumbers ER. Regulation of the renin-angiotensin system (RAS) in BeWo and HTR-8/SVneo trophoblast cell lines. Placenta. 2012;33(8):634–9. doi: 10.1016/j.placenta.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [36].Novakovic B, Yuen RK, Gordon L, Penaherrera MS, Sharkey A, Moffett A, Craig JM, Robinson WP, Saffery R. Evidence for widespread changes in promoter methylation profile in human placenta in response to increasing gestational age and environmental/stochastic factors. BMC Genomics. 2011;12:529. doi: 10.1186/1471-2164-12-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Blair JD, Langlois S, McFadden DE, Robinson WP. Overlapping DNA methylation profile between placentas with trisomy 16 and early-onset preeclampsia. Placenta. 2014;35(3):216–22. doi: 10.1016/j.placenta.2014.01.001. [DOI] [PubMed] [Google Scholar]
- [38].An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, Sali A, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148(3):1059–1079. doi: 10.1210/en.2006-0683. [DOI] [PubMed] [Google Scholar]
- [40].Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15(9):866–877. doi: 10.1177/1933719108322425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nature reviews Genetics. 2009;10(5):295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- [42].Palacin M, Kanai Y. The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch. 2004;447(5):490–494. doi: 10.1007/s00424-003-1062-7. [DOI] [PubMed] [Google Scholar]
- [43].Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39(4):457–66. doi: 10.1038/ng1990. [DOI] [PubMed] [Google Scholar]
- [44].Bird A. DNA methylation patterns and epigenetic memory. Genes & development. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- [45].Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature reviews Genetics. 2012;13(7):484–92. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- [46].Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, Onyango P, Cui H, Gabo K, Rongione M, Webster M, Ji H, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41(2):178–86. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.