Abstract
The present study aimed to extract total phenolic compounds (TPC), total flavonoid compounds (TFC), and ascorbic acid (AA) from the fruit of rugosa rose (Rosa rugosa Thunb.) by ultrasound-assisted extraction (UAE), and to evaluate their antioxidant activities. UAE significantly increased the extract yield compared with that obtained using the conventional method. TPC, TFC, and AA were extracted, depending on the extraction conditions (temperature, time, and ethanol concentration), in the range of 50.73–96.69, 15.93–31.88, and 3.06–6.08 mg/g, respectively. TPC and TFC were effectively extracted at a relatively high temperature (50 °C) than AA was (30 °C). The solvent condition used to extract TPC, TFC, and AA was 50% ethanol. The UAE condition for the highest antioxidant activity was obtained 30 °C, 30 min, and 50% ethanol, which were the same condition for the highest AA extraction. Among the extracts, AA showed a strong correlation with antioxidant activity at p-value of 0.001.
Keywords: Rugosa rose (Rosa rugosa Thunb.), Ultrasound-assisted extraction, Antioxidant, Total phenolic compounds, Total flavonoid compounds, Ascorbic acid
Introduction
The rugosa rose (Rosa rugosa Thunb.), a species belonging to the Rosaceae family, is a summer green shrub and native plant of Northeastern China, Japan, Korea, and Southeastern Siberia. It grows commonly on the coast and in sand dunes [1]. The plant is a halophyte and is resistant to severe climatic conditions and high salinity. In particular, it can grow at a low temperature (− 20 °C), therefore, it has been introduced to Europe and North America as an ornamental plant. In Asia, the rugosa rose is often used in traditional medicine, and in cosmetic, food, and textile industries, because of its high contents of phenolic compounds and vitamin C. The essential oil extracted from rugosa rose flowers is used as a cosmetic and food ingredients because of its scent and medicinal properties. In addition, extracts of the fruit and leaves are used in herbal remedies as vasodilators and antioxidants [2]. The biologically active compounds in the rugosa rose differ depending on the growth conditions, species, and plant part. The plant contains various compounds including phenolic compounds, terpenoids, fatty acids, and sugars [3]. The fruit and its extracts contain significant quantities of flavonoids (catechin, quercetin, and rutin), phenolic acid (methyl gallate, ferulic acid, and syringic acid), and ascorbic acid (AA) [4, 5].
Hydroxyl groups generated from these compounds remove free radicals and reactive oxygen species (ROS) by acting as reducing agents or hydrogen-atom donors. Oxygen is essential to most living organisms for energy production in biological processes. However, some of the oxygen (2–3%) taken into the human body is converted to ROS and free radicals, which accelerate oxidative damage to various biomolecules such as DNA, proteins, small cellular molecules, and membrane lipids [6]. This oxidative damage can lead to diseases such as cancer, Alzheimer’s disease, and arteriosclerosis [7]. Antioxidants are widely used to remove ROS and other free radicals. Among known antioxidants, butylated hydroxytoluene (BHT) and butylated hydroxylanisole (BHA) have been used in the food industry because they are effective and economical. However, the use of these chemicals is limited or prohibited because their excessive consumption might lead to cancer. Therefore, increased research effort has been directed toward discovering of antioxidants from natural products [8].
Various extraction methods have been suggested for isolating natural biologically active components, depending on the target use. Soxhlet extraction, heating reflux extraction, and maceration have been used as traditional extraction methods. However, these methods have some disadvantage such as their time consumption, inefficiency, and uneconomical nature. To overcome these problems, an ultrasound-assisted extraction (UAE) method has been introduced to extract biologically active compounds [9]. UAE increases the yield of the extract with short extraction times. The acoustic cavitation generated by UAE destroys the cell wall and reduces the particle size, thereby enhancing the contact between the solvents used in UAE and the target compounds present in the cell wall [10]. In addition, there are some advantages to this method, such as simplicity, speed, and low energy consumption, compared with traditional extraction methods.
In this study, the UAE method was used to extract total phenolic compounds (TPC), total flavonoid compounds (TFC), and ascorbic acid (AA) from rugosa rose fruit with specific and optimized extraction factors (solvent concentration, extraction time, and temperature) for each compound. Furthermore, their antioxidant activities were investigated, and statistical comparisons were performed between each extract and their antioxidant activities.
Materials and methods
Materials
The rugosa rose fruit were harvested in November 2015 from Chonnam National University (35°10′25.6″N, 126°54′00.8″E), South Korea. The fruit were immediately washed in water and freeze dried. The dried fruits were ground, and then passed through a 40 mesh sieve. Finally, the samples were sealed and stored at 4 °C until further analysis.
Ultrasound-assisted extraction (UAE) procedure
Extraction of rugosa rose fruit was performed using an ultrasonic water bath (NXP-1505P, Kodo, Hwaseong-si, Korea) at power and frequency of 200 W and 40 kHz, respectively. The extraction temperature and time were controlled using an operating system with a range of 30–50 °C and 30–50 min, respectively. The extraction variables of time, temperature, and solvent concentration were also investigated. The rugosa rose fruit samples were extracted with ethanol [50, 75, and 95% (w/w)]. The sample to solvent ratio was 1:20 (w/w). The extraction was conducted at different temperatures (30, 40, and 50 °C) and times (30, 40, and 50 min). The liquid fraction (extracts) was separated via vacuum filtration and subsequently concentrated using a rotary evaporator (N-1000, EYELA, Tokyo, Japan). The concentrated extract was collected with 7 mL of distilled water and freeze-dried to obtain a powdered extract, which was diluted to 1 mg/mL with distilled water and subsequently analyzed for the TPC, TFC, and AA contents. For antioxidant activity determination, the powder was diluted with ethanol to the desired concentration.
To compare UAE with conventional extraction, conventional extraction was performed in a shaking water bath under the same conditions used for UAE.
Analysis of total phenolic compounds (TPC)
The TPC content of the rugosa rose fruit extracts was determined using a slight modification of the method described by Scalbert et al. [11]. Briefly, 0.2 mL of the extract was mixed with 1 mL of Folin Ciocalteu’s reagent (10%, w/w) and then 0.8 mL of sodium carbonate (Na2CO3, 7.5%) was added to the mixture, which was kept in the dark at room temperature for 2 h. The absorbance of the mixture was measured at 760 nm using an UV–Vis spectrophotometer (UV 1800, Shimadzu, Kyoto, Japan). The TPC were determined using a linear regression equation, which was obtained from a standard curve constructed using various concentrations of vanillin.
Analysis of total flavonoid compounds (TFC)
The TFC content of the rugosa rose fruit extracts was determined using a slight modification of the method described by Zhishen et al. [12]. Briefly, 0.5 mL of the extract was diluted to 4 mL with distilled water; 0.15 mL of sodium nitrate (NaNO2, 5%) was added to the extract, and then the mixture was incubated at room temperature for 5 min. Finally, 0.15 mL of aluminum chloride (AlCl3, 10%) and 1 mL of sodium hydroxide (NaOH, 1 M) were added to complete the reaction. The absorbance of the mixture was measured at 510 nm using a UV–Vis spectrophotometer (UV 1800, Shimadzu, Kyoto, Japan). The calibration curve was prepared using quercetin as the standard.
Analysis of ascorbic acid (AA)
The AA content of the rugosa rose fruit extracts was detected using a HPLC system (Waters e2695, Alliance, MA, USA), which was fitted with a C18 column (4.6 × 250 mm) and a UV/Vis detector (Waters 2489, Milford, MA, USA). We used 0.1% phosphoric acid as the mobile phase at a flow rate of 1 mL/min [13].
Antioxidant activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the rugosa rose fruit extracts was evaluated using a modification of the method described by Brand-Williams et al. [14]. Different concentration of the extracts in ethanol (0.25–3.0 mg/mL, 50 μL) were added to 0.4 mM DPPH solution (1 mL), and the total volume of the mixture was made up to 2.5 mL with ethanol. The mixture was kept in the dark for 30 min, and then the absorbance of the reaction mixture was determined at 517 nm using a UV–Vis spectrophotometer (SHIMADZU, Kyoto, Japan). Butylated hydroxytoluene (BHT) was used as the positive control, and the activity was calculated using the following formula:
where Ac and As are the absorbance values of the control and sample at 517 nm.
The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activity was measured at 734 nm [15]. To generate ABTS+, ABTS (7 mM) was added to potassium persulfate (2.45 mM) at a 1:1 ratio (v/v). The mixture was placed in the dark for 16 h and the resulting ABTS+ was diluted with distilled water to obtain an absorbance of 0.7 ± 0.02 at 734 nm using a UV–Vis spectrophotometer (SHIMADZU, Japan). The diluted ABTS+ solution (1.98 mL) was mixed with the extract (20 μL), and the absorbance was measured at 734 nm.
The reducing power was analyzed by mixing each extract thoroughly with ethanol (0.5 mL) and 0.2 M sodium phosphate buffer (pH 6.6, 2.5 mL), and then 1% potassium ferricyanide (2.5 mL, w/v) was added to the mixture. The reaction was performed at 50 °C for 20 min after which the reaction was stopped by adding 10% trichloroacetic acid (2.5 mL, w/v), followed by centrifugation for 10 min (1000×g). Finally, the supernatant (2.5 mL), distilled water (2.5 mL), and 0.1% ferric chloride (w/v, 0.5 mL) were mixed thoroughly and the absorbance was measured at 700 nm [16].
Statistical analysis
All the experiments were performed in triplicate, and a two-way analysis of variance (ANOVA) was carried out to analyze the data using the statistical package for the social sciences (SPSS) software version 23 (SPSS Inc., Chicago, IL, USA). Pearson’s correlation coefficient was used to determine the correlation between the variables used, and the differences were considered significant at a p-value < 0.05.
Results and discussion
TPC, TFC, and AA contents in extracts of rugosa rose fruit depend on the extraction conditions
The results of the analysis of the TPC, TFC, and AA contents of the extracts of rugosa rose fruits depended on the UAE conditions (temperature, time, and ethanol concentration) and are presented in Table 1. The extract yield was significantly affected by the extraction time, temperature, and solvent concentration. The yield of TPC was determined as 54.02–64.49 mg/g at 30 °C for 30 min. In addition, the values ranged from 54.32–75.23 mg/g at 30 °C for 50 min. This result suggested that the reaction time had little effect on the extract yield. At higher reaction temperatures (40 and 50 °C), the yield of TPC in the extract decreased with increasing reaction time because the oxidative degradation of phenolic compounds is enhanced when a sample is exposed to high reaction temperatures for a prolonged reaction time. These results are in agreement with those of a previous study by Ma et al. [17]. Another study found that the yield of TPC and anthocyanin increased with increasing reaction time at a low temperature (10 °C), while the yield decreased above 20 °C [18].
Table 1.
Total phenolic compounds, total flavonoids compounds, and ascorbic acid concentrations in extracts of rugosa rose fruit prepared using ultrasound-assisted extraction (mg/g dry weight)
| Ethanol | TPC | TFC | AA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (min) | |||||||||
| 30 | 40 | 50 | 30 | 40 | 50 | 30 | 40 | 50 | |
| 30 °C | |||||||||
| 0% | 58.90 (3.28) | 72.55 (7.90) | 60.02 (5.35) | 20.20 (2.72) | 22.10 (5.35) | 24.38 (6.65) | 5.63 (0.24) | 5.28 (0.41) | 5.00 (0.21) |
| 50% | 64.49 (4.96) | 73.04 (6.54) | 75.23 (6.72) | 21.03 (5.03) | 24.43 (6.72) | 25.30 (9.53) | 6.38 (0.84) | 5.81 (0.64) | 5.40 (0.57) |
| 75% | 60.32 (4.43) | 72.99 (4.22) | 62.28 (4.76) | 20.28 (5.31) | 22.53 (4.76) | 23.72 (1.86) | 4.72 (0.66) | 4.90 (0.28) | 3.95 (0.16) |
| 95% | 54.02 (1.26) | 56.21 (4.50) | 54.32 (3.30) | 17.68 (3.08) | 18.55 (3.30) | 19.83 (4.70) | 3.94 (0.26) | 3.57 (0.37) | 3.06 (0.33) |
| 40 °C | |||||||||
| 0% | 70.00 (3.66) | 67.65 (6.27) | 58.65 (4.76) | 20.45 (3.41) | 19.65 (4.76) | 21.18 (2.02) | 5.23 (0.32) | 4.95 (0.47) | 5.10 (0.35) |
| 50% | 93.53 (6.77) | 80.83 (2.20) | 78.55 (2.67) | 26.95 (3.97) | 28.93 (2.67) | 23.12 (5.65) | 5.93 (0.54) | 5.43 (0.10) | 4.58 (0.27) |
| 75% | 83.45 (2.04) | 69.78 (3.67) | 72.87 (4.52) | 24.27 (3.30) | 20.88 (4.52) | 22.88 (3.64) | 4.27 (0.94) | 4.69 (0.49) | 3.64 (0.65) |
| 95% | 61.46 (4.28) | 52.38 (3.77) | 50.73 (4.36) | 19.07 (6.12) | 19.30 (4.36) | 20.30 (1.35) | 3.45 (0.20) | 3.81 (0.59) | 3.42 (0.15) |
| 50 °C | |||||||||
| 0% | 70.16 (5.59) | 68.12 (3.56) | 71.21 (4.89) | 19.43 (6.55) | 26.10 (4.89) | 20.52 (1.36) | 4.03 (0.35) | 4.84 (0.26) | 4.77 (0.19) |
| 50% | 95.69 (4.33) | 90.14 (3.64) | 94.11 (3.83) | 26.65 (9.53) | 31.88 (3.83) | 27.93 (2.69) | 5.98 (0.28) | 5.27 (0.30) | 4.42 (0.38) |
| 75% | 82.20 (6.99) | 73.67 (2.07) | 77.80 (3.65) | 26.03 (1.86) | 26.28 (3.65) | 22.77 (8.72) | 3.90 (0.13) | 4.49 (0.15) | 3.85 (0.18) |
| 95% | 67.27 (3.60) | 61.12 (3.78) | 56.19 (5.58) | 21.72 (4.70) | 19.07 (5.58) | 15.93 (1.86) | 3.31 (0.36) | 3.22 (0.10) | 3.17 (0.30) |
Numbers in parentheses are standard deviations of triplicate measurements
TPC total phenolic compounds (mg vanillin equivalents/g extract), TFC total flavonoid compounds (mg quercetin equivalents/g extract), AA ascorbic acid (mg/g extract)
The sonochemical reaction is complex and correlates with the reaction time, temperature, solvent concentration, pressure, and the solid/liquid ratio [19]. TPC analysis revealed that the optimal extraction time was 40 min at 30 °C, whereas 30 min was sufficient at higher temperatures (40 and 50 °C) regardless of the solvent concentration. The extract yield decreased by 15.45% at 40 °C when the extraction was carried for a long reaction time (50 min) compared with that obtained after 30 min at the same temperature.
Rugosa rose fruit extracts prepared under different extraction conditions differed significantly in their TFC. The yield of TFC in the extract was in the range of 17.68–25.30 mg/g at 30 °C, and the highest yield was obtained at 50 min as 19.83–25.30 mg/g. The yield of TFC increased with increasing reaction time at 30 °C, while the yield was the highest (29.01–31.88 mg/g) at 50 °C for 40 min.
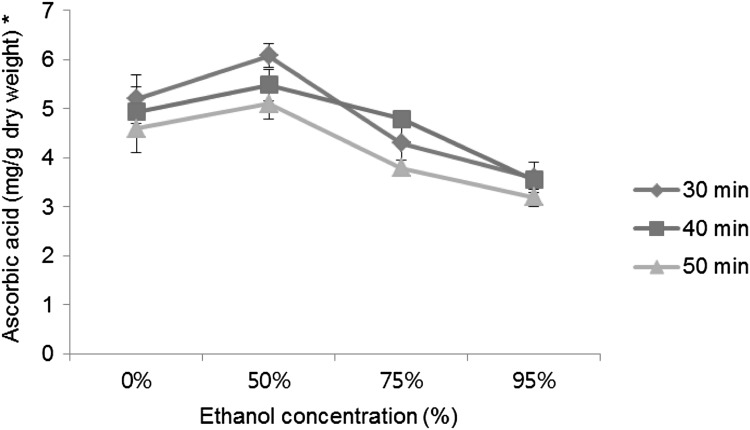
AA is a major compound among the organic acid constituents of rugosa rose fruit extract [2]. AA is a low molecular weight compound compared with TPC and TFC. The concentrations of AA in the extracts were 3.31–6.38, 3.22–5.81, and 3.17–5.40 mg/g at 30, 40, and 50 min, respectively. The yield decreased with increasing reaction time at the studied temperatures. However, these results did not agree with the results reported by Davey et al. [20], who found values ranging from 7.27 to 10 mg/g. The difference could have been caused by differences in the extraction methods, the maturity of the fruit, or the climatic conditions [2]. Ultrasonic waves create cavitation, and the resultant bubbles are filled with water vapor and gasses (O2 and N2). The degradation of AA is related to the generation of cavitation bubbles [21, 22]. Therefore, the intense ultrasound condition had a deleterious effect on the extraction of AA from rugosa rose fruits.
The yield of TPC and TFC obtained with the different concentrations of the extraction solvents increased in the following order: 50 > 75 > 0 > 95%. The results indicated that 50% ethanol effectively extracted the TPC and TFC, which was likely attributable to the polarity and good solubility of the extracts. In general, aqueous mixtures of ethanol at 50–70% have been described as the optimum concentrations for the extraction of TPC and TFC because water can easily penetrate and diffuse into plant cells [23]. Therefore, the use of pure water or ethanol was not efficient to extract TPC and TFC. Moreover, pure water and 50% ethanol were better solvents to extract AA than the other tested solvents because of AA’s good water solubility.
Overall, the optimal condition for the extraction of TPC, TFC, and AA was 50% ethanol at 50 °C for 30 min; 50% ethanol, 50 °C, and 40 min; and 50% ethanol, 30 °C, and 30 min, respectively.
Antioxidant activity of extract prepared using UAE
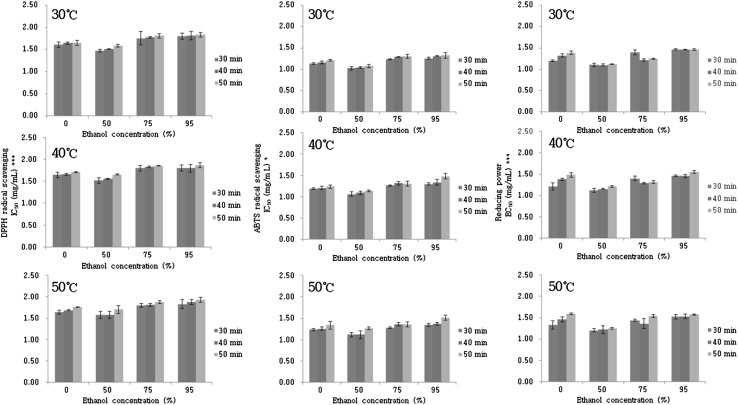
The antioxidant activities of rugosa rose fruit were evaluated using DPPH and ABTS radical scavenging assays, as well as evaluation of reducing power (Fig. 1). The results are presented as IC50 values. The solvent concentration had the greatest effect on the antioxidant activities. High activities were observed with 50% ethanol. The results agree with the optimal extraction condition (solvent) for TPC, TFC, and AA. In the DPPH radical scavenging assay, the activity decreased slightly with increasing reaction temperature. The IC50 values ranged from 1.48 to 1.83 mg/mL at 30 °C. In contrast, the values ranged from 1.52 to 1.93 mg/mL at 50 °C. Similar trends were observed in the ABTS radical scavenging assay and reducing power analysis.
Fig. 1.
Antioxidant activities of extracts depended on solvent concentration, extraction time, and temperature during ultrasound-assisted extraction (significant at *p < 0.05 and ***p < 0.001 level of probability)
Regarding the reaction time, the antioxidant activities determined using the DPPH and ABTS assay decreased with increasing in reaction time up to 50 min. This might be attributable to the reduction in extract yields (such as for AA), which are related to the antioxidant activities, following a prolonged extraction time. During the prolonged extraction time, the extract components are exposed to detrimental effects, such as oxidation [24]. The slight difference in the antioxidant activities observed using the various methods was attributed to the structural conformation of the extract components [25].
Overall, the optimal conditions for antioxidant activities were 30 °C, 30 min, and 50% ethanol using the UAE method, which corresponded to the optimal extraction conditions for AA.
Comparison of UAE and conventional extraction
To compare the investigated method with the conventional extraction method, the compounds (TPC, TFC, and AA) were extracted at 30 °C for 30 min, with 50% ethanol without ultra-sonication. The results are shown in Table 2. The extract yield was significantly higher using UAE compared with that obtained using conventional method. The extract yields were 64.46, 21.03, and 6.38 mg/g for TPC, TFC, and AA, respectively. The improvement in extract yield could be attributed to the propagation of ultrasound waves and cavitation [26]. The cavitation bubbles attack the plant cell wall, which enhances the solvent diffusion through the cell wall [10]. Shear force was generated during ultra-sonication by the ultrasound waves, which increased the mass transfer of the original material into each extract solution. In addition, ultra-sonication contributes to reducing the particle size of the raw material which increases the surface area. Therefore, the increase in mass transfer improved the extraction yield [27].
Table 2.
Comparison of each yield of rugosa rose fruit extract from conventional extraction and ultrasound-assisted extraction at 30 min, 30 °C, and ethanol 50% (mg/g dry weight)
| TPC | TFC | AA | |
|---|---|---|---|
| CE | 4.79 (0.33) | 1.19 (0.34) | 1.30 (0.05) |
| UAE | 64.49 (4.94) | 21.03 (5.03) | 6.38 (0.84) |
Numbers in parentheses are standard deviations of triplicate measurements
TPC total phenolic compounds (mg vanillin equivalents/g extract), TFC total flavonoid compounds (mg quercetin equivalents/g extract), AA ascorbic acid (mg/g extract), CE conventional extraction, UAE ultrasound-assisted extraction
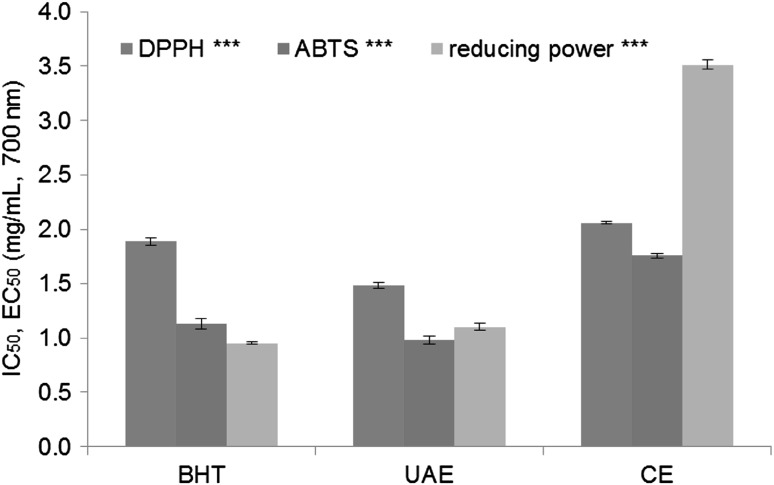
Figure 2 shows the results of the comparison of the antioxidant activities of rugosa rose fruit extracts and the positive control (BHT). The antioxidant activities of the extracts prepared using UAE were slightly higher than those of the positive control in the DPPH and ABTS assays. However, the antioxidant activity was relatively low in the extract prepared using the conventional method. This result reflected the fact that the extraction yield depended on the extraction method.
Fig. 2.
Comparison of antioxidant activities of extracts prepared using ultrasound-assisted extraction (UAE) and conventional extraction (CE) (BHT; butylated hydroxyanisole, optimal extraction condition was 50% ethanol, 30 °C, and 30 min) (significant at *p < 0.05 and ***p < 0.001 level of probability)
Statistical comparisons between the extract (TPC, TFC, and AA) and antioxidant activity
The correlation analysis between the extract (TPC, TFC, and AA) and antioxidant activities (using the DPPH, ABTS, and reducing power assays) was carried out, and the results are shown in Table 3. Statistically significant correlation coefficients between each compound and the antioxidant activities were observed at a p-value of 0.001. The positive correlation coefficient between the DPPH and ABTS (0.568) or reducing sugar (0.458) indicated that they were significantly correlated with each other, at a p-value of 0.001 [28]. A high correlation coefficient values (> 0.6) indicates a strong correlation between each factor [29]. AA showed higher correlation coefficients with antioxidant activities, which ranged from 0.500 to 0.627 compared with the other compounds. This suggested that AA plays a major role in the observed antioxidant activity. In contrast, the value for TPC was relatively low for the antioxidant activities, at 0.147–0.535.
Table 3.
Correlation coefficients between each extract (TPC, TFC, and AA) and antioxidant activities
| Evaluation characteristics | DPPH | ABTS | Reducing power | TPC | TFC | AA |
|---|---|---|---|---|---|---|
| DPPH | 1 | – | – | – | – | – |
| ABTS | 0.568* | 1 | – | – | – | – |
| Reducing power | 0.458* | 0.595* | 1 | – | – | – |
| TPC | 0.147* | 0.384* | 0.535* | 1 | – | – |
| TFC | 0.149* | 0.388* | 0.547* | 0.794* | 1 | – |
| AA | 0.627* | 0.622* | 0.500* | 0.459* | 0.424* | 1 |
TPC total phenolic compounds, TFC total flavonoid compounds, AA ascorbic acid, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
* p-value < 0.001
Optimal condition for AA extraction based on the statistical analysis
AA contributed significantly to the antioxidant activity (Table 3). Two-way ANOVA was used to determine the effect of the interaction between the extraction conditions and AA (Fig. 3). The extraction time and solvent concentration were the independent variables when the temperature was fixed to 30 °C. The result was significant, at a p-value of 0.05, suggesting that the extraction time and solvent concentration affected the extraction yield of AA. However, there was no significant interaction between the independent variables. This suggests that each factor affected AA extraction independently. The extract yield of AA differed significantly depending on the solvent concentration (partial η 2 = 0.873); however, the difference was relatively low for the extraction time (partial η 2 = 0.314). The Turkey test revealed there was no significant difference between 30 and 40 °C reaction temperatures. Therefore, the optimal condition for AA extraction was an extraction time of 30 min, an ethanol concentration of 50%, and extraction temperature of 30 °C. These conditions agree with the extraction conditions for the antioxidant activities.
Fig. 3.
Two-way analysis of variance (ANOVA) of extraction of ascorbic acid depended on solvent concentration and extraction time (significant at *p < 0.05 and ***p < 0.001 level of probability)
In this study, the UAE method significantly increased the extract yield and antioxidant activities compared with the conventional method. In particular, the antioxidant activity of the extract prepared using UAE was slightly higher than that of the positive control (BHT). Collectively, these results suggested that extracts of rugosa rose fruit have potential as natural antioxidant agents for use in food, cosmetics, and the pharmaceutical industry.
Acknowledgements
This research was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0020141).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jang HR, Park BJ, Park SA, Pee OJ, Park SY, Park KY. Optimization of shoot cultures and bioactive compound accumulation in Rosa rugosa during acclimatization. J. plant Biotechnol. 2016;43:104–109. doi: 10.5010/JPB.2016.43.1.104. [DOI] [Google Scholar]
- 2.Demir N, Yildiz O, Alpaslan M, Hayaloglu AA. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT - Food Sci. Technol. 2014;57:126–133. doi: 10.1016/j.lwt.2013.12.038. [DOI] [Google Scholar]
- 3.Hashidoko Y. The phytochemistry of Rosa rugose. Phytochemistry. 1996;43:535–549. doi: 10.1016/0031-9422(96)00287-7. [DOI] [Google Scholar]
- 4.Razungles A, Oszmianski J, Sapis JC. Determination of Carotenoids in Fruits of Rosa sp. (Rosa Canina and Rosa Rugosa) and of Chokeberry (Aronia Melanocarpa) J. Food Sci. 1989;54:774–775. doi: 10.1111/j.1365-2621.1989.tb04709.x. [DOI] [Google Scholar]
- 5.Czyzowska A, Klewicka E, Pogorzelski E, Nowak A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J Food Comp Anal. 2015;39:62–68. doi: 10.1016/j.jfca.2014.11.009. [DOI] [Google Scholar]
- 6.Cabiscol E, Tamarit J, Ros J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2010;3:3–8. [PubMed] [Google Scholar]
- 7.Kang HS, Chung HY, Kim JY, Son BW, Jung HA, Choi JS. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004;27:194–198. doi: 10.1007/BF02980106. [DOI] [PubMed] [Google Scholar]
- 8.Hayes JD, Chanas SA, Henderson CJ, McMahon M, Sun C, Moffat GJ, Yamamoto M. The Nrf2 transcription factor contributes both to the basal expression of glutathione S-transferases in mouse liver and to their induction by the chemopreventive synthetic antioxidants, butylated hydroxyanisole and ethoxyquin. Biochem. Soc. Trans. 2000;28:33–41. doi: 10.1042/bst0280033. [DOI] [PubMed] [Google Scholar]
- 9.Vilkhu K, Mawson R, Simon L, Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry - A review. Innov. Food Sci. Emerg. Technol. 2008;9:161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- 10.Saleh IA, Vinatoru M, Mason TJ, Abdel-Azim NS, Aboutabl EA, Hammouda FM. A possible general mechanism for ultrasound-assisted extraction (UAE) suggested from the results of UAE of chlorogenic acid from Cynara scolymus L. (artichoke) leaves. Ultrason. Sonochem. 2016;31:330–336. doi: 10.1016/j.ultsonch.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Scalbert A, Monties B, Janin G. Tannins in wood: comparison of different estimation methods. J. Agric. Food Chem. 1989;37:1324–1329. doi: 10.1021/jf00089a026. [DOI] [Google Scholar]
- 12.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 13.Nováková L, Solich P, Solichová D. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. Trac-Trends Anal. Chem. 2008;27:942–958. doi: 10.1016/j.trac.2008.08.006. [DOI] [Google Scholar]
- 14.Brand-Williams W, Cuvelier ME, Berset CLWT. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 15.Re R, Pellegrini N, Proteggente A, Pannanla A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira IC, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007;100:1511–1516. doi: 10.1016/j.foodchem.2005.11.043. [DOI] [Google Scholar]
- 17.Ma YQ, Chen JC, Liu DH, Ye XQ. Simultaneous extraction of phenolic compounds of citrus peel extracts: effect of ultrasound. Ultrason. Sonochem. 2009;16:57–62. doi: 10.1016/j.ultsonch.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Carrera C, Ruiz-Rodríguez A, Palma M, Barroso CG. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta. 2012;732:100–104. doi: 10.1016/j.aca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Suslick KS, Hammerton DA, Cline RE. Sonochemical hot spot. J. Am. Chem. Soc. 1986;108:5641–5642. doi: 10.1021/ja00278a055. [DOI] [Google Scholar]
- 20.Davey MW, Montagu MV, Inzé D, Sanmartin M. Kanellis A, Smirnoff N, Benzie IJJ. Strain JJ, Favell D, Fletcher J. Plant L-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000;80:825–860. doi: 10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6. [DOI] [Google Scholar]
- 21.Portenlänger G, Heusinger H. Chemical reactions induced by ultrasound and γ-rays in aqueous solutions of L-ascorbic acid. Carbohydr. Res. 1992;232:291–301. doi: 10.1016/0008-6215(92)80061-5. [DOI] [Google Scholar]
- 22.Tiwari BK, OʼDonnell CP, Patras A, Cullen PJ. Anthocyanin and ascorbic acid degradation in sonicated strawberry juice. J. Agri Food Chem. 2008;56:10071–10077. doi: 10.1021/jf801824v. [DOI] [PubMed] [Google Scholar]
- 23.Cheng VJ, Bekhit AEDA, McConnell M, Mros S, Zhao J. Effect of extraction solvent, waste fraction and grape variety on the antimicrobial and antioxidant activities of extracts from wine residue from cool climate. Food Chem. 2012;134:474–482. doi: 10.1016/j.foodchem.2012.02.103. [DOI] [Google Scholar]
- 24.Wang J, Sun B, Cao Y, Tian Y, Li X. Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 25.Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012;130:1036–1043. doi: 10.1016/j.foodchem.2011.07.127. [DOI] [Google Scholar]
- 26.Ramarathnam N, Ochi H, Takeuchi M. Antioxidative defense system in vegetable extracts Natural antioxidants. pp. 76–87. In: Natural Antioxidants: Chemistry, Health Effects, and Applications. Fereidoon S (ed). AOCS press, Champaign, Illinois, USA (1997)
- 27.Pinelo M, Arnous A, Meyer AS. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006;17:579–590. doi: 10.1016/j.tifs.2006.05.003. [DOI] [Google Scholar]
- 28.Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007;72:159–166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 29.Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol. Bull. 2009;135:589–607. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]