Abstract
As the mechanism of aged black garlic (ABG) extract affecting lipid metabolism in adipocytes remains unclear, this study evaluated the effect of ABG extract on lipid metabolism and the expression of related proteins in mature 3T3-L1 adipocytes. ABG extract treatments at 0, 0.625, 1.25, and 2.5, and 5 mg/mL had no effect on cell morphology or viability in adipocytes. ABG extract suppressed lipogenesis and induced lipolysis in a dose-dependent manner compared to control. Furthermore, ABG extract at 2.5 and 5 mg/mL significantly reduced protein expression of proliferator activated receptor γ (PPARγ) and perilipin in mature 3T3-L1 adipocytes. The hormone-sensitive lipase (HSL) and Ser563-pHSL levels were also significantly reduced by treatment with 5 mg/mL of ABG extract. Taken together, these results suggest that ABG extract has anti-lipogenic and lipolytic effects in mature 3T3-L1 adipocytes, indicating a potential in anti-obesity therapies.
Keywords: Lipolysis, Lipogenesis, Mature 3T3-L1 adipocyte, Aged black garlic
Introduction
Adipose tissue plays a critical role in maintaining energy homeostasis of the body, and white adipose tissue in mammals has the capacity to store and release energy in the form of lipids [1]. Obesity is a condition characterized by the accumulation of excess fat tissue [2]. Therefore, adipocyte is one of the most important targets for research and development of new strategies for obesity treatment.
Proliferator activated receptor γ (PPARγ) play key roles in balancing lipid metabolism by controlling the expression of target genes associated with lipid accumulation, fatty acid oxidation, and lipolysis [3]. Thus, some treatments for obesity may try to affect lipid metabolism modulation. One of the molecular targets in this scheme may be PPARγ, as it is a transcription factor mainly expressed in the adipose tissue, and it affects adipocyte differentiation both in vivo and in vitro [3], indicating that PPARγ is involved in both adipogenesis and lipogenesis.
Lipolysis is a regulated catabolic process involving cooperated participation of several lipases, cofactors, and lipid droplet-associated proteins. Hormone-sensitive lipase (HSL), an important lipase, is known as a triglyceride lipase. It mainly hydrolyzes triacylglycerols to release fatty acids and diglycerides [4]. On modulating HSL, a lipid droplet-associated protein, perilipin, is a crucial component of lipolysis as it controls the action of HSL [5].
Garlic (Allium sativum L.) has long been used for culinary and medicinal purposes from very early times [6]. Despite its beneficial effects, raw garlic has a peculiar smell and acrid taste. Aged black garlic (ABG) is made by ripening raw garlic at high temperatures and humidity to remove its peculiar smell and taste [7]. Through this ripening process, bioactive compounds are also newly formed or altered [8]. It has been reported that black garlic has a stronger anti-oxidant and anti-lipidemic effect compared to raw garlic [9]. ABG extract have also been reported to have an anti-adipogenic effect in 3T3-L1 preadipocytes [10].
Only few studies, however, have reported the lipid-lowering effect of black garlic [11]. The objective of this study was to determine the anti-obesity effect of aged black garlic extract (ABG) on lipid metabolism in mature 3T3-L1 adipocytes.
Materials and methods
Preparation of aged black garlic (ABG) extract
The ABG extract was provided by Medience Incorporated (Chuncheon, Korea). ABG at 1 kg was homogenized and extracted with 20% methanol (MeOH; 5 L) in an extractor for 6 h. The mixture was then filtered with Whatman No. 3 filter paper. The total filtrate was concentrated by evaporation, and the residue of ABG was dissolved in deionized water. The total yield was 41% and containing 3.7% poly phenolic compounds.
Cell culture and cell viability assay
3T3-L1 preadipocytes (American Type Culture Collection, Manassas, VA, USA) were maintained in DMEM containing 10% newborn calf serum (NCS), 100 IU/mL penicillin, and 100 μg/mL streptomycin in a humidified atmosphere of 37 °C, 5% CO2. To induce adipogenesis, the cells were cultured for 2 days until confluency. This induction was in presence of the differentiation mixture containing 5 μg/mL insulin, 0.5 μM dexamethasone, and 0.5 μM 3-isobutyl-1-methylxanthine (IBMX) in DMEM with 10% fetal bovine serum (FBS). Thereafter, the medium was replaced with 10% FBS/DMEM containing 5 μg/mL insulin every 2 days. Cell viability assay was performed according to the method described by Kim et al. [16].
Oil Red O staining
Oil Red O staining was performed using Kim’s protocol [12] with minor modifications. The morphology of the cells was examined under an inverted microscope (ZEISS, Oberkochen, Germany) and the images were captured using a digital camera (Nikon, Tokyo, Japan). The values were calculated as percentages of the untreated control and expressed as mean ± standard deviation (SD). Glycerol concentrations were calculated based on a standard calibration curve.
Glycerol release assay
Mature 3T3-L1 adipocytes were treated with ABG extract for 48 h. Released free glycerol was then assayed using the Glycerol Assay Kit according to the manufacturer’s protocol (MAK117, Sigma-Aldrich, St. Louis, MO, USA). The supernatants were collected and added with reagent. They were then added to a 96-well plate. The absorbance at 570 nm was measured using a luminescence microplate reader.
Western blot analysis
Cell extracts were prepared by adding a protein extraction solution (PRO-PREP, Intron Biotechnology, Sungnam, Korea). Protein concentration in cell lysates was determined according to the method described by Bradford [13]. Blots were incubated with primary antibodies (including PPARγ (1:200), p-HSL-Ser563 (1:500), HSL (1:1000), and perilipin (1:1000) at 4 °C overnight (Cell Signaling, Beverly, MA, USA). After washing, the blots were incubated with goat anti-rabbit IgG-HRP secondary antibody (NOVUS Biologicals, Littleton, CO, USA) (1:200,000) in a blocking solution. The target protein was detected using ECL western blotting detection reagent (GE Healthcare, Chicago, IL, USA).
Statistical analysis
Data are presented as mean ± SD. To determine the statistical significance, data were analyzed using student’s t test with Microsoft Excel 2010 program (Microsoft Inc., Redmond, WA, USA). A p value of less than 0.05 was considered statistically significant.
Results and discussion
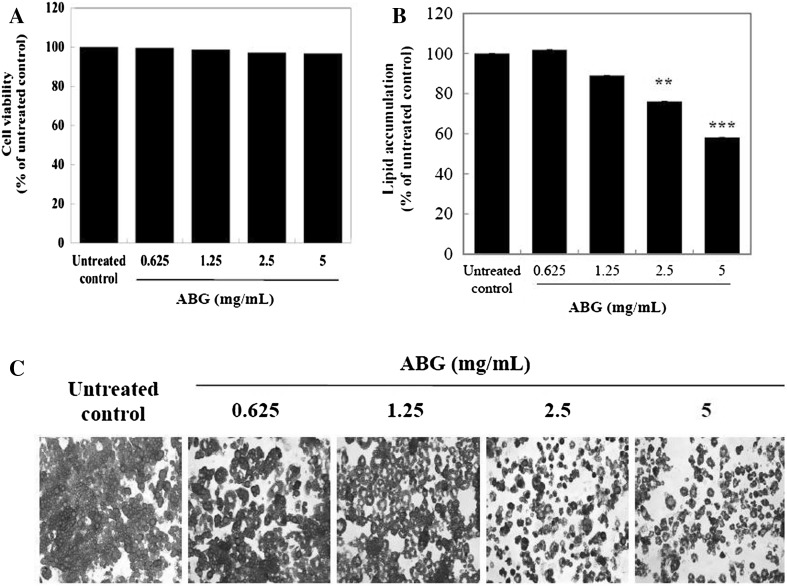
To investigate the effect of ABG extract on viability in mature 3T3-L1 adipocytes, the cell was treated with various concentrations of ABG extract for 72 h. As shown in Fig. 1(A), ABG extract at 0.625, 1.25, and 2.5 and 5 mg/mL did not display any cytotoxic effect. Thus, ABG extract at 0.625–5 mg/mL was considered to be non-cytotoxic to mature 3T3-L1 adipocytes in this study.
Fig. 1.
Effects of ABG extract on viability and lipid accumulation in mature 3T3-L1 adipocytes. (A) Mature 3T3-L1 adipocytes were incubated for 72 h with various concentrations of ABG extract (0–5.0 mg/mL). Then cell viability assay was performed using CCK-8 assay kit. (B) Mature 3T3-L1 adipocytes were stained with Oil Red O after 7 days of treatment with 0-5.0 mg/mL of ABG extract. (C) Microscopic pictures were taken at 200 × magnification. The OD values were measured from isopropanol elution of the Oil Red O stained cells. **p < 0.01, ***p < 0.005 compared to that of untreated control
ABG extract at 2.5 and 5.0 mg/mL resulted in 24 and 42% of decrease (p < 0.01 and p < 0.005) in oil droplet staining, respectively, when compared to that of the control [Fig. 1(B)]. These results suggest that ABG extract might have anti-lipogenic effect in mature 3T3-L1 adipocytes. ABG extract also containing 3.7% polyphenol compounds. Some polyphenols reduces fat mass in animal models as well as human fat cells through suppression of adipocyte differentiation, reduces lipogenesis, and increases lipolysis in adipocytes [14]. Aged garlic extract (AGE) contains several active compounds, including S-allylcysteine (SAC) and allixin. SAC, the most abundant organosulfur compound have been reported to have anti-oxidative, anti-hypertensive, and anti-inflammatory effects [8]. However, there are few studies on the lipolysis of AGE.
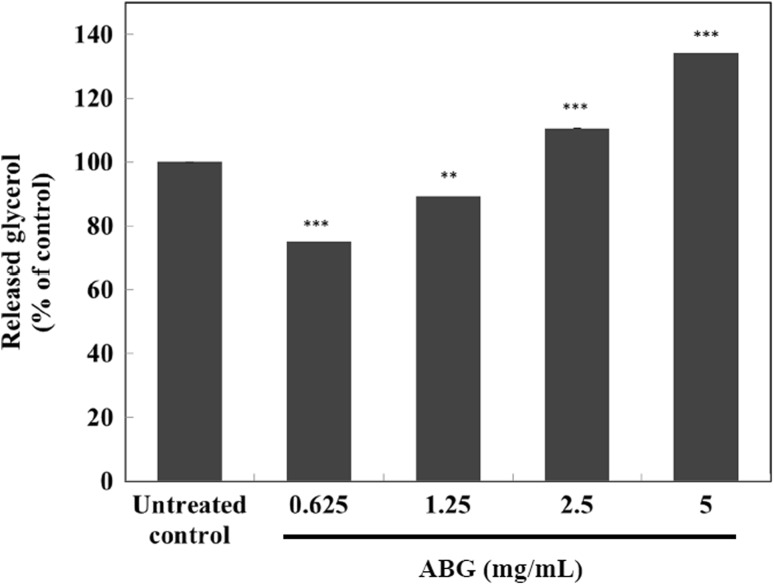
To analyze the lipolytic effect of ABG extract in mature 3T3-L1 adipocytes, glycerol release was measured as indicator of lipolysis as described in the Materials and Methods section. ABG extract at 2.5 and 5.0 mg/mL resulted in 11 and 34% increases (both p < 0.005), respectively, when compared to that of the control, in a dose-dependent manner (Fig. 2).
Fig. 2.
Effect of ABG extract on glycerol release in mature 3T3-L1 adipocytes. Extracellular free glycerol was measured with a glycerol quantification kit. Values are expressed as mean ± SD of three separate experiments. **p < 0.01, ***p < 0.005 compared to that of untreated control
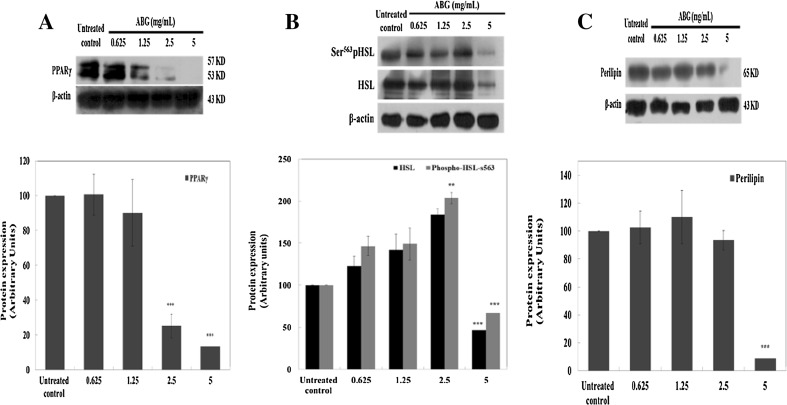
The expression levels of PPARγ were reduced by up to 75 and 87% (both p < 0.005), respectively, by 2.5 and 5 mg/mL of ABG extract [Fig. 3(A)]. The expression levels of Ser563-pHSL transcriptional factor during lipolysis were significantly (p < 0.005) down-regulated (up to 33%) by 5 mg/mL of ABG extract [Fig. 3(B)]. HSL total protein level was also significantly (p < 0.005) down-regulated (up to 53%) by 5 mg/mL treatment with ABG extract [Fig. 3(B)]. The protein level of perilipin, a barrier protein of lipid droplet (LD), was also significantly (p < 0.005) down-regulated (up to 91%) by treatment with 5 mg/mL of ABG extract [Fig. 3(C)]. The rate of lipolysis in adipocyte is determined by both the expression of lipolytic enzymes and the integrity of lipid droplets [15]. PPARγ is required for maintaining mature 3T3-L1 adipocyte function, including triglyceride synthesis and storage [16]. In the present study, the expression of PPARγ protein was significantly down-regulated by treatment with 2.5 and 5 mg/mL of ABG extract in mature 3T3-L1 adipocyte [Fig. 3(A)]. These results showed that ABG extract inhibited lipid accumulation in mature 3T3-L1 adipocytes.
Fig. 3.
Effect of ABG extract on the expression of lipogenic and lipolytic proteins in mature 3T3-L1 adipocytes. Expression levels of (A) PPARγ, (B) p-HSL-Ser563 and HSL, and (C) perilipin are shown. Band intensities of PPARγ, HSL, and perilipin were normalized to their respective ß-actin fractions, whereas p-HSL-Ser563 was normalized to HSL levels. Values are expressed as mean ± SD of three separate experiments. **p < 0.01, ***p < 0.005 compared to that of untreated control
HSL, a major lipase, is a triglyceride lipase that is usually present in the cytoplasm [4]. Phosphorylated HSL is responsible for increasing HSL hydrolytic activity/translocation by forming a complex with PKA-phosphorylated perilipin located on the surface of LDs, leading to lipolysis [4]. In this study, HSL and phosphorylated HSL (Ser563) protein levels were significantly down-regulated by treatment with 5 mg/mL of ABG extract in mature 3T3-L1 adipocytes [Fig. 3(B)].
It has been reported that partial knockdown of PPARγ can down-regulate triglyceride lipase (ATGL) and HSL, the two major lipases in adipocytes [17]. Thus, inhibition of PPARγ can explain the inhibitory effect of ABG in lipogenesis. Perilipin is a structural lipid droplet-associated protein that normally inhibits HSL, and perilipin is a target gene of PPAR-γ [18]. In present study, it was possible that reduced expression of PPAR-γ partially promoted lipolysis through down-regulating its target gene perilipin in ABG-treated mature 3T3-L1 adipocytes [Fig. 3(C)].
In conclusion, the present study suggests that ABG extract could suppress lipogenesis by reducing the expression of PPARγ while enhancing lipolysis by upregulating HSL phosphorylation at Ser563 and downregulating perilipin in mature 3T3-L1 adipocytes. Thus, ABG extract has anti-obesity effects by stimulating lipolysis in adipocytes and by inhibiting lipogenesis in adipocytes. These results suggest that ABG may be useful for the treatment of obesity.
Acknowledgments
We are grateful to Dr. Kyung-min Sohn for useful discussions.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported by the authors.
References
- 1.Smorlesi A, Frontini A, Giordano A, Cinti S. The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes. Rev. 2012;13(Suppl 2):83–96. doi: 10.1111/j.1467-789X.2012.01039.x. [DOI] [PubMed] [Google Scholar]
- 2.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;53:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 3.Sung SH, Lee M. Anti-adipogenic activity of a new cyclic diarylheptanoid isolated from Alnus japonica on 3T3-L1 cells via modulation of PPARgamma, C/EBPalpha and SREBP1c signaling. Bioorg. Med. Chem. Lett. 2015;25:4648–4651. doi: 10.1016/j.bmcl.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Drira R, Sakamoto K. Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes. Eur. J. Nutr. 2014;53:743–750. doi: 10.1007/s00394-013-0578-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim SO, Sakchaisri K, Asami Y, Ryoo IJ, Choo SJ, Yoo ID, Soung NK, Kim YS, Jang JH, Kim BY, Ahn JS. Illudins C2 and C3 stimulate lipolysis in 3T3-L1 adipocytes and suppress adipogenesis in 3T3-L1 preadipocytes. J. Nat. Prod. 2014;77:744–750. doi: 10.1021/np400520a. [DOI] [PubMed] [Google Scholar]
- 6.Butt MS, Sultan MT, Butt MS, Iqbal J. Garlic: nature’s protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 7.Lanzotti V. The analysis of onion and garlic. J. Chromatogr. A. 2006;1112:3–22. doi: 10.1016/j.chroma.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Orozco-Ibarra M, Munoz-Sanchez J, Zavala-Medina ME, Pineda B, Magana-Maldonado R, Vazquez-Contreras E, Maldonado PD, Pedraza-Chaverri J, Chanez-Cardenas ME. Aged garlic extract and S-allylcysteine prevent apoptotic cell death in a chemical hypoxia model. Biol. Res. 2016;49:7. doi: 10.1186/s40659-016-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Li N, Lu X, Liu P, Qiao X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016;96:2366–2372. doi: 10.1002/jsfa.7351. [DOI] [PubMed] [Google Scholar]
- 10.Park JA, Park C, Han MH, Kim BW, Chung YH, Choi YH. Inhibition of adipocyte differentiation and adipogenesis by aged black garlic extracts in 3T3-L1 preadipocytes. J. of Life Science. 2011;21:720–728. doi: 10.5352/JLS.2011.21.5.720. [DOI] [Google Scholar]
- 11.Ha AW, Ying T, Kim WK. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr. Res. Pract. 2015;9:30–36. doi: 10.4162/nrp.2015.9.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JK, Nam H, Kim YY, Jung H, Choi YS, Oh JS, Chun SK, Suh JG. Anti-adipogenic effects in 3T3-L1 cells of acetone extracts and fractions from Styrax japonica fruit. Food Sci. Biotechnol. 2015;24:1513–1521. doi: 10.1007/s10068-015-0195-8. [DOI] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-gamma in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 15.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti P, English T, Karki S, Qiang L, Tao R, Kim J, Luo Z, Farmer SR, Kandror KV. SIRT1 controls lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J. Lipid Res. 2011;52:1693–1701. doi: 10.1194/jlr.M014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JJ, Toy WC, Liu S, Cheng A, Lim BK, Subramaniam T, Sum CF, Lim SC. Acetyl-keto-beta-boswellic acid induces lipolysis in mature adipocytes. Biochem. Biophys. Res. Commun. 2013;431:192–196. doi: 10.1016/j.bbrc.2012.12.136. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014;68:1075–1087. doi: 10.1038/ejcn.2014.143. [DOI] [PubMed] [Google Scholar]