Abstract
Functional food has been highly demanded lately because of its benefits in counteracting diseases. Fucoidan and agave fructan are ingredients that enhance the growth of beneficial bacteria in the gut (prebiotics). This mixture has great potential to develop innovative products but it has never been explored before. Because of fucoidan is more expensive than agave fructan, the innovative proposed mixture is vulnerable to adulteration. This research was aimed to assess the accuracy of Fourier transform infrared spectroscopy with attenuated total reflectance (ATR–FTIR) coupled with chemometrics to identify and predict concentration of both polysaccharides in powder mixtures (0–100%). Absorption bands at 1240–1255 and 836–840 cm−1 were attributed to fucoidan and a strong peak at ~ 936 cm−1 confirmed the fructan presence. Peak areas were best fitted into linear models ( ≥ 0.92, RMSE ≤ 3.54%). This achievement may be useful to certificate ingredients contained in fucoidan–fructan mixtures, preventing adulteration.
Keywords: Fructan, Sulfated polysaccharide, Undaria pinnatifida, Chemometrics
Introduction
Agave is an important crop in Mexico, being the total production of 1846,345 MT in 2015 [1]. Agave plants belong to the family Agavaceae (genus Agave) and their leaves are over 1 m long in adult plants [2]. Mature agave plants (8–12 years) accumulate 13–17% (w/w) of fresh weight fructans [3]. Fructans are reserve carbohydrates that enhance the plant cold and drought tolerance. They are composed of fructose (β (2–1 and/or β 2–6 linkages) [4] and are fermented in colon because they can not be hydrolyzed by enzymes contained in human salivary and intestine. Fructans have shown good performance as stabilizers, sweeteners, moisturizers, gellifiers, natural prebiotics and dietary fiber [5]. Little research has been conducted to date about fructans despite the interest in their use as functional food ingredients continues to grow in the food industry.
Another ingredient is fucoidan, which is a biologically active compound, an anionic polysaccharide that is obtained from the extracellular matrix from edible brown seaweeds. It is composed of l-fucopyranose units, sulfated ester groups and small proportions of d-xylose, d-mannose, d-galactose, l-rhamnose, arabinose, glucose, d-glucuronic acid and acetyl groups [6, 7]. Fucoidan has antioxidant, anti-inflammatory, anti-allergic, anti-thrombotic, anti-obesity, in vitro anti-coagulant, anti-viral, anti-uropathy and anti-tumor activities [6, 8]. Moreover, fucoidan inhibits the growth of bacteria known to develop multi-drug resistance and chronic active gastritis and does not cause liver toxicity [7, 9]. Research about sulfated polysaccharides such as heparin, heparin oligosaccharides and fucoidan has been registered since 1950 and the amount of published papers has increased lately. On the other hand, agave fructan was not studied until 1990. Although research in this topic has been increased, it only represents 2% of the highest amount of sulfated polysaccharides published works [10].
Fucoidan and agave fructans are polysaccharides recognized as safe (GRAS) by the USA Food and Drug Administration (FDA). Based on their approval, innovative products can be developed by using them as a mixture, which has not been tested nor explored before. Fucoidan is more expensive than agave fructan and the mixture is target of adulteration for economic gain. Because of this, consumers may be in risk to pay higher or lower prices to acquire substandard products of reduced nutritional value with risk to health [11].
Besides developing new functional products, there is a need to provide rapid, non-destructive and reliable testing tools for the ingredients certification in the food industry [11]. Fourier transform infrared (FTIR) spectroscopy is a technology that is widely used by food industry to monitor food adulteration. It has many advantages such as simplicity, reliability, ease of maintenance and use [12].
The aim of this short communication was to propose a new powder mixture composed of agave fructan and fucoidan. Moreover, the polysaccharides were identified and their concentrations were predicted by ATR–FTIR coupled with chemometrics to prevent economic adulteration.
Materials and methods
Agave fructan powder was procured from Ingredion Mexico Co. (Guadalajara, Jalisco, Mexico). ≥ 85% Fucoidan from Undaria pinnatifida powder was purchased from Haewon Biotech Inc. (Chongro- gu, Seoul, Korea). α-d-fructose and β-d-fructose were purchased from MP Biomedicals, Inc. (Solon, OH, USA).
Samples were prepared by mixing fructan and fucoidan with different ratio (Table 1) at 25 °C by duplicate. Mixtures were analyzed by infrared attenuated total reflectance (FTIR/ATR) spectroscopy, performed by Perkin Elmer Inc. (Waltham, MA, USA) equipment operating at 4 cm−1 resolution. The mirror velocity was 0.08 cm−1 and 35 interferograms were co-added before Fourier transformation. Spectra were collected from 4000 to 650 cm−1 in the absorbance mode and normalized that the absorption band at ca. 1008 cm−1 equaled to 1. Normalization did not alter the proportion of signals in the origin spectra.
Table 1.
Polysaccharides concentration of powder mixtures
| Samples code | Agave fructan | Fucoidan | ||
|---|---|---|---|---|
| g | % | g | % | |
| a | 1.000 | 100.0 | 0.000 | 0.0 |
| b | 0.995 | 99.5 | 0.005 | 0.5 |
| c | 0.990 | 99.0 | 0.010 | 1.0 |
| d | 0.950 | 95.0 | 0.050 | 5.0 |
| e | 0.900 | 90.0 | 0.100 | 10.0 |
| f | 0.800 | 80.0 | 0.200 | 20.0 |
| g | 0.600 | 60.0 | 0.400 | 40.0 |
| h | 0.500 | 50.0 | 0.500 | 50.0 |
| i | 0.400 | 40.0 | 0.600 | 60.0 |
| j | 0.200 | 20.0 | 0.800 | 80.0 |
| k | 0.000 | 0.0 | 1.000 | 100.0 |
Chemometrics and partial least square multivariate regression data analysis were performed using the software version 8 OriginPro (Northampton, MA, USA). The models' ability to fit all experimental data, the and the standard deviation (RMSE) were calculated.
Results and discussion
Agave fructan and fucoidan mixtures FTIR spectra
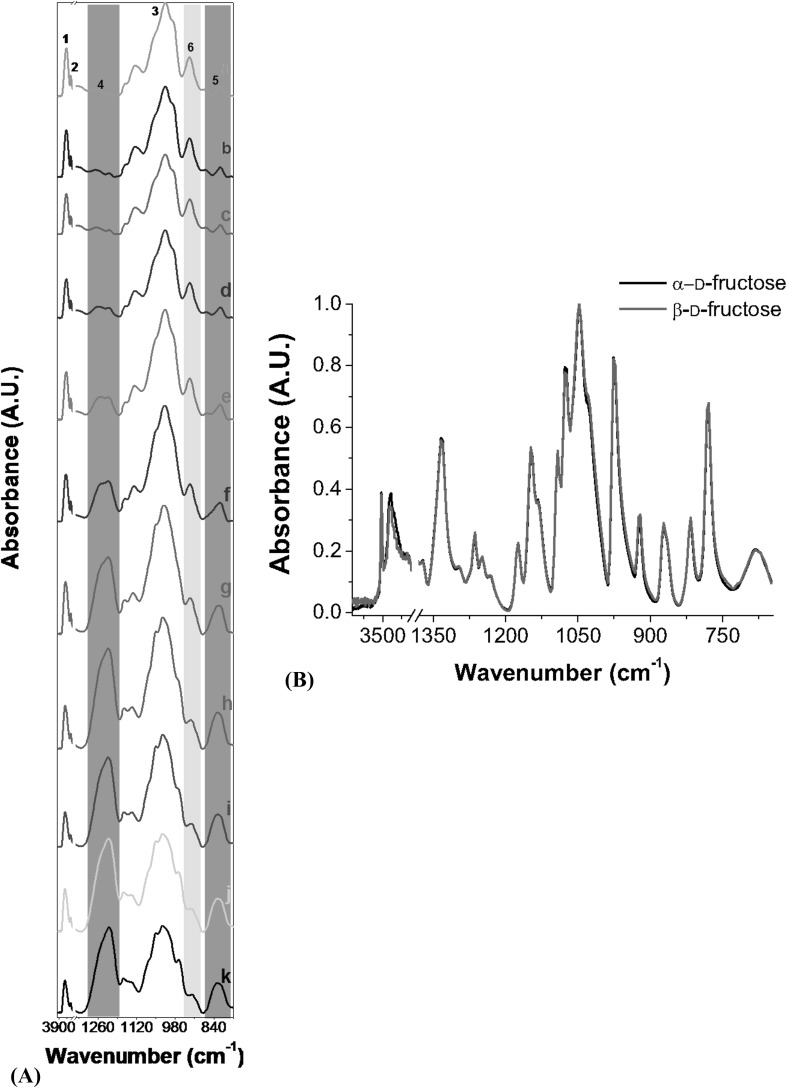
Agave fructan-fucoidan mixtures FTIR spectra are shown in Fig. 1(A). All powder samples showed absorption bands at ~ 3400 (1), ~ 2920 (2) and 900–1200 cm−1 regions (3). Sharp differences were observed at 1240–1255 (4) and 836–840 cm−1 (5) regions. These signals at 4 and 5 regions increased as fucoidan concentration was increased in the mixtures. The opposite effect was observed in these regions when agave fructan concentration was increased in samples. Another strong absorption band was evidenced at ~ 936 cm−1 (6). This signal showed the highest absorbance values in samples composed of agave fructan in major proportion, being decreased as the amount of this ingredient was reduced. Absorption bands found at region 1 are characteristic of O–H stretching and region 2 peaks evidenced the C–H stretching and C–C ring stretching. Region 3 signals belong to the carbohydrate fingerprint, which are attributed to COC group vibrations in cyclic structures, as have been confirmed by several research works [13]. S=O stretching and C–O–S bending vibration of sulfate substituents at the axial C-4 position signals were found in regions 4 and 5, respectively, and were attributed to fucoidan. These two signals have been essential for fucoidan characterization by FTIR in many works. For instance, Pielesz et al. [14] used these signals for fucoidan detection from Fucus vesiculosus Linnaeus.
Fig. 1.
(A) ATR–FTIR spectra of agave fructan and fucoidan powders. Samples nomenclature is shown in Table 1. Numbers in spectra represent the different regions used to describe absorption bands. (1) ~ 3400 (2) ~ 2920 (3) 900–1200 (4) 1240–1255 (5) 836–840 and (6) ~ 936 cm−1. (B) ATR–FTIR spectra of α-d-fructose and β-d-fructose standards
The absorption band found in region 6 was attributed to fructans. This peak also was found in FTIR spectra from barley, and was used to measure the fructan content by Cozzolino et al. [15]. Sample a (without fucoidan) showed small peaks at regions 4 and 5, which were attributed to fructose [Fig. 1(B)]. These peaks were less visible as fucoidan signals were superimposed in samples b–k as a result of the increased concentration of the sulfated polysaccharide.
Both polysaccharides could be fully identified in FTIR spectra. More research needs to be carried out to know if this approach is applicable to the food system to determine possible interference with other components.
Integration of powder mixtures FTIR absorption bands and model fitting
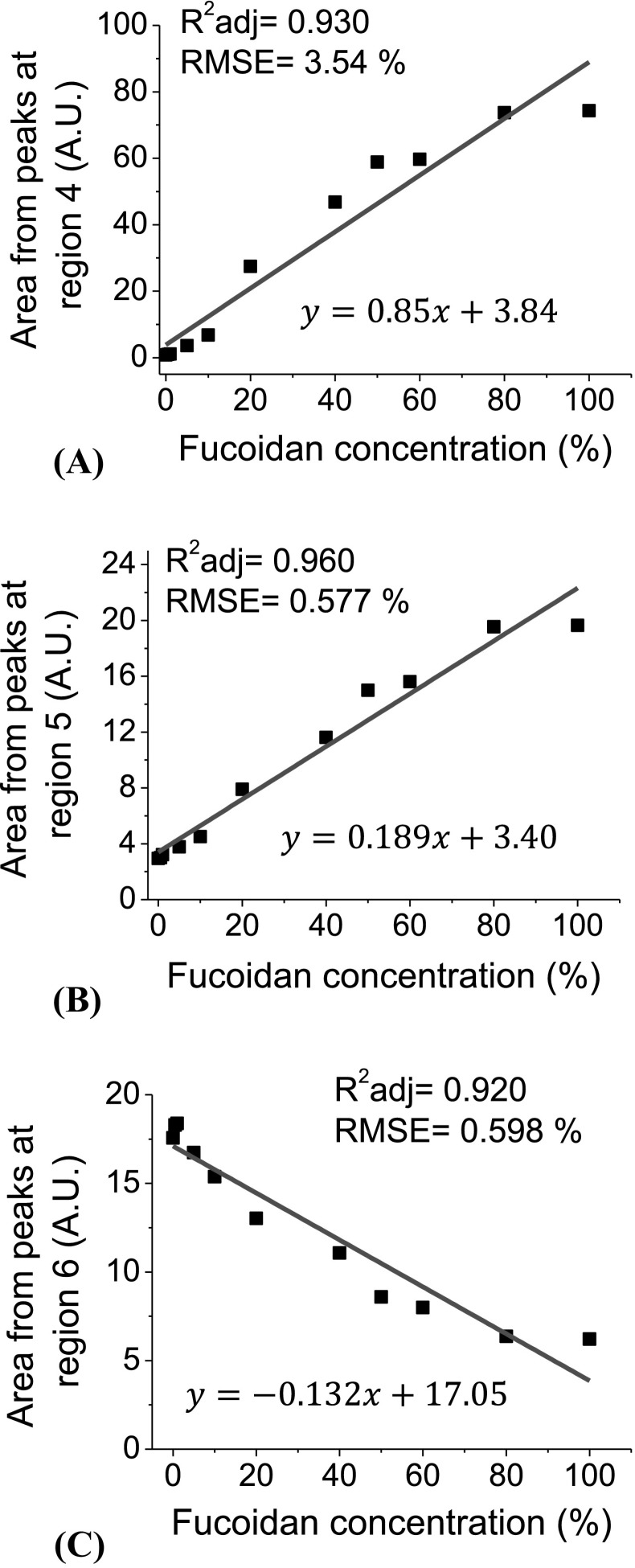
Three regions were selected for area calculation, (4, 5 and 6). Absorbance and area behavior was consistent. The area behavior obtained from absorbance peaks of samples composed of different amounts of agave fructan and fucoidan was linear. Linear models were preferred to prepare calibration curves, to preserve the linearity in the error. After linear regression, was ≥ 0.92 and RMSE was ≤ 3.54% in the three regions [Fig. 2(A), (B), (C)].
Fig. 2.
FTIR area peaks attributed to fucoidan (A, B) and agave fructan (C) using linear fitting. Solid lines represent the models fit
Since FDA has established as safe the intake of 0.03 g of fucoidan per serving, the most recommended mixture powder from this work would be sample C (1% fucoidan and 99% agave fructan).
An innovative mixture of agave fructan and fucoidan has been proposed in this work. Moreover, the assessment on concentration prediction of the innovative powder mixtures was successfully achieved by ATR–FTIR technique. Integrated FTIR peaks attributed to the presence of both ingredients showed linear model fit. Despite the ATR–FTIR technique could be easily adapted to routine analysis in agave fructan and fucoidan mixtures, more research is needed to establish possible interference with other ingredients in the food system.
Acknowledgements
G. Espinosa-Velázquez received a scholarship for his graduate degree from the National Council of Science and Technology (CONACYT, Mexico).
Contributor Information
Ana Mayela Ramos-de-la-Peña, Phone: 52 844 122 49 33, Email: ramos.amay@itesm.mx.
Juan Carlos Contreras-Esquivel, Phone: 52 844 122 49 33, Email: coyotefoods@hotmail.com.
References
- 1.Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA), Servicio de Información Agroalimentaria y Pesquera (SAP). 83 (2016)
- 2.Cedeño M. Tequila production. Crit. Rev. Biotechnol. 1995;15:1–11. doi: 10.3109/07388559509150529. [DOI] [PubMed] [Google Scholar]
- 3.Lopez MG, Mancilla-Margalli NA, Mendoza-Diaz G. Molecular structures of fructans from Agave tequilana Weber var. azul. J. Agr. Food Chem. 2003;51:7835–7840. doi: 10.1021/jf030383v. [DOI] [PubMed] [Google Scholar]
- 4.Santiago-Garcia PA, Mellado-Mojica E, Leon-Martinez FM, Lopez MG. Evaluation of Agave angustifolia fructans as fat replacer in the cookies manufacture. LWT Food Sci. Technol. 2017;77:100–109. doi: 10.1016/j.lwt.2016.11.028. [DOI] [Google Scholar]
- 5.Espinosa-Andrews H, Urias-Silva JE. Thermal properties of agave fructans (Agave tequilana Weber var. Azul). Carbohyd. Polym. 87: 2671–2676 (2012)
- 6.Lu KY, Li R, Hsu CH, Lin CW, Chou SC, Tsai ML, Mi FL. Development of a new type of multifunctional fucoidan-based nanoparticles for anticancer drug delivery. Carbohyd. Polym. 2017;165:410–420. doi: 10.1016/j.carbpol.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 7.Shang Q, Song G, Zhang M, Shi J, Xu C, Hao J, Li G, Yu G. Dietary fucoidan improves metabolic syndrome in association with increased Akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods. 2017;28:138–146. doi: 10.1016/j.jff.2016.11.002. [DOI] [Google Scholar]
- 8.Vo T, Kim S. Fucoidan as a natural bioactive ingredient for functional foods. J. Funct. Foods. 2013;5:16–27. doi: 10.1016/j.jff.2012.08.007. [DOI] [Google Scholar]
- 9.Chua EG, Verbrugghe P, Tay CY. Fucoidans disrupt adherence of helicobacter pylori to AGS cells in vitro. Evidence-Based Complementary and Alternative Medicine. 1–6 (2015)
- 10.SciFinder®. Chemical Abstracts Service, Columbus, OH, USA. (2017)
- 11.Hudson A. Food incidents: lessons from the past and anticipating the future. New Food. 5 (2016)
- 12.Rodriguez-Saona LE, Allendorf ME. Use of FTIR for rapid authentication and detection of adulteration of food. Annual Reviews. 2011;2:467–483. doi: 10.1146/annurev-food-022510-133750. [DOI] [PubMed] [Google Scholar]
- 13.Contreras-Esquivel JC, Espinoza-Pérez JD, Montanez JC, Charles-Rodríguez AV, Renovato J, Aguilar CN, Rodríguez-Herrera R, Wicker L. Extraction and characterization of pectin from novel sources. pp. 215–229. In: Advances in Biopolymers. Fishman ML, Qi PX, Wicker L (eds). American Chemical Society, Washington, DC, USA (2006)
- 14.Pielesz A, Biniaś W. Cellulose acetate membrane electrophoresis and FTIR spectroscopy as methods of identifying a fucoidan in Fucus vesiculosus Linnaeus. Carbohyd. Res. 2010;345:2676–2682. doi: 10.1016/j.carres.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Cozzolino D, Roumeliotis S, Eglinton J. Feasibility study on the use of attenuated total reflectance MIR spectroscopy to measure the fructan content in barley. Anal. Methods. 2014;6:7710–7715. doi: 10.1039/C4AY01560F. [DOI] [Google Scholar]