Abstract
Enzyme technology has many potential applications in the baking industry because carbohydrate-active enzymes specifically react with carbohydrate components, such as starch, in complex food systems. Amylolytic enzymes are added to starch-based foods, such as baking products, to retain moisture more efficiently and to increase softness, freshness, and shelf life. The major reactions used to modify the structure of food starch include: (1) hydrolysis of α-1, 4 or α-1, 6 glycosidic linkages, (2) disproportionation by the transfer of glucan moieties, and (3) branching by formation of α-1, 6 glycosidic linkage. The catalytic reaction of a single enzyme or a mixture of more than two enzymes has been applied, generating novel starches, with chemical changes in the starch structure, in which the changes of molecular mass, branch chain length distribution, and the ratio of amylose to amylopectin may occur. These developments of enzyme technology highlight the potential to create various structured-starches for the food and baking industry.
Keywords: Amylolytic enzymes, Starch modification, Baking industry, Disproportionation enzyme, Anti-staling agent, Glycosyl hydrolase, Glycosyl transferase
Introduction
Starch is found widely in nature and is the most important energy source for human nutrition. It consists of amylopectin (α-1, 4 linked glucan and α-1, 6 linked branches) and amylose (α-1, 4 linked glucan). The physicochemical properties of natural starch do not meet the requirements of nutritional and industrial-baking applications. Starch is required to tolerate various process conditions, including pH, extreme heat, and cold when treated with sterilization, microwave ovens, and storage in a refrigerator. Starch should also have a high bioavailability like resistant starch (RS) or branched amylopectin. Therefore, modified starches for the baking industry are tailor-made to overcome shortcomings such as viscosity loss on processing, retrogradation, and instability of the gelatinized starch structure during low temperature storage of native starches.
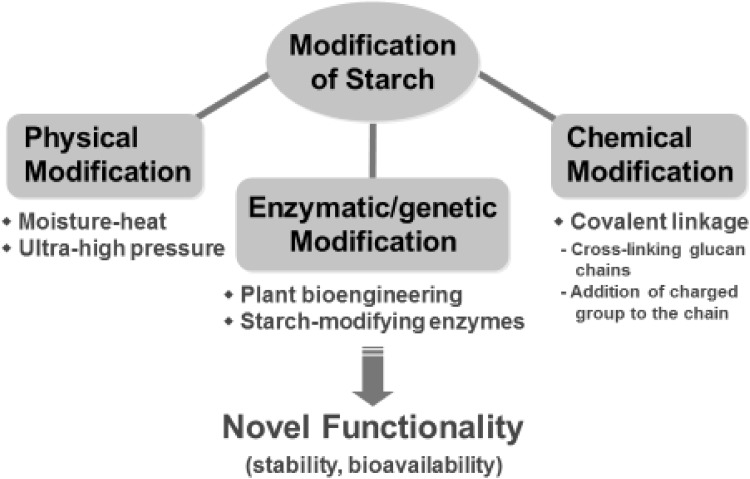
In general, the modification of starches has been traditionally conducted by physical and/or chemical methods (Fig. 1). Physical methods are simple and inexpensive, including heat-moisture treatments, freezing, and ultra-high pressure treatments, while chemical modification involves the introduction of functional groups into the starch molecules, e.g., etherification and esterification, cross-linking and acid treatment [1–9]. In contrast, the genetic modification of starch employs transgenic technology that targets the enzymes involved in starch biosynthesis and degradation pathways in plants. In recent decades, enzymatic modifications have been adopted, partly replacing the chemical and physical methods for the preparation of modified starch, because enzymes are safer and healthier than chemical method for both the environment and food consumers. Food enzymes are the most widely used and still represent the major share of the enzyme market. Currently, starch-converting/or modifying enzymes used in the production of maltodextrin, glucose and fructose syrups or modified starches comprise about 30% of the world’s enzyme production [10]. Thus, carbohydrate enzymes play an important role as processing essences in the food and baking industry. Their roles include the production of food ingredients, the enhancement of product quality, and the improvement of the efficiency of food processing [11].
Fig. 1.
Various processes of starch modification
In addition, enzyme reactions are not only mild and very specific for substrates of starch present in complex food systems but they also give high yields and fewer by-products [12, 13]. For example, enzymes have been efficiently used in the baking industry to improve the aspect of bread quality such as crumb softness and loaf microstructure, as well as the shelf life of products. The application of enzymes in bread or rice cake manufacturing has shown their value in quality control and the efficiency of production. The baking enzyme industry is expected to be worth $695.1 million by 2019 [14]. Currently, food and beverage production is the main consumer for the industrial enzyme market and it’s worth is estimated to reach $2.3 billion by 2020 [14].
One approach to enzymatic starch modification is to design a starch with a new structure, in which the molecular mass, branch chain length distribution, and amylose/amylopectin ratio can be changed by enzyme reactions, when the enzymes react with gelatinized starch. These techniques usually produce starches with altered physicochemical properties and modified structural attributes for various food and non-food applications.
Similarly, native starch granules can be enzymatically modified, enabling the enzymatic hydrolysis to occur in some regions of a starch granule. The regions that are susceptible to enzymatic attack are the less well-organized amorphous regions, whereas the crystalline lamellae are more resistant to enzymatic erosion [15, 16]. The small starch granules have novel properties, including the capability to hold and release sensitive materials, such as flavors [17]. Small granules from wheat starches (2 μm average diameter) can mimic lipid micelles, providing a fat-like texture [18]. Waxy maize starch nano-crystals have also been prepared by α-amylase [19–21]. Kim et al. [19] reviewed the applications of the starch nanoparticles in food industry as a filler in composites, that can improve not only the mechanical properties but also the biodegradability of the composites.
Retrogradation of starchy foodstuffs, which has been referred to as staling, is a major problem that must be solved. Retrogradation is a process that occurs in gelatinized starch as it moves from an initial amorphous state to a more ordered or crystalline state, resulting in an increase in the firmness of foodstuffs. Starch retrogradation is a major factor in the staling of bread and other starchy foods during storage, having an unacceptable influence on the texture of foods. The increase in the firmness of crumb texture results in food becoming unsuitable for consumption. Therefore, the staling of starch-based foods, such as bread and rice cake, has been the focus of many investigations. Attempts to slow the rate of retrogradation have initiated the development of various enzymes, emulsifiers, oligosaccharides, and polysaccharides [22]. Enzyme treatment is a means of directly modifying starch structure, providing changes in the molecular size, the ratio of amylose to amylopectin, molecular weight, and branch chain length distribution [23–25]. Consequently, the branch chains produced by enzyme reactions are not able to be recrystallized and associated with other branch chains for the formation of new hydrogen bonds. Additionally, it has been suggested to modify amylopectin molecules in particular ways, so that the re-association of these molecules becomes less effective at increasing matrix rigidity during storage [24].
A number of review articles on the subject of starch modification and conversion by various techniques are available [26–30]. However, there are not many reviews specific to the enzymatic modification of starches in the baking industry [31], although there have been many biotechnology studies of food enzymes. Therefore, this review addresses the fundamental functions of the key enzymes and their applications for starch modifications in the baking industry.
Important starch modifying enzymes for baked goods
Glycoside hydrolase and transglycosylase
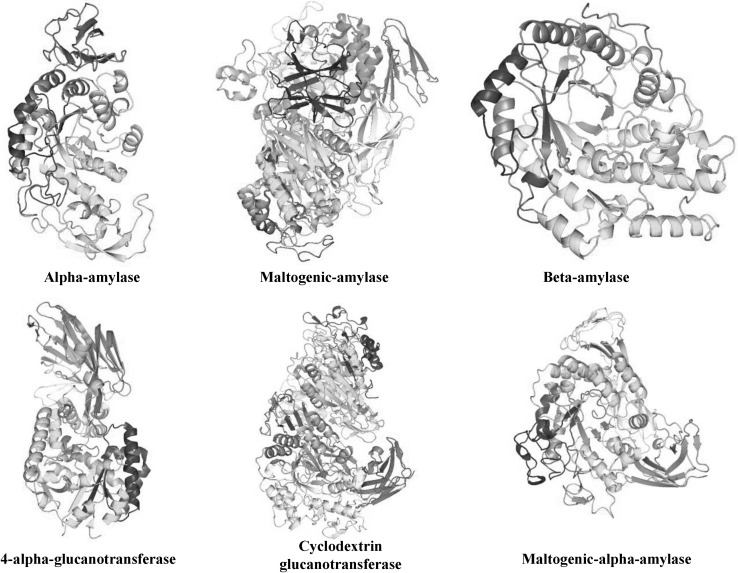
Coutino and Henrissat [32] classified all the enzymes that act on starch into several families (CAZY website: http://afmb.curs-mrs.fr/CAZY). Family 13, 57, and 77 include most of the technologically important enzymes that have been used for starch modification [33]. A classification of glycoside hydrolases into families based on amino acid sequence similarities has been proposed by Henrissat and Coutinho [34–36]. Glycoside hydrolases (GHs) are also referred to as glycosidases, and sometimes also as glycosyl hydrolases. Family 13 (GH13) includes enzymes such as α-amylase (EC 3.2.1.1), pullulanase (EC 3.2.1.41), isoamylase (EC 3.2.1.68), glucan branching enzyme (EC 2.4.1.18), and cyclodextrin glycosyltransferase (EC 2.4.1.19), whereas Family 77 (GH77) contains amylomaltase and 4-α-glucanotransferase (EC 2.4.1.24) [30]. The GH13 enzymes share a common secondary structure such as a (β/α)8 barrel or TIM barrel structure (Fig. 2). Most of this group of enzymes have four (or five) highly conserved regions in their primary sequence and share common amino acid residues in the active site [34, 35, 37, 38]. Three-dimensional (3D) structures of the α-amylase family have been determined using protein crystallization and X-ray crystallography (Fig. 2).
Fig. 2.
Three-dimensional structures of various amylases for starch modification
Van der Maarel et al. [30] reviewed the properties of the starch-converting enzymes belonging to the α-amylase family or family 13 glycosyl hydrolases. Glycoside hydrolases (EC 3.2.1.-) are enzymes that catalyze the hydrolysis of the glycosidic linkage of glycosides, leading to the formation of a sugar hemiacetal or hemiketal and the corresponding free aglycon (Fig. 3). In contrast, transglycosylases are enzymes that can catalyze the transformation of one glycoside to another. Transglycosylases use the same mechanism as various retaining glycoside hydrolases [39, 40]. Thus, reaction of the nucleophile of a retaining glycoside hydrolase with a substrate gives a glycosyl-enzyme intermediate that can be attacked either by water to give the hydrolysis product, or by another acceptor to give a new glycoside or oligosaccharide, as shown in Fig. 3 [41]. Both glycoside hydrolase and transglycosylase are involved in the modification of starches. In general, some transglycosylases possess substantial glycoside hydrolase activity and some glycoside hydrolases possess transglycosylase activity [39, 42, 43]. As shown in Fig. 3 the glycosyl enzyme intermediate can react with water to cause hydrolysis (glycoside hydrolase activity) or with a sugar acceptor to cause transglycosylation (transglycosylase activity) [44].
Fig. 3.
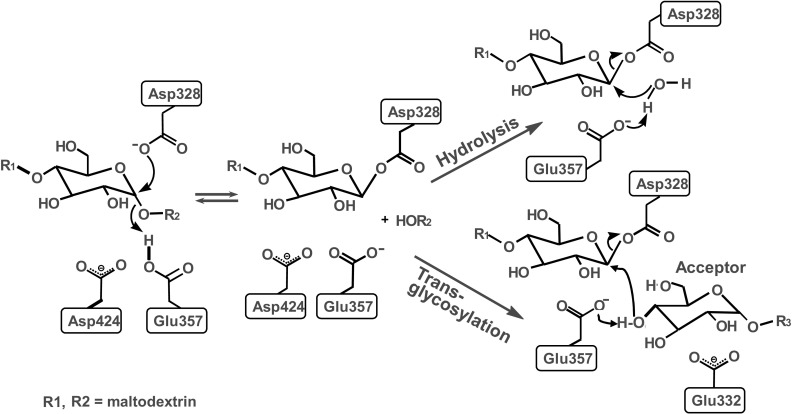
Catalytic mechanism of hydrolysis and transglycosylation reaction [44]
Glycosyltransferase
Glycosyltransferases belong to the superfamily of glycoside hydrolases. They are found in three GH families, 13, 57, and 77 [45–47]. Among these enzymes, the GH13 family, including 4-α GTase and cyclodextrin glucanotransferase, is the largest (Table 1). In Table 1, the starch-converting/or starch modifying enzymes including glycosyl hydrolases (EC 3.2.1.X) and glycosyl transferases (EC 2.4.X.Y.) are listed, showing the catalytic reaction, substrate specificity, and their applications.
Table 1.
Enzymes for starch modification
| Enzyme | Microorganisms/plants | Principal catalytic reaction | applications | References |
|---|---|---|---|---|
| α-glucanotrans ferase (Amylomaltase/D-enzyme) (EC 2.4.1.25) |
Thermus thermophilus
Thermus scotoductos Thermus aquatieus Ramie leaf |
Inter-/intra molecular trans-glycosylation by cleaving and reforming of α-1, 4-glycosidic linkage | Fat replacer in mayonnaise Prevention of staling of rice cake and bakery products Thermoreversible gel Lump-free cooked rice and porridge |
[31] [83] [84] [73] [74], [103] [75], [50] |
| Cyclodextrin glucanotroms-ferase (EC 2.4.1.19) |
Bacillus stearothermo-philus
Alkalophilc Bacillus sp.Ι-5 |
Intra molecular transglycosylation of maltodextrin via formation of α-1, 4-glycosidic linkage | Retard of bread/rice cake retrogradation Lump-free cooked rice and porridge |
[82] [75] |
| Branching enzyme (EC 2.4.1.18) |
Rhodothermus obamensis
Aquifex aeolicus Deinococcus radiodurans |
Hydrolysis of α-1, 4 linkage and formation of α-1, 6- branches | Thermostable glycogen Branched maltodextrin Slow digestable starch Highly branched amylopectin |
[104] [105] [106] [107] [108] [109] [110] |
| Amylosucrase (2.4.1.4) |
Neisseria polysaccharea
Deinococcus geothermalis |
Sequential transglycosylation of glucosyl unit from sucrose onto acceptor molecule | Resistant starch Slowly digestible starch |
[95] [91, 92] [90] [93] |
| α-amylase (EC 3.2.1.1) | Bacilus magaterium | Hydrolyis of α-1. 4-glycosidic linkage | Retard of bread retrogradation Enzyme-hydrolyzed -HP starch |
[51] [71] |
| β-amylase (EC 3.2.1.2) | Barley (malt) Ramie leaf |
Shortening the external side chains of amylo-pectin by cleaving maltose | Prevention of starch/rice cake retrogradation Improvement of emulsification properties Highly branched nano-starch particle |
[66] [63] [23] [111] [65] |
| Maltogenic amylase Neopullulanase Cyclodextrinase (EC 3.2.1.54) (EC 3.2.1.135) |
Bacillus stearothermphilus
Thermus sp. |
Maltose as major product from starch and cyclodextrin panose production from pullulan by hydrolysis of α-1,4-linkage and formation of α-1,6- linkage by transglycosylation | Selective hydrolysis of amylose Low-armylose starch |
[56] [57] |
| Maltogeic α-amylase (Novamyl ®) (EC 3.2.1.133) | Bacillus stearothermophilus | Maltose production from amylose, amylerpeot and clyclodextrin in exo/or endo-like manner | Prevention of staling of bakery products Non-digestible starch |
[79] [112] [89] [86] |
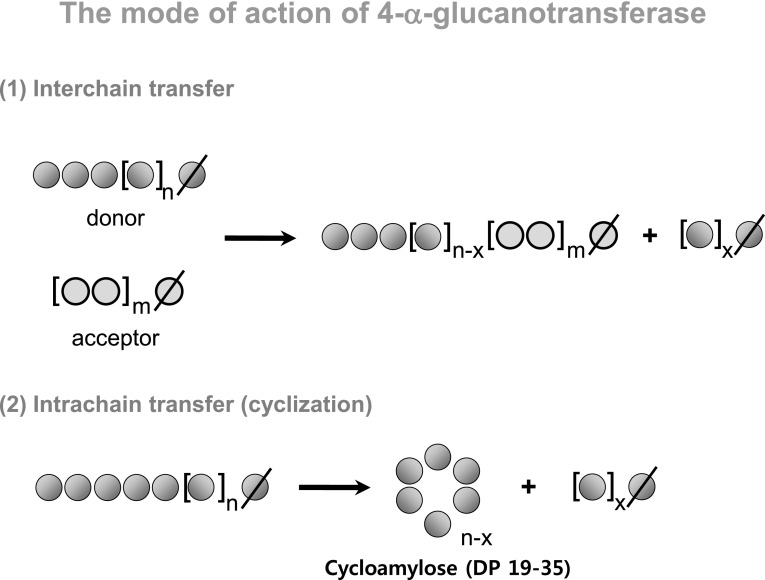
Alpha-glucanotransferase (α-GTase) or amylomaltase catalyzes the transfer of a glucan fragment from the non-reducing end of a glucan (donor) molecule to another glucan acceptor molecule, thereby resulting in disproportionation in both donor and acceptor glucan molecules (Fig. 4). The glucan moiety of the donor molecules forms an enzyme–substrate intermediate, and the non-reducing end of the acceptor glucan enters the acceptor subsite + 1 and attacks the enzyme–substrate intermediate with its 4-hydroxyl group. For cyclodextrin glucanotransferase, intra-molecular transglycosylation is dominant, creating various types of cyclodextrins. The α-GTases found in plants have been referred to as D-enzymes and resemble those found in microorganisms [48, 49]. One of the microbial α-GTases, MalQ, has been intensively investigated to understand the maltose utilization system in Escherichia coli. The enzyme catalyzes the transfer of glucosyl and maltodextrinyl residues from the non-reducing end of maltodextrin to acceptor molecules. It is also involved in the starch degradation pathway in plants, which is a process similar to the maltose utilization system in E. coli.
Fig. 4.
The mode of action of 4-α-glucanotransferase
Thermostability of starch modifying enzymes for baking process—intermediate temperature-stable enzymes
Enzyme-mediated hydrolysis or transglycosylation can occur after the starch molecule is gelatinized. Therefore, the enzyme must be active and heat-stable around the gelatinization temperature of the target starch. In the baking and rice cake making process, anti-staling enzymes are usually added to wheat flour or rice powder before preparing the dough or during the mixing of the dough, and they actively react with starch at a certain temperature at which starch gelatinization occurs. These enzymes are then inactivated during the baking or steaming process due to the high process temperature, and thus do not excessively hydrolyze starch or cause gumminess in the products. Therefore, the intermediate temperature stable enzymes are desirable as anti-staling agents [50]. However, most intermediate temperature plant amylases are not sufficiently active due to instability at the gelatinization temperature of about 60 °C. In contrast, the gumminess problem occurs when using bacterial heat stable amylases because they are inactivated at temperatures higher than 75 °C [51, 52]. For example, α-GTase from Thermus brokianus has an optimum temperature of 70 °C, with a loss of enzymatic activity above 80 °C [53].
He et al. [50] isolated β-amylase from ramie leaf that had intermediate temperature-stable enzyme properties. Its optimum temperature was 65 °C, which is suitable for the baking industry. Kraus and Hebeda [51] also isolated an intermediate temperature-stable α-amylase from Bacillus megaterium, with an optimum activity at a temperature of about 65–72 °C, which retained less than 50% of the activity at temperatures above 75 °C. The enzyme-treatment retarded the staling of baked goods without causing gumminess or adversely affecting the organoleptic characteristics of the baked goods.
In the food and bio-industry, plant-originated amylases are preferred, due to their high product specificity and safety. However, their use is limited to some extent because most of the enzymes are extracted from cereal grains, which are human staple foods. Moreover, the thermal properties are not ideal for application as an intermediate temperature-stable enzyme. More studies are required to identify novel plant leaf enzymes that are highly stable and could be a potential alternative to traditional plant amylases. The T50, i.e., the temperature at which enzyme activity decreases by a half after a 30-min treatment, for ramie leaf β-amylase is 66 °C, which is higher than for wheat flour β-amylase (50 °C), soybean β-amylase (63.2 °C), and barley β-amylase (56.8 °C) [54]. The ramie leaf β-amylase has superior thermostability to other plant β-amylases. The thermostability of wheat β-amylase is also significantly enhanced in the presence of additives [54].
Starch modification by various carbohydrate enzymes
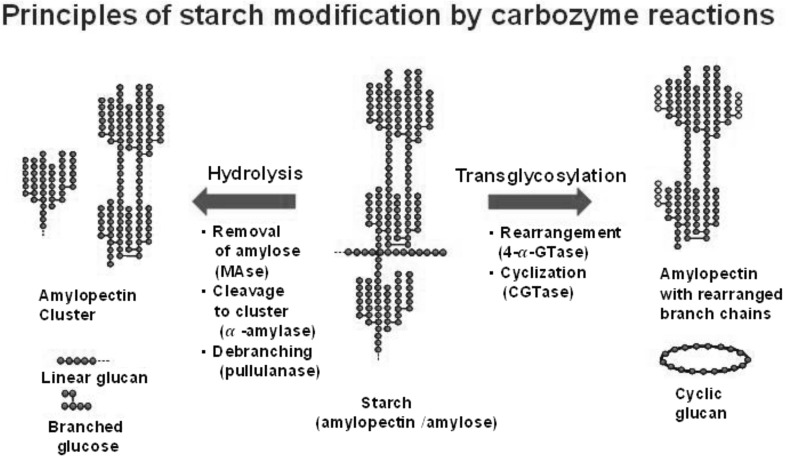
In Fig. 5, a general scheme for the enzymatic modification of amylose and amylopectin molecules by glycosyl hydrolase and glycosyyl transferase is outlined. Amylose and amylopectin molecules are the target substrates for glycosidic hydrolases, such as maltogenic amylase (MAase), α-amylase, and pullulanase. They generate various products, including the amylopectin cluster, with reduced molecular mass, linear glucans, and branched maltooligosaccharides. Transglycosidases, α-glucanotransferase, and cyclodextrin glucanotransferase transfer the glucan moiety of amylase and/or amylopectin (donor) to other molecules (acceptor), resulting in the formation of amylopectin with rearranged branch chains and cyclic glucans.
Fig. 5.
Schematic diagram for starch modification by carbohydrate enzymes
Reducing amylose content by the selective hydrolysis of amylose
Rice is traditionally classified into three categories according to its amylose content (International Rice Research Institute, IRRI): 25–30% is classified as high, while 10–20% is classified as low, with intermediate amylose rice (20–25%) preferred in most rice growing areas in the world. It is known that the ratio of amylose to amylopectin may greatly influence the taste of cooked rice. Cooked rice with an amylose content of 5–15% is suitable for low temperature storage at around 4 °C. Therefore, it is desirable to prepare low amylose-rice products in vitro by the modification of rice starch. One possible technique would be to develop an enzyme, which can selectively hydrolyze amylose, but not amylopectin.
The active site of maltogenic amylase in dimeric form is known to create a narrow and deep groove [55, 56], and it has been proposed that the substrate preference of the enzyme toward carbohydrate molecules can be explained by this unique active site conformation. Similarly, Kamasaka et al. [57] obtained a higher selectivity for neopulluanase on relatively small amylose molecules in starch rather than on large amylopectin molecules. The distinguishable action of maltogenic amylase or cyclodextrinase (CDase) can be applied to produce low-amylose starch by selectively-degrading amylopectin molecules. A CDase isolated from alkalophilic Bacillus sp. I–5 had a kcat/km value on amylose of 14.6 s−1(mg/mL)−1, whereas for amylopectin it was 0.92 s−1(mg/mL)−1, demonstrating an exceptionally high preference toward amylose [56]. However, amylases with a high selectivity toward amylose frequently cannot be used in rice cooking due to their low optimal temperature. To overcome this disadvantage, a thermostable maltogenic amylase from Thermofilum pendens (optimum temperature > 90 °C) has been developed [58]. The substrate specificity of the enzyme had a k cat/Km value on amylose of 0.98 s− 1 mL mg− 1, whereas for amylopectin it was 0.30 s− 1 mL mg− 1, indicating a high preference toward amylose. When cooking with this enzyme, the retrogradation rates of cooked rice decreased significantly during storage at 4 °C. Furthermore, to enhance the specificity toward amylose, Gly50, Asp109, and Val431, located at the interface of the dimer of maltogenic amylase from Thermus sp., were replaced with bulky amino acids, creating the mutant enzymes of G501I/D109E/V431I. The kcat/km values of the mutants for amylose increased significantly compared to the values for the wild-type enzyme, whereas the values for amylopectin decreased [59]. Thus, the substituted bulky amino acid residues such as isoleucine and glutamate resulted in a narrower shape of the catalytic site, which enhanced the ability of the enzyme to distinguish between small and large molecules.
Amylose-free starch has been produced by genetic modification in plants that targets the enzymes involved in starch biosynthesis [60]. Amylose-free starch and high-amylose starch in cereals have been produced by traditional plant breeding technology or biotechnology [61, 62]. In addition, amylopectin with an altered structure was synthesized by the inhibition of SS II and SS III isoforms in rice species [29]. Amylose-free short-chain amylopectin starches have also been developed and the starches have excellent freeze–thaw stability [60].
Modification of starch by hydrolysis reaction of enzymes: α- and β-amylases
Beta-amylase is an exo-splitting enzyme and releases maltose successively from the non-reducing end of glucan chains, thereby shortening the external side chains of amylopectin. A shortening of the external chains of waxy maize and potato amylopectin was performed with β-amylase. Partial beta-amylolysis produced a significant fraction of chains with 2-6 glucose units. No retrogradation occurred when the external chains of both amylopectins had 11 or less glucose units on average [63]. The inhibition of retrogradation may be caused primarily by the presence of very short external chains, which hinders the re-association of the long external chains. Thus, the enzyme is suggested to retard bread staling by reducing the tendency of the amylopectin to retrograde in baking products [63]. Moreover, plant-originated β-amylase has significant technological importance for the baking, brewing, and starch industries due to its safety and high specificity compared to microbial enzymes [23, 54]. A plant β-amylase with a temperature optimum > 60 °C would be useful for the baking industry as an intermediate temperature-stable amylase. Ramie leaf-treated rice cake exhibited a significant increase of shorter branch chains (DP < 15) of amylopectin, but a decreased number of longer branch chains (DP = degree of polymerization > 16). Furthermore, ramie leaf treatment significantly reduced retrogradation during 48 h of storage at 4 °C [23]. The hardness of the ramie leaf rice cakes was lower than that of the control without ramie leaf addition, resulting in the production of a rice cake with a milder taste. As an alternative plant-originated enzyme, ramie leaf β-amylase has the potential for use as a novel anti-staling additive.
The activities of amylase isozymes affect the palatability of cooked rice. Therefore, enzymes from the rice endosperm can serve as biomarkers for evaluating the palatability of steamed rice [51, 64]. An intermediate temperature-stable bacterial α-amylase retards the staling of baking products, without causing gumminess or adversely affecting the organoleptic characteristics of baking products. The enzyme survives incorporation into dough, remains active at > 60 °C, but is inactivated rapidly at > 75 °C; thus, it has no tendency to hydrolyze starch excessively or cause gumminess in baking products. In contrast, the Bacillus subtilis heat-stable alpha-amylase causes gumminess because it is not inactivated at 75 °C [51].
The effects of amylase treatment on the surface of starch particles have been investigated. β-amylase resulted in a thinning effect at the surface of soluble starch particles [65]. The molecular weight distributions of enzyme-treated starch particles and their chain length distributions showed that β-amylolysis had a thinning effect at the outermost surface of soluble starch particles, thereby resulting in an increase of DP 2–5 chains, but a decrease in the external long chains. The digestion analysis showed that enzyme-treated starch particles had a low digestion rate, and could be used for preparing highly branched nano-particles as a delivery carrier for functional compounds [65].
Yao et al. [66] investigated the effect of partial β-amylolysis on the retrogradation of rice starch and suggested the potential application of β-amylase in the preparation of rice products with an extended shelf life. The results indicated that partial β-amylolysis using β-amylase retarded amylopectin retrogradation by shortening the branch chain length of amylopectin. In addition, the maltose produced in β-amylolysis might slightly attenuate the inhibition effect of retrogradation.
Amylases producing maltose, maltotriose, maltotetraose, and longer maltooligosaccharides are claimed to extend the shelf life of baking products by delaying retrogradation of the starch. Of these enzymes, α-amylases have an anti-staling effect on bread by producing various maltooligosaccharides [67, 68]. However, the production of branched maltooligosaccharides of DP 20–100 by α-amylase may result in gumminess in bread [67]. To reduce the production of branched maltooligosaccharides, Carroll et al. [69] used a debranching enzyme, pullulanase, with α-amylase for making bread. Pullunase hydrolyzes the branched maltooligosaccharides of DP 20–100 produced by the α-amylase into smaller maltooligosaccharides, which improves the quality of the baked product. Min et al. [70] employed a novel maltooligosaccharide-producing amylase that produces mainly maltooligosaccharides as an anti-staling agent for baking bread.
A dual modification of starch has been performed, in which the starch in the granular was treated with a mixture of fungal α-amylase and glucoamylase and then chemically modified to produce enzyme-hydrolyzed-hydroxypropyl (HP) starch [71]. Enzyme-hydrolyzed-HP starch has significantly different functional properties compared to hydroxypropyl starch prepared from untreated (native) starch.
Modification of starch by transglycosylation reaction of alpha-glucanotransferase (α-GTase)
Maize granular starch has been modified by Thermus scotoductus α-GTase and the physicochemical properties of the products have been characterized. The molecular weight of amylopectin decreased from 4.4 × 108 Da to 7.1 × 105 Da, indicating that the inner chain (C or B chains) of amylopectin was cleaved and the outer chains (A or B chains) were rearranged. As a result, small amylopectin clusters with shortened branch chains were produced. The branch chain length distribution of rice starch was examined after α-GTase treatment. Both the number of long (DP > 25) and short-branch chains significantly increased. The increase in the number of long-branch chains may be attributed to amylose and glucan moieties (fragments), which were transferred to the branch chains of amylopectin. Consequently, the α-GTase-modified starches displayed a smaller proportion of branch chains with DP 7–20, but a larger proportion of branch chains of DP > 20 than the control [72, 73]. Additionally, the enzyme had the capability to produce cycloamylose with DP 19–35 (Fig. 5). The physicochemical properties of α-GTase-modified rice flours were examined [74]. α-GTase-modified rice flour had a higher water holding capacity than a control rice flour. Furthermore, the textural properties of noodles prepared with 4α-GTase-treated rice flour after a freeze–thaw cycle were retained when compared to a control noodle. As a result, the α-GTase-treated rice flour appeared to have good potential as a non-sweet cryoprotectant of frozen products.
Lump-free cooked rice was prepared using a disproportionation reaction of α-GTase [75]. Thermostable disproportionation enzymes active at around the gelatinization temperature of rice starch (70–80 °C) were applied to improve the quality of cooked rice, with lump-free properties. A starch binding domain-attached α-GTase from Thermus aquaticus was prepared from an E. coli transformant carrying a recombinant plasmid. The stickiness of the cooked rice showed that the enzyme treatment had the effect of reducing the lumping properties of cooked rice grains and rice porridge. The enzyme treatment also provided a creamier, and more uniform texture of rice porridge. A high-performance anion exchange chromatography (HPAEC) analysis of the starch structure in cooked rice and rice porridge revealed that the proportion of both short (DP < 6) and long-branch chains (DP > 20) increased in the enzyme-treated samples. In addition, when rice was cooked with α-GTase, the amylose content of the cooked rice grain decreased by 36%. The amylose content on the surface of the cooked rice grains treated with the enzyme was reduced by 50% compared with that of the untreated cooked rice. These results revealed that the glucan fragment of amylose was transferred to amylopectin branch chains by a transferring reaction. Thus, the decrease in the amylose content on the surface is likely to be responsible for reducing the stickiness of cooked rice grains and rice porridge. Furthermore, the retrogradation rate determined by a differential scanning calorimeter was significantly lowered in the enzyme-treated samples after storage for 10 h at 4 °C. Thus, α-GTase may have great potential for application to various rice-based products in the food industry.
Starch modification of bakery products by cyclodextrin glucanotransferase and maltogenic α-amylase, Novamyl®
Currently, Bacillus stearothermophilus maltogenic α-amylase (Novamyl®), is widely used in the baking industry as an anti-staling agent due to its ability to lower the retrogradation of gelatinized starch [76], and is known to be different from exoglucanases like β-amylase and glucoamylase. The reaction products of the enzyme are maltose and oligosaccharides, whereas β-amylase and glucoamylase produce maltose and glucose, respectively. Interestingly, maltogenic α-amylase does not require a non-reducing end, and attacks amylose, amylopectin, and cyclodextrins in an endo-like manner [77]. The 3-D structure of the enzyme (Fig. 2) has a five-domain organization, which is more usually associated with CGTase and sequence homology to CGTases [78, 79]. Like CGTase, maltogenic α-amylase cleaves cyclodextrins and forms transglycosylation products, but is subject to product inhibition by maltose [77]. An enzyme mixture comprising maltogenic α-amylase and amyloglucosidase has been used to prevent the staling of baked bread [22].
Cyclodextrin glucanotransferase (CGTase) produces various cyclodextrins (α-, β- and γ-) via the intramolecular transglycosylation of maltooligosaccharides. Lee et al. [80] reported the potential application of CGTase as an anti-staling agent for bread by eliminating the cyclization activity. CGTase from B. stearothermophilus ET1 was genetically modified to enhance its potential as an anti-staling enzyme. The cyclization activity of the mutants dramatically decreased, but showed a two-fold higher hydrolysis activity than the wild-type enzyme. Consequently, CD was barely detected in the loaf treated with the mutant enzymes and the mutant-treated bread contained more of the larger maltooligosaccharides, such as maltopentaose and maltohexaose, than the control. Retrogradation rates decreased significantly in the loaves treated with the mutants. In a similar way, Shim et al. [81] reported another mutant to improve a CGTase activity as an anti-staling enzyme. A CGTase cloned from alkalophilic Bacillus sp. I-5 was mutated in which the cyclizing activity of the mutant enzyme decreased by 10-fold, while the hydrolysis activity increased by 15-fold. The application of the mutant enzyme reduced the retrogradation rate of bread as much as Novamyl® during a seven-day storage at 4 °C [81]. Furthermore, CGTase [3–18] that was previously engineered to enhance the hydrolyzing activity with little cyclodextrin formation activity toward starch was primarily manipulated to be displayed on the cell surface of Saccharomyces cerevisiae [82]. Saccharomyces cerevisiae carrying pδCGT integrated into the chromosome exhibited a high starch-hydrolyzing activity. The volumes of the bread loaves and rice cakes prepared using S. cerevisiae/pδCGT increased by 20 and 45%, respectively, with no detectable CD. The retrogradation rates of the bread and rice cakes decreased significantly during storage. The results revealed that CGTase [3–18] displayed on the surface of yeast hydrolyzed starch into glucose and maltose that could be used at a moderate rate by yeasts, resulting in the production of evenly-distributed gas bubbles during bread baking.
Thermoreversible starch gel and other modified starch products
The production of thermoreversible starch gels is a potential industrial application of α-GTase. A normal untreated starch gel cannot be dissolved in water after it has retrograded, whereas starch treated with α-GTase obtains thermoreversible gelling characteristics. The action of α-GTase reduces the amylose content and broadens the branch-chain length distribution in amylopectin, which results in the production of both shorter and longer branch chains in modified amylopectin. α-GTase-treated potato starch was shown to exhibit thermoreversible gelation at a concentration of 3% (w/v) [49]. Van der Maarel et al. [49] described this process using α-GTase from the hyperthermophilic bacterium Thermus thermophilus. Hansen et al. [83] modified starches from various plant sources including potato, maize, waxy maize, wheat, and pea. Modifying starch with α-GTase resulted in a broadening of the amylopectin chain length distribution. The increase in longer chains appeared to be a combination of the effect of amylose on amylopectin chain transfer and transfer of cluster units within the amylopectin molecules. Lee et al. [84] studied the effects of a thermostable α-GTase from T. scotoductus on the thermo-reversibility and freeze–thaw stability of cooked rice paste. The enzyme–treated gel exhibited a highly improved freeze–thaw stability. Currently, α-GTase is not commercially available and so the thermoreversible starch gel cannot be produced on an industrial scale.
Low-fat spreads were developed using a thermoreversible gelling agent and α-GTase-modified rice starch [85]. Formulations with 15 or 20% of the modified starch paste resulted in highly stable oil-in-water low-fat spreads, with an acceptable spreadability. Furthermore, the modified starch-based low-fat spreads were found to be highly thermoreversible. The thermoreversible low-fat spread can be used in some baking such as cakes, However, more investigations will be needed to make it suitable for baking to achieve optimum results.
A highly branched rice starch amylopectin cluster was prepared using a mixture of T. scotoductus α-GTase and maltogenic amylase. The hydrolysis rate of the modified product toward glucoamylase and α-amylase significantly decreased [86]. Cho et al. [87] found that the production of cycloamyloses (DP 3–40) by T. aquaticus α-GTase increased as the amylose content of the starch samples increased.
Enzyme resistant starch formation
Resistant starch (RS) is defined as the fraction of starch that is highly recrystallized or retrograded. When consumed as dietary intake, the undigested RS in the small intestine reaches the large intestine, thereby serving as a fermentation substrate for beneficial colonic bacteria. The short fatty acids, such as the propionic and butyric acids produced by colonic fermentation, have beneficial physiological effects, with therapeutic and nutritional values akin to dietary fiber [88]. A number of investigations on resistant starches have been conducted using a combination of maltogenic α-amylase, with other enzymes [86, 89].
Physicochemical properties of amylosucrase-modified starch were also investigated, focusing on structural changes and digestion [90–95]. The starch modification by amylosucrase resulted in a decrease in the proportion of short- branch chains, whereas for long-branch chains there was an increase, thereby transferring glucosyl units to the non-reducing ends of other branch chains of amylopectins. The in vitro digestion analyses of amylosucrase-modified starch revealed that the elongated branch chains were the major areas with a high content of slowly digestible starch and RS, due to formation of a partly ordered crystalline structure via associations between elongated branch chains.
Branched oligosaccharide (BOS) (isomalto-oligosaccharide: IMO) production by starch-converting enzymes
BOS or IMO constitute a mixture of glucose oligomers, which contain more than one α-1, 6-glucosidic linkages in the molecule. It includes isomaltose, panose, isomaltotriose, isomaltotetraose, isomaltopentaose, and higher branched oligosaccharides. BOS is naturally found in some starch-based foods, and is being manufactured commercially from starch by the enzyme reaction. While human intestinal enzymes readily digest α-(1, 4)-glycosidic bonds, α-(1, 6)-linkages are barely hydrolyzed and so exhibit digestion-resistant properties. A BOS mixture can be used as a substitute for sucrose and other saccharides in the food industry due to the low viscosity, mild sweet taste, and limited freezing point depression [96]. They are also effective at reducing microbial contamination as well as the retrogradation of starch-based foods because they have a high moisture-holding capacity and lower the water activity of foods [97].
On a commercial scale the manufacturing process consists of two steps. First, the starch is converted, by enzymatic hydrolysis, into a high sugar concentrate of di-, tri, and oligosaccharides, having α-(1, 4)-glycosidic linkages and, secondly, α-(1, 4)-glycosidic linkages of the product are further converted into α-(1, 6)-glycosidic linkages by an enzymatic transglycosylation reaction, forming BOS. Maltogenic amylase is capable of transferring an alpha-1, 4-sugar moiety released from starch to acceptor sugar molecules by an alpha-1, 6-glucosidic linkage when the substrates are present in excess. In a similar way, these catalytic properties of enzymes can be applied to produce highly branched oligosaccharide mixtures, with various glucosidic linkages. It has been shown that BOS improves the shelf life of starch-based foods by lowering water activity [97].
In addition to branched oligosaccharides being manufactured by a two-step procedure that uses two alpha-amylases on starch solution [98], some amylolytic enzymes possess a glucose transferring activity as well as a hydrolyzing activity [99]. When using these bifunctional enzymes, a one-step process is required to produce branched oligosaccharides [100, 101]. The amount of BOS in the mixture used in these studies was comparable to the amount in the mixtures on the market.
A continuous process for the production of maltodextrins of a specific length, such as maltohexaose, maltopentaose, and maltooctaose, was developed using Pyrococcus furiosus thermostable amylase. The resulting products were of high quality, with a purity of more than 95%, and have the potential to be widely used in the food and bio-industry [102].
Enzyme technology is a promising tool for the starch modification of starch-based foods such as baking products because carbohydrate enzymes can react in a substrate-specific way toward the target starches in the complex matrix of food items. Although the enzymatic method has many advantages over other methods, such as chemical and physical treatments, for most microbial enzymes there is a need to prove their safety in terms of human health when they are used in baking products. In contrast, plant-originated enzymes can be applied as safe food ingredients. However, their uses are limited due to limited number of natural sources and their temperature instability during food and starch processing. Thus, new enzyme resources from plants or safety-proven microorganisms should be further investigated in the future. As mentioned above, the rapid retrogradation problem of rice starch-based foods can be overcome technologically. One potential way to achieve this is by the combination of two or more enzymes, or by enzyme treatments with chemical/or physical methods that have been recently proven to be more effective for starch modification. The mechanisms involved in starch retrogradation and enzyme reaction should be further investigated under the various storage conditions used for baking products.
Acknowledgements
Authors thank Dr. Eui-Jeon Woo, Korea Institute of Bioscience and Biotechnology for the 3-D structure model of amylases.
References
- 1.Chung HJ, Liu Q, Hoover R. The impact of annealing and heat-moisture treatments on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinizedcorn, pea and lentil starches. Carbohydr. Polym. 2009;75:436–447. doi: 10.1016/j.carbpol.2008.08.006. [DOI] [Google Scholar]
- 2.Chung HJ, Liu Q, Hoover R. Effect of single and dual hydrothermal treatments on the crystalline structure, thermal properties and nutritional fractions of pea, lentil and navy starches. Food Res. Int. 2010;43:501–508. doi: 10.1016/j.foodres.2009.07.030. [DOI] [Google Scholar]
- 3.Hoover R, Manuel H. Effect of heat-moisture treatments on the structure and physicochemical properties of legume starches. Food Res. Int. 1996;29:731–750. doi: 10.1016/S0963-9969(97)86873-1. [DOI] [Google Scholar]
- 4.Hoover R, Vasanthn T. The effect of annealing on the physicochemical properties of wheat, oat, potato and lentil starches. J. Food Biochem. 1994;17:303–325. doi: 10.1111/j.1745-4514.1993.tb00476.x. [DOI] [Google Scholar]
- 5.Hirsch JB, Kokini JL. Understanding the mechanism of cross-linking agents (POCl3, STMP, and EPI) through swelling behavior and pasting properties of cross-linked waxy maize starches. Cereal Chem. 2002;79:102–107. doi: 10.1094/CCHEM.2002.79.1.102. [DOI] [Google Scholar]
- 6.Perry PA, Donald AM. The effects of low temperatures on starch granule structure. Polymer. 2000;41:6361–6373. doi: 10.1016/S0032-3861(99)00813-7. [DOI] [Google Scholar]
- 7.Kavitha R, BeMiller JN. Characterization of hydroxypropylated potato starch. Carbohydr. Polym. 1998;37:115–121. doi: 10.1016/S0144-8617(98)00056-3. [DOI] [Google Scholar]
- 8.Richardson S, Gorton AC. High-performance anion exchange chromatography-electrospray mass spectrometry for investigation of the substituent distribution in hydroxypropylated potato amylopectin starch. J. Chromat. 2001;917:111–121. doi: 10.1016/S0021-9673(01)00690-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang YJ, Wang L. Characterization of acetylated waxy maize starches prepared under catalysis by different alkali and alkaline-earth hydroxides. Starch. 2002;54:25–30. doi: 10.1002/1521-379X(200201)54:1<25::AID-STAR25>3.0.CO;2-T. [DOI] [Google Scholar]
- 10.Miguel ASM, Martins-Meyer TS, Figueiredo EVC, Paulo Lobo BW, Dellamora-Ortiz GM. Enzymes in bakery: current and future trends. pp. 287–321. In: Food Industry. Muzzalupo I, InTech, London, U.K. (2013)
- 11.Biegelis R. Carbohydrases. pp. 121–158. In: Enzymes in food processing. Nagodawithana T, Reed G, Academic Press Inc. New York, U.S.A. (1993)
- 12.Kennedy, JF, Knill CJ, Taylor, DW. Maltodextrins. pp. 65–82. In: Handbook of starch hydrolysis products and their derivatives. Keasley MW and Dziedzic SZ, Blackie Academic & Professional, Glasgow, U.K. (1995)
- 13.Butler DP, van der Maarel MJEC, Steeneken PAM. Starch-acting enzymes. pp 128–155. In: Starch in food: structure, function and applications. Eliasson AC, CRC Press, Boca Raton, U.S.A. (2004)
- 14.Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3. Biotech. 2016;6:174. doi: 10.1007/s13205-016-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang WJ, Powell AD, Oates CG. Pattern of enzyme hydrolysis in raw sago starch: Effects of processing history. Carbohydr. Polym. 1995;26:91–97. doi: 10.1016/0144-8617(94)00090-G. [DOI] [Google Scholar]
- 16.Oates CG, Powell AD. 1996. Bioavailability of carbohydrate material stored in tropical fruit seed. Food Chem. 56: 405–414 (1996)
- 17.Tari TA, Annapure US, Singhal RS, Kulkarni PR. Starch-based spherical aggregates: Screening of small granule sized starches for entrapment of a model flavouring compound, vallin. Carbohydr. Polym. 2003;53:45–51. doi: 10.1016/S0144-8617(02)00293-X. [DOI] [Google Scholar]
- 18.Daniel JR, Whistler R L. 1990. Fatty sensory qualities of polysaccharides. Cereal Foods World 35: 825 (1990)
- 19.Kim JY, Park DJ, Lim ST. Fragmentation of waxy rice starch granules by enzymatic hydrolysis. Cereal Chem. 2008;85:182–187. doi: 10.1094/CCHEM-85-2-0182. [DOI] [Google Scholar]
- 20.Angellier CH, Boisseau SM, Dufresne A. Mechanical properties of waxy starch nanocrystal reinforced natural rubber. Macromolecules. 2005;38:9161–9170. doi: 10.1021/ma0512399. [DOI] [Google Scholar]
- 21.Angellier CH, Putaux JL, Molina BS, Dufresne A, Bertoft E, Perez S. The molecular structure of waxy maize starch nanocrystals. Carbohydr. Res. 2009;344:1558–1566. doi: 10.1016/j.carres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Else AJ, Tronsmo KM, Nieman LA, Moonen JHE. Use of anti-staling enzyme mixture in the preparation of baked bread. U.S. Patent 2013/0059031 A1 (2013)
- 23.Nguyen DH, Tran PL, Ha HS, Lee JS, Hong WS, Le QT, Oh BC, Park SH. Presence of β-amylase in ramie leaf and its anti-staling effect on rice cake. Food Sci. Biotechnol. 2015;24:37–40. doi: 10.1007/s10068-015-0006-2. [DOI] [Google Scholar]
- 24.Hickman BE, Janaswamy S, Yao Y. Properties of starch subjected to partial gelatinization and β-amylolysis. J. Agric. Food Chem. 2009;57:666–674. doi: 10.1021/jf8030698. [DOI] [PubMed] [Google Scholar]
- 25.Seo NS, Roh SA, Auh JH, Park JH, Kim YR, Park KH. Structural characterization of rice starch in rice cake modified by Thermus scotoductus 4-α-glucanotransferase (TSαGTase) J. Food Sci. 2007;72:C331–C336. doi: 10.1111/j.1750-3841.2007.00428.x. [DOI] [PubMed] [Google Scholar]
- 26.Din Z, Xiong H, Fei P. Physical and chemical modification of starches – A review. Crit. Rev. Food Sci. Nutr. 2017;57:2691–2705. doi: 10.1080/10408398.2015.1087379. [DOI] [PubMed] [Google Scholar]
- 27.BeMiller JN, Huber KC. Physical modification of food starch functionalities. Ann. Review. Food Sci. Technol. 2015;6:19–69. doi: 10.1146/annurev-food-022814-015552. [DOI] [PubMed] [Google Scholar]
- 28.Ashogbon AO, Akintayo ET. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch. 2014;66:41–57. doi: 10.1002/star.201300106. [DOI] [Google Scholar]
- 29.Neelam K, Vijay S, Lalit S. Various techniques for the modification of starch and the applications of its derivatives. Int. Res. J. Pharm. 2012;3(5):25–31. [Google Scholar]
- 30.van der Maarel MJEC, van der Veen B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002;94:137–155. doi: 10.1016/S0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 31.van der Maarel MJEC, Leemhuis H. Starch modification with microbial alpha-glucanotransferase enzymes. Carbohydr. Polym. 2013;93:116–121. doi: 10.1016/j.carbpol.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 32.Coutinho PM, Henrissat B. Carbohydrate-active enzymes: An integrated database approach. In: Recent advances in carbohydrate bioengineering. Gilbert HJ, Davies GJ, Henrissat B, Svensson B (ed), Royal Society of Chemistry, Cambridge, England (1999)
- 33.Park KH, Park JH, Lee SY, Yoo SH, Kim JW. Enzymatic modification of starch for food industry. In: Carbohydrate-active enzymes: structure, function and applications. Park KH (ed). CRC Press, Inc., Boca Raton, FL, USA. Woodhead Pub. Ltd. Cambridge, England (2008)
- 34.Henrissat B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991;280:309–316. doi: 10.1042/bj2800309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henrissat B, Davies G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997;7:637–644. doi: 10.1016/S0959-440X(97)80072-3. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003;328:307–317. doi: 10.1016/S0022-2836(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 37.Janecek S. New conserved amino acid region of alpha-amylase in the 3rd loop of their (beta/alpha)8-barrel domains. Biochem. J. 1992;288:1066–1070. doi: 10.1042/bj2881069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janecek S. Close evolutionary relatedness among functionally distantly related members of the (beta/alpha) 8-barrel glycosyl hydrolases suggested by similarity of their fifth conserved sequence region. FEBS Lett. 1995;377:6–8. doi: 10.1016/0014-5793(95)01309-1. [DOI] [PubMed] [Google Scholar]
- 39.Cha HJ, Yoon HG, Kim YW, Lee HS, Kim JW, Kwon K, Cha SS, Cho MJ, Oh BH, Park KH. Molecular and enzymatic characterization of a maltogenic amylase that hydrolyzes and transglycosylates acarbose. Eur. J. Biochem. 1998;253:251–262. doi: 10.1046/j.1432-1327.1998.2530251.x. [DOI] [PubMed] [Google Scholar]
- 40.Sinnott ML. Catalytic mechanisms of enzymic glycosyl transfer. Chem. Rev. 1990;9:1171–1202. doi: 10.1021/cr00105a006. [DOI] [Google Scholar]
- 41.Bissaro B, Monsan P, Fauré R, O’Donohue MJ. Glycosynthesis in a waterworld: new insight into the molecular basis of transglycosylation in retaining glycoside hydrolases. Biochem J. 2015;467:17–35. doi: 10.1042/BJ20141412. [DOI] [PubMed] [Google Scholar]
- 42.Crout DH, Vic G. Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr. Opin. Chem. Biol.: 98–111 (1998) [DOI] [PubMed]
- 43.Singh S, Packwood J, Samuel CJ, Critchley P, Crout D. Glycosidase-catalyzed oligosaccharide synthesis: preparation of N-actylchitooligosaccharides using the β-N-acetylhexosaminidase of Aspergillus oryzae. Carbohydr. Res. 1995;279:293–305. doi: 10.1016/0008-6215(95)00302-9. [DOI] [PubMed] [Google Scholar]
- 44.Williams S. “Glycoside Hydrolase Family: Transglycosylase” in CAZypedia, available from http://www.cazypedia.org/, Accessed May 1, 2017.
- 45.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaper T, van der Maarel MJEC, Euverink GJ, Dijkhuizen L. Exploring and exploiting starch-modifying amylomaltases from thermophiles. Biochem. Soc. Trans. 2004;32:279–282. doi: 10.1042/bst0320279. [DOI] [PubMed] [Google Scholar]
- 47.Vocadlo DJ, Davies GJ. Mechanistic insights into glycosidase chemistry. Curr. Opin. Chem. Biol. 2008;12:539–555. doi: 10.1016/j.cbpa.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Maarel MJEC, Capron I, Euverink GJW, Bos HT, Kaper T, Binnema DJ, Steeneken PAM. A novel thermostable gelling product made by enzymatic modification of starch. Starch. 2005;57:465–472. doi: 10.1002/star.200500409. [DOI] [Google Scholar]
- 50.He L, Park SH, Nguyen DHD, Duong HX, Duong TPC, Tran PL, Jong-Tae Park JT, Ni L, Park KH. Characterization and thermal inactivation kinetics of highly thermostable ramie leaf β–amylase. Enzyme Microbial. Technol. 2017;101:17–23. doi: 10.1016/j.enzmictec.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Kraus JK, Hebeda RE. Method for retarding staling of baked goods, U.S. Patent: 5,209,938 (1993)
- 52.Shen GJ, Saha BC, Bhatnagar YE, Zeikus J. Purification and characterization of a novel thermostable beta-amylase from Clostridium thermosulphurogenes. Biochem. J. 1988;254:835–840. doi: 10.1042/bj2540835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bang BY, Kim HJ, Kim HY, Baik MY, Ahn SC, Kim JH, Park CS. Cloning and overexpression of 4-α-glucanotransferase from Thermus brockianus (TBGT) in E. coli. J. Microbiol. Biotechnol. 2006;16:1809–1813. [Google Scholar]
- 54.Daba T, Kojima K, Inouye K. Characterization and solvent engineering of wheat -amylase for enhancing its activity and stability. Enzyme Microb. Technol. 2012;51:245–251. doi: 10.1016/j.enzmictec.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Kim JS, Cha SS, Kim HJ, Kim TJ, Ha NC, Oh ST, Cho HS, Kim MJ, Lee HS, Kim JW, Choi KY, Park KH, Oh BH. Crystal structure of a maltogenic amylase: Provides insights into a catalytic versatility. J. Biol. Chem. 1999;274:26279–26286. doi: 10.1074/jbc.274.37.26279. [DOI] [PubMed] [Google Scholar]
- 56.Auh JH, Chae HY, Kim YR, Shim KH, Yoo SH, Park KH. Modification of rice starch by selective degradation of amylose using alkalophilic Bacillus cyclomaltodextrinase. J. Agric. Food Chem. 2006;54:2314–2319. doi: 10.1021/jf051887r. [DOI] [PubMed] [Google Scholar]
- 57.Kamasaka H, Sugimoto K, Takata H, Nishimura T, Kuriki T. Bacillus stearothermophilus neopulluanase selective hydrolysis of amylose to maltose in the presence of amylopectin. Appl. Environ. Microbiol. 2002;68:1658–1664. doi: 10.1128/AEM.68.4.1658-1664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li X, Li D, Tian H, Park, K.H. Reducing retrogradation of gelatinized rice starch and rice meal under low temperature storage by addition of extremely thermostable maltogenic amylase during their cooking. Food Res. Int. 62: 1134–1140 (2014)
- 59.Park SH, Kang HK, Shim JH, Woo EJ, Hong JS, Kim JW, Oh BH, Lee BH, Cha H, Park KH. Modulation of substrate preference of Thermus maltogenic amylase by mutation of the residues at the interface of a dimer. Biosci. Biotechnol. Biochem. 2007;71:1564–1567. doi: 10.1271/bbb.70017. [DOI] [PubMed] [Google Scholar]
- 60.Jobling S. Improving starch for food and industrial applications. Cur. Opinion Plant Biol. 2004;7:210–218. doi: 10.1016/j.pbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Sun YW, Jiao GA, Liu ZP, Zhang X, Li JY, Guo XP, Du WM, Du JL, Francis F, Zhao YD, Xia LQ. Generation of high-amylose rice through CRISPR/Cas9- mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 2017;8:298–312. doi: 10.3389/fpls.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh K, Ozaki H, Okada K, Hori H, Takeda Y, Mitsui T. Introduction of Wx transgene into rice wx mutants leads to both high and low-amylose rice. Plant Cell Physiol. 2003;44:473–480. doi: 10.1093/pcp/pcg068. [DOI] [PubMed] [Google Scholar]
- 63.Wursch P, Gummy D. Inhibition of amylopectin retrogradation by partial beta-amylolysis. Carbohydr. Res. 1994;256:129–137. doi: 10.1016/0008-6215(94)84232-9. [DOI] [PubMed] [Google Scholar]
- 64.Tsujii Y, Nagafuku N, Miyake A, Uchino M, Takano K. Presence and activity of various amylases in rice: Effect on texture and leached sugar composition during cooking. Food Sci. Technol. Res. 2013;19:81–87. doi: 10.3136/fstr.19.81. [DOI] [Google Scholar]
- 65.Miao M, Li R, Huang C, Jiang B, Zhang T. Impact of β-amylase degradation on properties of sugary maize soluble starch particles. Food Chem. 2015;177:1–7. doi: 10.1016/j.foodchem.2014.12.101. [DOI] [PubMed] [Google Scholar]
- 66.Yao Y, Zhang J, Ding X. Partial β-amylolysis retards starch retrogradation in rice products. J. Agric. Food Chem. 2003;51:4066–4071. doi: 10.1021/jf0209488. [DOI] [PubMed] [Google Scholar]
- 67.De Stefani, VA, Turner EW. Modified enzyme system to inhibit bread firming method for preparing same and use of same in bread and other bakery products. U.S. Patent 4 299 848 (1981)
- 68.Sahlstrom S, Brathen E. Effects of enzyme preparations for baking, mixing time and resting time on bread quality and bread staling. Food Chem. 1997;58:75–80. doi: 10.1016/S0308-8146(96)00216-6. [DOI] [Google Scholar]
- 69.Carroll, JO, Boyce COL, Wong TM, Strance CA. Retardation of bread staling and avoidance of gummy mouthfeel by incorporation of 0.25–5 SKB units/100 grams of cereal or bacterial alpha-amylase and 10-50 PUN of a pullulanase per 100 grams of flour in the dough. U.S. Patent 4 654 216 (1987)
- 70.Min BC, Yoon SH, Kim JW, Lee YW, Kim YB, Park KH. Cloning of novel maltooligosaccharide-producing amylases as an antistaling agent for bread. J. Agric. Food Chem. 1998;46:779–782. doi: 10.1021/jf970755y. [DOI] [PubMed] [Google Scholar]
- 71.Karim AA, Sufha EH, Zaidul IS. Dual modification of starch via partial enzymatic hydrolysis in the granular state and subsequent hydroxypropylation. J. Agric. Food Chem. 2008;56:10901–10907. doi: 10.1021/jf8015442. [DOI] [PubMed] [Google Scholar]
- 72.Park JH, Park KH, Jane JL. Physicochemical properties of enzymatically modified maize starch using 4-alpha-glucanotransferase. Food Sci. Biotechnol. 2007;16:902–909. [Google Scholar]
- 73.Park JH, Kim HJ, Kim YH, Cha H, Kim YW, Kim TJ, Kim YR, Park KH. The action mode Thermus aquaticus YT-1 4-alpha-glucanotransferase and its chimeric enzymes introduced with starch-binding domain on amylose and amylopectin. Carbohydr. Polym. 2007;67:164–173. doi: 10.1016/j.carbpol.2006.05.018. [DOI] [Google Scholar]
- 74.Kim YL, Mun S, Park KH, Shim JY, Kim YR. Physicochemical functionality of 4-α-glucanotransferase-treated rice flour in food application. Int. J. Biol. Macromol. 2013;60:422–426. doi: 10.1016/j.ijbiomac.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen DHD, Tran PL, Li D, Han JA, Hwang JY, Hong WS, Lee JS, Kim YR, Yoo SH, Park JT, Choi YJ, Lee SY, Park KH. Modification of rice grain starch for lump-free cooked rice using thermostable disproportionating enzymes. Food Res. Int. 2014;63:55–61. doi: 10.1016/j.foodres.2014.04.007. [DOI] [Google Scholar]
- 76.Diderichsen B, Christiansen L. Cloning of a maltogenic alpha-amylase from Bacillus stearothermophilus. FEMS Lett. Microbiol. 1988;56:53–60. doi: 10.1111/j.1574-6968.1988.tb03149.x. [DOI] [Google Scholar]
- 77.Christophersen C, Otzen DE, Noman BE. Christensen, Schaefer T. Enzymatic characterization of Novamyl, a thermostable α-amylase. Starch. 1998;50:39–45. doi: 10.1002/(SICI)1521-379X(199801)50:1<39::AID-STAR39>3.0.CO;2-S. [DOI] [Google Scholar]
- 78.Dauter Z, Dauter M, Brzozowski AM, Christensen S, Borchert TV, Beier L, Wilson KS, Davies GJ. X-ray structure of Novamyl, the five-domain “maltogenic” α-amylase from Bacllus sterothermophilus: Maltose and acarbose complexes at 1.7Å resolution. Biochemistry. 1999;38:8385–8392. doi: 10.1021/bi990256l. [DOI] [PubMed] [Google Scholar]
- 79.Bier L, Svensen A, Andersen C, Frandsen TP, Borchert TV, Cherry JR. Conversion of the maltogenic α-amylase Novamyl into CGTase. Prot. Eng. 2000;13:500–513. doi: 10.1093/protein/13.7.509. [DOI] [PubMed] [Google Scholar]
- 80.Lee SH, Kim YW, Lee S, Auh JH, Yoo SS, Kim TJ, Kim JW, Kim ST, Rho HJ, Choi JH, Kim YB, Park KH. Modulation of cyclizing activity and thermostability of cyclodextrin glucanotransferase and its application as antistaling enzyme. J. Agric. Food Chem. 2002;50:1411–1415. doi: 10.1021/jf010928q. [DOI] [PubMed] [Google Scholar]
- 81.Shim JH, Kim YW, Kim TJ, Cha HY, Park JH, Cha HJ, Kim JW, Kim YR, Schaefer T, Spendler T, Moon TW, Park KH. Improvement of cyclodextrin glucanotransferase as an anti-staling enzyme by error-prone PCR. Prot. Eng. Design Select. 2004;17:205–211. doi: 10.1093/protein/gzh035. [DOI] [PubMed] [Google Scholar]
- 82.Shim JH, Seo NS, Roh SA, Kim JW, Cha HJ, Park KH. Improved bread-baking process using Saccharomyces cerevisiae displayed with engineered cyclodextrin glucanotransferase. J. Agr. Food Chem. 2007;55:4735–4740. doi: 10.1021/jf070217d. [DOI] [PubMed] [Google Scholar]
- 83.Hansen MR, Blennow A, Pedersenc S, Nørgaarda L, Engelsen SB. Gel texture and chain structure of amylomaltase-modified starches compared to gelatin. Food Hydrocoll. 2008;22:1551–1566. doi: 10.1016/j.foodhyd.2007.10.010. [DOI] [Google Scholar]
- 84.Lee KY, Kim YR, Park KH, Lee HG. 2006. Effects of α-glucanotransferase treatment on the thermo-reversibility and freeze-thaw stability of a rice starch gel. Carbohydr. Polym. 63: 347–354 (2006)
- 85.Viet HD, Mun SH, Kim YL, Rho SJ, Park KH, Kim YR. Novel formulation of low-fat spread using rice starch modified by α-glucanotransferase. Food Chem. 2016;208:132–141. doi: 10.1016/j.foodchem.2016.03.101. [DOI] [PubMed] [Google Scholar]
- 86.Lee CK, Le QT, Kim YH, Shim JH, Lee SJ, Park JH, Lee KP, Song SH, Auh JH, Lee SJ, Park KH. Enzymatic synthesis and properties of highly branched rice starch amylose and amylopectin cluster. J. Agric. Food Chem. 2008;56:126–131. doi: 10.1021/jf072508s. [DOI] [PubMed] [Google Scholar]
- 87.Cho KH, Auh JH, Kim JH, Ryu JH, Park KH. ParkCS, Yoo SH. Effect of amylose content on corn starch modification by Thermus aquaticus 4-α-glucanotransferase. J. Microbiol. Biotechnol. 2009;19:1201–1205. doi: 10.4014/jmb.0811.644. [DOI] [PubMed] [Google Scholar]
- 88.Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ao Z, Sinsek S, Zhang G, Venkatachalam M. Starch with a slow digestion property produced by altering its chain length, brach chain density, and crystalline structure. J. Agric. Food Chem. 2007;55:4540–4547. doi: 10.1021/jf063123x. [DOI] [PubMed] [Google Scholar]
- 90.Jane J, Chen YY, Lee LF, McPherson AE, Wong KS, Radosavljevic M, Kasemsuwan T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999;76:629–637. doi: 10.1094/CCHEM.1999.76.5.629. [DOI] [Google Scholar]
- 91.Kim H, Choi S, Park CS, Moon TW. Low digestion property of amylosucrase-modified waxy adlay starch. Food Sci Biotechnol. 2016;25:457–460. doi: 10.1007/s10068-016-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim HR, Choi SJ, Park CS, Moon TW. Kinetic studies of in vitro digestion of amylosucrase-modified waxy corn starches based on branch chain length distributions. Food Hydrocolloids. 2017;65:46–56. doi: 10.1016/j.foodhyd.2016.10.038. [DOI] [Google Scholar]
- 93.Zhang H, Zhou X, Hea J, Wang T, Xiaohu Luo X, Wang L, Wang R, Chen Z. Impact of amylosucrase modification on the structural and physicochemical properties of native and acid-thinned waxy corn starch. Food Chem. 2017;220:413–419. doi: 10.1016/j.foodchem.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 94.Ryu JH, Lee BH, Seo DH, Baik MY, Park CS, Wang R, Yoo SH. Production and characterization of digestion-resistant starch by the reaction of Neisseria polysaccharea amylosucrase. Starch. 2010;62:221–228. doi: 10.1002/star.200900182. [DOI] [Google Scholar]
- 95.Shin HJ, Choi SJ, Park CS, Moon TW. Preparation of starches with low glycaemic response using amylosucrase and their physicochemical properties. Carbohydr. Polym. 2010;82:489–497. doi: 10.1016/j.carbpol.2010.05.017. [DOI] [Google Scholar]
- 96.Osaki S, Yoshino Z, Tsujisaka Y, Takaku H. Manufacture of oligosaccharides. pp 210–217. In Handbook of amylases and related enzymes: their sources, isolation methods, properties and applications. The amylase research society of Japan, Osaka, Japan (ed.), Pergamon Press, New York (1988)
- 97.Kwon MR, Park CS, Auh JH, Cho BM, Yang NS, Park KH. Phospholipid hydrolysate and antistaling amylase effects on retrogradation of starch in bread. J. Food Sci. 1994;59:1072–1076. doi: 10.1111/j.1365-2621.1994.tb08193.x. [DOI] [Google Scholar]
- 98.Kim IC, Yoo SH, Lee SJ, Oh BH, Kim JW, Park KH. Synthesis of branched oligosaccharides from starch by two amylase cloned from Bacillus licheniformis. Biosci. Biotechnol. Biochem. 1994;58:416–418. doi: 10.1271/bbb.58.416. [DOI] [Google Scholar]
- 99.Kim IC, Cha JH, Kim JR, Jang SY, Seo BC, Cheong TK, Lee DS, Choi YD, Park KH. Catalytic properties of the cloned amylase from Bacillus lichenformis. J. Biol. Chem. 1992;267:22108–22114. [PubMed] [Google Scholar]
- 100.Kim IC, Yoo SH, Lee SJ, Oh BH, Kim JW, Park KH. Synthesis of branched oligosacchareides from starch by two amylases cloned from Bacillus licheniformis. Biosci. Biotech. Biochem. 1994;58:416–418. doi: 10.1271/bbb.58.416. [DOI] [Google Scholar]
- 101.Cheong TK, Kim TJ, Kim MJ, Choi YD. Kim IC, Kim JW, Park KH. Modulation of Bacillus amylolytic enzymes and production of branched oligosaccharides. pp 43–60. In: Enzymes for Carbohydrate Engineering: Progress in Biotechnology, Vol. volume 12, Park KH, Robyt JF, Choi YD (e Ed.) pp 43–60. Elsevier, Amsterdam, Netherland (1996)
- 102.Cuong NP, Lee WH, Oh IN, Thuy NM, Kim DG, Park JT, Park KH. 2016 Continuous production of pure maltodextrin from cyclodextrin using immobilized Pyrococcus furiosus thermostable amylase. Process Biochem. 2016;51:282–287. doi: 10.1016/j.procbio.2015.11.022. [DOI] [Google Scholar]
- 103.Mun S, Kim YL, Kang CG, Park KH, Shim JY, Kim YR. Development of reduced-fat mayonnaise using 4GTase-modified rice starch andxanthan gum. Intl. J. Biol. Macromol. 2009;44:400–407. doi: 10.1016/j.ijbiomac.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Shinohara ML, Ihara M, Abo M, Hashida M, Takagi S, Beck TC. A novel thermostable branching enzyme from an extremely thermophilic bacterial species. Rhodothermus obamensis. Appl. Microbiol. Biotechnol. 2001;57:653–659. doi: 10.1007/s00253-001-0841-3. [DOI] [PubMed] [Google Scholar]
- 105.Takata H, Ohdan K, Takaha T, Kuriki T, Okada S. (2003). Properties of branching enzyme from hyperthermophilic bacterium, Aquifex aeolicus, and its potential for production of highly-branched cyclic dextrin. J. Appl. Glycosci. 50: 15–20 (2003)
- 106.van der Maarel MJEC, Vos A, Sanders P, Dijkhuizen L. (2003). Properties of the glucan branching enzyme of the hyperthermophilic bacterium Aquifex aeolicus. Biocat. Biotrans. 21: 199–207 (2003)
- 107.Palomo M, Kralj S, van der Maarel MJEC, Dijkhuizen L. The unique branching patterns of Deinococcus glycogen branching enzymes are determined by their N-terminal domains. Appl. Environ. Microbiol. 2009;75:1355–1362. doi: 10.1128/AEM.02141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Maarel MJEC, Binnema DJ, Semeijn C, Buwalda PL. Novel slowly digestible storage carbohydrate. EP1943908 (2007)
- 109.Dermaux L, Peptitjean C, Wills D. Soluble, highly branched glucosepolymers for enteral and parenteral nutrition and for peritoneal dialysis. WO2007099212 (2007)
- 110.Le QT, Lee CK, Kim YW, Lee SJ, Zhang R, Withers SG, Kim YR, Auj JH, Park KH. Amylolytically-resistant tapioca starch modified by combined treatment of branching enzyme and maltogenic amylase. Carbohydr. Polym. 2009;75:9–14. doi: 10.1016/j.carbpol.2008.06.001. [DOI] [Google Scholar]
- 111.Xu Y, Huang Q, Fu X, Jane J. Modification of starch octenylsuccinate by β-amylase hydrolysis in order to increase its emulsification properties. Food Hydrocolloid. 2015;48:55–61. doi: 10.1016/j.foodhyd.2015.02.010. [DOI] [Google Scholar]
- 112.Kelly RM, Dijkhuizen L, Leemhuis H. Starch and α-glucan acting enzymes, modulating their properties by directed evolution. J. Biotechnol. 2009;140:184–193. doi: 10.1016/j.jbiotec.2009.01.020. [DOI] [PubMed] [Google Scholar]