Abstract
It has been proposed that the hydrophilic and/or lipophilic characteristics of fatty acid derivatives affect their antibacterial activities according to their ability to incorporate into the bacterial cell membrane. To verify this hypothesis, six kinds of lauric acid derivatives esterified with different non-fatty acid moieties were selected to confirm whether antibacterial activity from their precursor (i.e., lauric acid) is retained or lost. Three compounds, monolaurin, sucrose laurate, and erythorbyl laurate, exerted bacteriostatic and bactericidal effects against Gram-positive bacteria, while the others showed no inhibitory activity. Interestingly, the calculated log P (octanol–water partition coefficient) values of monolaurin, sucrose laurate, and erythorbyl laurate were − 4.122, − 0.686, and 3.670, respectively, relatively lower than those of the other compounds without antibacterial activity. Moreover, the hydrophilic-lipophilic balance values of the three compounds with antibacterial activity were higher than those of the other compounds, corresponding to the log P result.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0353-x) contains supplementary material, which is available to authorized users.
Keywords: Lauric acid esters, Antibacterial activity, Non-fatty acid moiety, Octanol–water partition coefficient, Hydrophilic-lipophilic balance
Introduction
An emulsion is a heterogeneous dispersion of two immiscible liquids (e.g., water and lipid) wherein droplets in the dispersed phase are encapsulated within a continuous phase in the presence of a surface-active agent (i.e., emulsifier) [6, 28]. Lipid oxidation and microbial contamination have been regarded as critical factors affecting the safety (for consumption) and quality deterioration of the emulsion-based products used widely in the food and cosmetic industries [14]. Our research group previously investigated a promising concept for achieving multifunctional emulsifiers, having both antioxidant and antimicrobial activities, which are safe to consume and resistant to deterioration [20]. Under this strategy, erythorbyl laurate (6-O-lauroyl-erythorbic acid) was synthesized through lipase-catalyzed esterification between lauric acid (lipophilic antimicrobial) and erythorbic acid (hydrophilic antioxidant), and was anticipated to be an amphiphilic material with multi-functionalities [21]. Subsequently, we reported that erythorbyl laurate is surface-active and shows higher foaming stability than Tween 20 or Triton X-100. Furthermore, the amphiphilic structure of erythorbyl laurate allowed the antioxidant molecules to be concentrated at the oil–water interface where lipid oxidation occurs, which led to more effective retardation of lipid oxidation in the emulsion [20]. It has also been shown that erythorbyl laurate exerts both bacteriostatic and bactericidal effects on Gram-positive pathogens, including Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus, which might result from alterations in the permeability of the cytoplasmic membrane and bacterial cell wall rupture [19].
Lauric acid, a lipophilic moiety of erythorbyl laurate, is a medium-chain fatty acid with strong antimicrobial activity against a wide range of foodborne pathogens [1, 13]. The antibacterial activity of erythorbyl laurate was derived from its precursor (i.e., lauric acid). Interestingly, several studies have reported that various derivatives of antibacterial fatty acids show increased, reduced, or even no inhibitory activity compared with that of their precursors. Zhao et al. [29] evaluated the antibacterial activities of sugar fatty acid esters with different saccharide moieties and suggested that disaccharide monoesters of capric acid exhibit better antibacterial activity than the monosaccharide monoesters. A study on the methylation of free fatty acids reported that the hydroxyl in the carboxyl group seems to be important in the antibacterial activity of free fatty acids, as the methylated form of antibacterial fatty acids often has reduced or no activity [30]. In addition, lauric acid derivatives esterified with monohydric alcohols or cholesterol have no antibacterial activity [11]. Taken together, it is plausible that differences in non-fatty acid moieties among fatty acid derivatives significantly affect their antibacterial activities, although the underlying mechanism has not yet been clarified.
It is widely accepted that the primary target of antibacterial fatty acids is the bacterial cell membrane [5, 17, 19]. To be more specific, antibacterial fatty acids and their derivatives are incorporated into bacterial cell membranes, thus disrupting the selective permeability of the membranes and in turn exerting bacteriostatic and bactericidal effects on the cells. It has been proposed that hydrophilicity and/or lipophilicity of fatty acid derivatives may be related to interactions with bacterial cell membranes, resulting in antibacterial activity.
The objective of this study was to assess whether hydrophilicity and/or lipophilicity of fatty acid derivatives is critical for their antibacterial activities. As a simple model of fatty acid derivatives with different non-fatty acid moieties, we selected six kinds of lauric acid ester, including newly synthesized sesamol laurate, and investigated the association between hydrophilicity/lipophilicity of the molecules and their antibacterial activity.
Materials and methods
Materials
Sesamol (≥ 98.0%), erythorbic acid (≥ 99.0%), dodecanoic acid (lauric acid ≥ 99.0%), sucrose monolaurate (≥ 97.0%), isoamyl laurate (≥ 97.0%), and methyl laurate (≥ 99.5%) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Novozym®435 (i.e., lipase from Candida antarctica immobilized on macroporous acrylic resin; approximate density, 0.40 g/mL) with catalytic activity of 7000 PLU/g (1 PLU is the amount of enzyme that synthesizes 1 μ mol/min propyl laurate at 60 °C) was kindly provided by Novozymes (Bagsvaerd, Denmark). High-performance liquid chromatography (HPLC)-grade acetonitrile (J. T. Baker Co., Phillipsburg, NJ, USA) was dehydrated using 4Å molecular sieves (8–12 mesh; Sigma-Aldrich Co.) and filtered through a membrane filter (0.45 µm) prior to use. All other chemicals were of extra pure grade and were used without further purification.
Enzymatic synthesis and identification of sesamol laurate
Sesamol (0.6 mmol) and lauric acid (3.0 mmol) were added to a crimped-top glass vial containing 15 mL acetonitrile. A pre-incubation was performed at 70 °C for 15 min in a water bath with a magnetic stirrer (200 rpm) to dissolve both substrates in the acetonitrile. The reaction was initiated by adding immobilized lipase (150 mg, 1050 PLU) to the mixture. Temperature was maintained at 70 ± 1 °C during the entire reaction. After the enzymatic reaction was terminated, sesamol laurate was purified according to the method reported previously, with a slight modification [12, 25, 27]. The reaction mixture was filtered through a 0.45 µm-membrane filter to separate the immobilized lipase, and then lyophilized at − 76 °C to remove acetonitrile. The concentrate was washed three times with 10 mL n-hexane, and the supernatant was discarded to eliminate the residual lauric acid after centrifugation at 8000×g. Then, sesamol laurate was isolated from the mixture containing sesamol using preparative HPLC (LC-918; Japan Analytical Industry Co., Ltd., Tokyo, Japan) equipped with poly (vinyl alcohol) gel columns (500 × 20 mm, JAIGEL GS-510; Japan Analytical Industry Co., Ltd.). The mobile phase was acetonitrile/water (90:5, v/v) at a flow rate of 5.0 mL/min. The peak corresponding to sesamol laurate was separated by recycling and collected through a fraction nozzle. The sesamol laurate was identified by liquid chromatography-electrospray ionization-mass spectroscopy (LC–ESI–MS) (Thermo Finnigan, San Jose, CA, USA).
Quantitative analysis of sesamol laurate
Quantitative analysis of synthesized sesamol laurate was carried out using an HPLC instrument (LC-2002; Jasco Inc., Tokyo, Japan) equipped with a silica-based column (5 µm, I.D. 4.6 mm × 150 mm: Luna C18; Phenomenex, Torrance, CA, USA), a refractive index (RI) detector (RI-2031; Jasco Inc.), and an ultraviolet (UV) detector (UV-2075; Jasco Inc.). The mobile phase was acetonitrile: water: acetic acid (90:5:5, v/v/v) at a flow rate of 1.0 mL/min. Sesamol and sesamol laurate were detected using the UV detector at 290 nm, and lauric acid was detected using the RI detector. Peaks in the chromatograms were identified according to the retention times of the sesamol and lauric acid standards.
Preparation of erythorbyl laurate
Enzymatic synthesis, purification, and identification of erythorbyl laurate were performed according to our previous methods [20, 21]. Erythorbic acid (0.12 mmol) and lauric acid (0.60 mmol) were mixed in a glass vial with 20 mL acetonitrile. After pre-incubating the mixture at 50 °C for 30 min in an orbital shaking water bath (200 rpm), the reaction was initiated by adding the immobilized lipase (200 mg, 1400 PLU) to the mixture. After terminating the reaction, erythorbyl laurate was obtained by the solvent separation method. Quantitative analysis and identification were performed using the HPLC instrument equipped with a Spherisorb-ODS column (5 μm, 100 Å, I.D. 4.6 × 250 mm; Waters, Milford, MA, USA) and a matrix-assisted laser desorption/ionization time-of-flight system (Auto Flex II; Bruker Daltonics, Bremen, Germany).
Bacterial strains and culture conditions
The Gram-positive strains assessed in this study were Staphylococcus aureus ATCC 49444 and Listeria monocytogenes ATCC 7644. The Gram-negative strains were Escherichia coli ATCC 43889 and Salmonella enterica serovar Typhimurium (S. Typhimurium) ATCC 43971. The microorganisms were cultured in tryptic soy broth (TSB) at 37 °C for 12–18 h to yield 108 colony-forming units per milliliter (CFU/mL).
Evaluation of antibacterial activities
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the lauric acid esters were determined by the broth micro-dilution method to compare the antibacterial activities of the lauric acid esters [3, 15, 18, 26]. Serial dilutions were prepared to generate the desired concentrations of lauric acid esters in sterile TSB to a final volume of 100 µL in a 96-well plate (Corning Inc., Corning, NY, USA). Then, each well was inoculated with 100 µL of test microorganisms in TSB at a final concentration of 5.0 × 105 CFU/mL. The MICs were defined as the lowest concentration of lauric acid esters that inhibited growth of the bacteria after a 12-h incubation at 37 °C, and the MBC was defined as the lowest concentration leading to a 99.9% reduction in viable bacterial count in the subcultured well contents relative to the initial inoculum [22].
Octanol–water partition coefficient (log P)
The atom/fragment contribution method was used to estimate the octanol–water partition coefficient (log P) for evaluating the hydrophilicity of lauric acid esters [16]. The log P values of the esters were calculated by summing all atom/fragment contribution values (Supplementary Data S1) and correction factors (Supplementary Data S2) corresponding to the chemical structure of the esters using the following equation:
where fi is the coefficient for each atom/fragment, cj is the coefficient for each correction factor, and ni and nj represent the number of times the atom/fragment and correction factor occur in the chemical structure.
Hydrophilic-lipophilic balance (HLB)
The HLB values, which represent a numerical correlation of the emulsifying and solubilizing properties of surface active agents between the water and oil phases, were determined by the Davies method [7]. HLB values were calculated from the chemical structures by summing the group numbers of the hydrophilic and lipophilic groups (Supplementary Data S3). The equation for the calculation was:
Statistical analysis
All data are presented as mean ± SD of triplicate experiments. Analysis of variance was performed and differences in means were detected with Duncan’s multiple range test (p < 0.05). All statistical analyses were done using SAS 9.3 software (SAS Institute, Cary, NC, USA).
Results and discussion
Enzymatic synthesis and identification of sesamol laurate
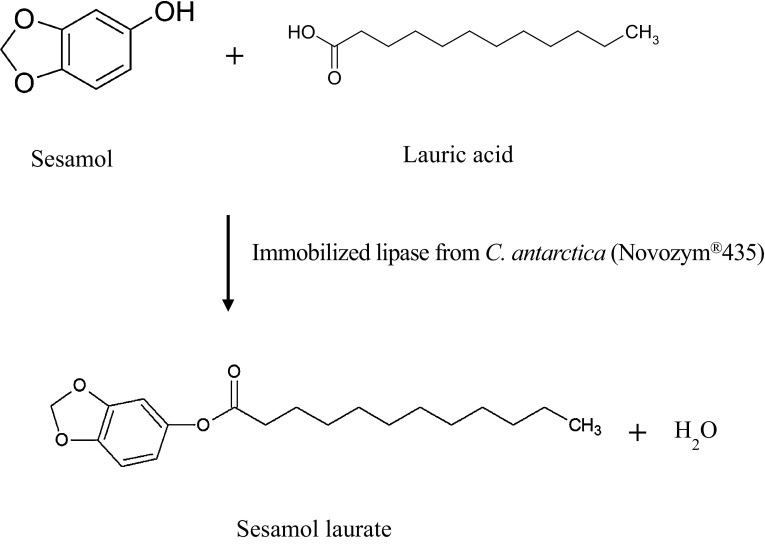
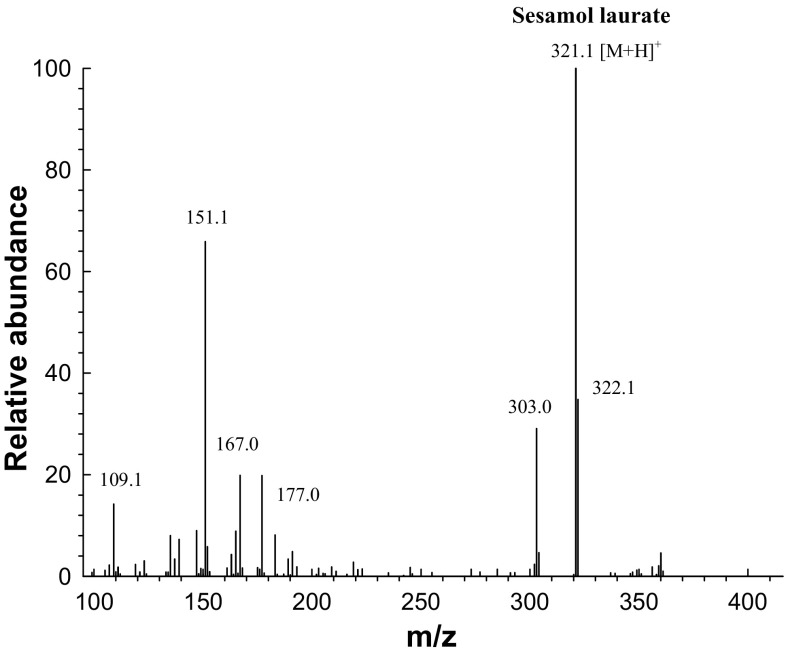
A novel derivative of lauric acid, sesamol laurate, was synthesized by lipase-catalyzed esterification in acetonitrile medium (Fig. 1). To monitor the enzymatic esterification between sesamol and lauric acid over time, the esterification products were analyzed at predetermined time intervals by HPLC. Typical HPLC chromatograms of each component of lipase-catalyzed esterification are represented in Supplementary Figure S4. The retention times of sesamol, lauric acid, and the resulting sesamol laurate were 1.76 ± 0.01, 3.75 ± 0.02, and 5.76 ± 0.10 min, respectively. After the preparative LC purification procedure, the resulting sesamol laurate obtained from the enzymatic esterification was identified by LC–ESI–MS. Mass spectrometry in full mode confirmed the presence of sesamol laurate. The spectra gave a molecular ion at m/z = 321.1 [M + H]+, corresponding exactly to the molecular mass of sesamol laurate (Fig. 2).
Fig. 1.
Scheme of lipase-catalyzed synthesis of sesamol laurate in acetonitrile
Fig. 2.
Liquid chromatography electrospray ionization mass spectrum of sesamol laurate synthesized through the lipase-catalyzed esterification (full scan mode)
Comparative evaluation of the antibacterial activities of lauric acid esters
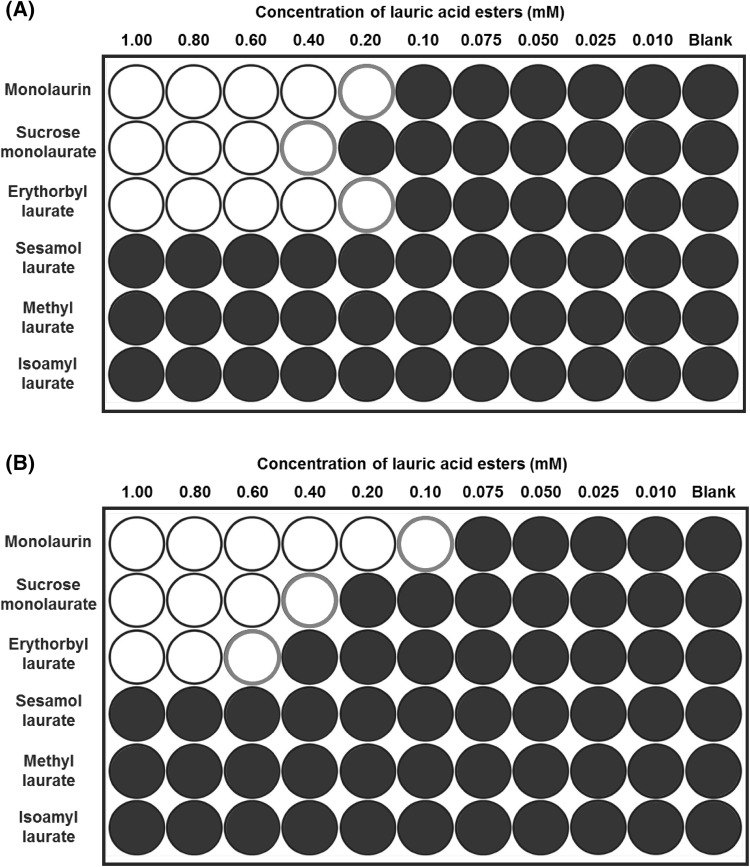
Six kinds of lauric acid derivative esterified with different molecules were tested to determine whether antibacterial activity from their precursor (i.e., lauric acid) was retained or lost (Table 1). The antibacterial activities of the lauric acid esters were evaluated in terms of both their bacteriostatic and bactericidal effects against foodborne pathogens, including Gram-positive (Staphylococcus and Listeria. spp.) and Gram-negative bacteria (Salmonella and Escherichia spp.). Figure 3 represents the summarized and symbolized results from the growth curves of selected bacteria treated with each laurate acid derivative (concentrations of 0.01–1.0 mM). The filled circles in Fig. 3 indicate a normal growth curve of the bacterium without inhibition by treatment, whereas the open circles represent bacteriostatic inhibition (no growth of the bacterium during the incubation period). As anticipated, none of the six lauric acid derivatives showed an inhibitory effect on Gram-negative bacteria, including S. Typhimurium or E. coli (data not shown). This result was entirely consistent with previous findings that lauric acid and its derivatives have selectively inhibitory activity against Gram-positive bacteria, but do not inhibit Gram-negative bacteria [4, 19].
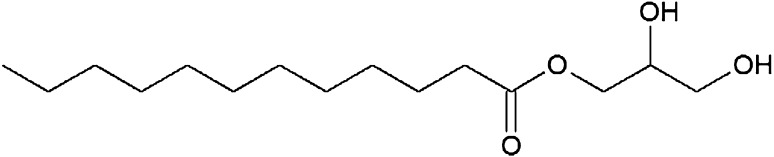
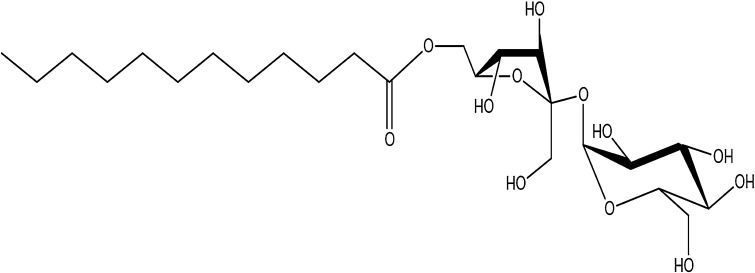
Table 1.
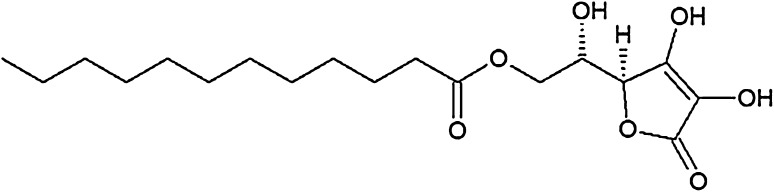
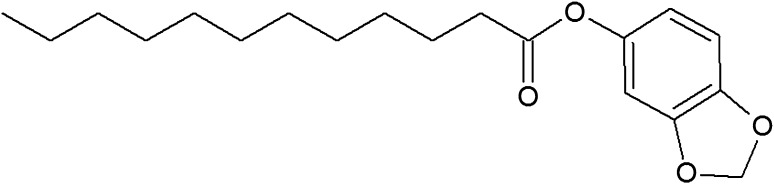
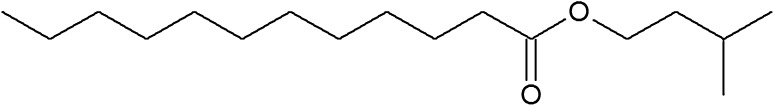
The chemical structure of lauric acid esters with different non-fatty acid moieties
Fig. 3.
Micro-dilution assay for determining the antibacterial susceptibility of lauric acid esters against Staphylococcus aureus ATCC 49444 (A) and Listeria monocytogenes ATCC 7644 (B). Filled circles indicate normal growth curves of the bacterium without inhibition by the treatment, whereas the open circles indicate bacteriostatic inhibition (no significant growth during the incubation period, changes in OD600 less than 0.1)
It is noteworthy that three of the six derivatives of lauric acid showed bacteriostatic effects against Gram-positive bacteria, such as S. aureus and L. monocytogenes, while the others had no inhibitory activity against the same bacteria. For example, the MICs of monolaurin, sucrose monolaurate, and erythorbyl laurate against L. monocytogenes (ATCC 7644) were 0.1, 0.4, and 0.6 mM, respectively. In addition, all three compounds at concentrations of 0.2–0.4 mM inhibited the growth of S. aureus (ATCC 49444). In contrast, sesamol laurate, methyl laurate, and isoamyl laurate did not show bacteriostatic effects against either strain of Gram-positive bacteria.
Interestingly, this tendency could be used to divide the compounds into two categories in terms of their inhibitory effect on Gram-positive bacteria. As shown in Fig. 4, the MBCs of monolaurin, sucrose monolaurate, and erythorbyl laurate were 0.1, 0.6, and 1.0 mM, respectively, against L. monocytogenes (ATCC 7644). Similarly, these compounds at concentrations of 0.2–0.4 mM showed bactericidal activities against S. aureus (ATCC 49444). On the other hand, sesamol laurate, methyl laurate, and isoamyl laurate did not show any bactericidal or bacteriostatic effects against either Gram-positive bacteria.
Fig. 4.
Minimum bactericidal concentration (MBC) test of lauric acid esters against Staphylococcus aureus ATCC 49444 (A) and Listeria monocytogenes ATCC 7644 (B)
The difference in non-fatty acid moieties among the lauric acid esters significantly affected their antibacterial activities; this result is in accordance with previous findings and the resulting hypotheses [11, 29, 30]. Hence, it was attempted to determine what factors are critical for retaining or losing the antibacterial activity derived from the precursor (i.e., lauric acid).
Critical factors affecting the antibacterial activity of lauric acid esters
The results of the susceptibility screening in this study revealed that lauric acid esters exerted selective antibacterial activity against Gram-positive, but not Gram-negative bacteria. This could be due to structural differences in bacterial membranes. Moreover, previous studies on the antibacterial mechanisms of fatty acids and their derivatives indicated that bacterial cell membranes are the primary target [5, 17, 19]. More specifically, antibacterial free fatty acids and their derivatives can be incorporated into cell membranes, leading to a disrupted electron transport chain and oxidative phosphorylation. Regarding the interaction between fatty acid derivatives and cell membranes, it has been assumed that the hydrophilicity and/or lipophilicity of lauric acid derivatives esterified with different non-fatty acid moieties affects their antibacterial activities according to their ability to be incorporated into the bacterial cell membrane. This hypothesis was partly based on the report by Kabara et al. [10] suggesting that the mechanism underlying the bactericidal action of long chain fatty acids and their derivatives is related to the balance between the hydrophilic and hydrophobic parts of the molecule.
To verify this hypothesis, log P and HLB values were adopted as the criteria for assessing the degree of hydrophilicity and/or lipophilicity of the lauric acid esters in this study. The octanol–water partition coefficient (log P) is a physical property used extensively to describe a chemical’s lipophilic or hydrophobic properties: it is the ratio of a chemical’s concentration in the octanol phase to its concentration in the aqueous phase of a two-phase system at equilibrium, which was estimated using the atom/fragment contribution method of Meylan and Howard [16]. Many studies have indicated that log P is useful for correlating a drug’s transport processes, its interactions with receptor molecules, and its observed changes with structure and various biological, biochemical, or toxic effects [8, 24]. As a result of calculating log P by the above method, log P values of antibacterial compounds, such as sucrose monolaurate, erythorbyl laurate, and monolaurin, were lower than those of the other compounds without antibacterial activity (Table 2). A low log P value corresponds to low lipophilicity of the molecules. The low lipophilicity of the non-fatty acid moiety was favorable for interacting with bacterial cell membranes (i.e., resulting in antibacterial activity).
Table 2.
Estimates of log P by the atom/fragment contribution method and of HLB by the Davies method
| Compounds | Log P1 | HLB2 | Antimicrobial activity |
|---|---|---|---|
| Sucrose monolaurate | − 4.122 | 16.090 | Bacteriostatic/bactericidal activities |
| Erythorbyl laurate | − 0.686 | 15.250 | |
| Monolaurin | 3.670 | 7.025 | |
| Methyl laurate | 5.284 | 3.700 | No activity |
| Sesamol laurate | 5.717 | 4.835 | |
| Isoamyl laurate | 7.175 | 1.800 |
1Partition coefficient (P), the ratio of concentrations of a compound in a mixture of two immiscible phases at equilibrium
2Hydrophilic-lipophilic balance (HLB), a measure of the degree to which a compound is hydrophilic or lipophilic
In terms of hydrophilicity of lauric acid HLB was estimated according to the Davies method, which is based on the hydrophilic group numbers assigned to various structural elements [23]. As expected, the HLB values of sucrose monolaurate, erythorbyl laurate, and monolaurin were 16.09, 15.25, and 7.03, respectively, which were relatively higher than those of the other compounds with no antibacterial activity (Table 2). As a high HLB theoretically corresponds to a low log P, this result derived from the HLB estimate can be understood in the same context as that of the log P calculation. It has been widely accepted that the antibacterial mechanisms of free fatty acids and their derivatives are mainly based on incorporation into bacterial cell membranes; hence, accounting for changes in membrane permeability, leakage of vital cell components, and the resulting growth inhibition or cell lysis [2, 9]. Therefore, we propose that lauric acid esters with relatively higher HLB and/or lower log P values facilitate incorporation into bacterial cell membranes, leading to bacteriostatic and/or bactericidal activities. In conclusion, these results suggest that hydrophilicity and/or lipophilicity of non-fatty acid moieties in lauric acid derivatives could be significant factors affecting those antibacterial activities.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (NRF-2017R1A2B4009230) and the Ministry of Education (NRF-R1A6A3A01012396).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Altieri C, Bevilacqua A, Cardillo D, Sinigaglia M. Effectiveness of fatty acids and their monoglycerides against gram-negative pathogens. Int J Food Sci Tech. 2009;44:359–366. doi: 10.1111/j.1365-2621.2008.01744.x. [DOI] [Google Scholar]
- 2.Arouri A, Mouritsen OG. Membrane-perturbing effect of fatty acids and lysolipids. Prog Lipid Res. 2013;52:130–140. doi: 10.1016/j.plipres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Bechert T, Steinrücke P, Guggenbichler J-P. A new method for screening anti-infective biomaterials. Nat Med. 2000;6:1053–1056. doi: 10.1038/79568. [DOI] [PubMed] [Google Scholar]
- 4.Dayrit FM. The Properties of Lauric Acid and Their Significance in Coconut Oil. J Am Oil Chem Soc. 2015;92:1–15. doi: 10.1007/s11746-014-2562-7. [DOI] [Google Scholar]
- 5.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 6.Friberg S, Larsson K, Sjoblom J. Food emulsions. 4. Boca Raton: CRC Press; 2003. [Google Scholar]
- 7.Guo X, Rong Z, Ying X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J Colloid Interface Sci. 2006;298:441–450. doi: 10.1016/j.jcis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins AL, Keserü GM, Leeson PD, Rees DC, Reynolds CH. The role of ligand efficiency metrics in drug discovery. Nat Rev Drug Discov. 2014;13:105. doi: 10.1038/nrd4163. [DOI] [PubMed] [Google Scholar]
- 9.Hyldgaard M, Sutherland DS, Sundh M, Mygind T, Meyer RL. Antimicrobial Mechanism of Monocaprylate. Appl Environ Microbiol. 2012;78:2957–2965. doi: 10.1128/AEM.07224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabara J, Vrable R, Jie MLK. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- 11.Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/AAC.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karmee SK. Lipase catalyzed synthesis of ester-based surfactants from biomass derivatives. Biofuels, Bioprod Biorefin. 2008;2:144–154. doi: 10.1002/bbb.60. [DOI] [Google Scholar]
- 13.Lieberman S, Enig MG, Preuss HG. A review of monolaurin and lauric acid: natural virucidal and bactericidal agents. Alternative and Complementary Therapies. 2006;12:310–314. doi: 10.1089/act.2006.12.310. [DOI] [Google Scholar]
- 14.Luther M, Parry J, Moore J, Meng J, Zhang Y, Cheng Z, Yu LL. Inhibitory effect of Chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 2007;104:1065–1073. doi: 10.1016/j.foodchem.2007.01.034. [DOI] [Google Scholar]
- 15.Magalhães L, Nitschke M. Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control. 2013;29:138–142. doi: 10.1016/j.foodcont.2012.06.009. [DOI] [Google Scholar]
- 16.Meylan WM, Howard PH. Atom/fragment contribution method for estimating octanol–water partition coefficients. J Pharm Sci. 1995;84:83–92. doi: 10.1002/jps.2600840120. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsuji T, Kao MC, Fang J-Y, Zouboulis CC, Zhang L, Gallo RL, Huang C-M. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobmann P, Bourke P, Dunne J, Henehan G. In vitro antimicrobial activity and mechanism of action of novel carbohydrate fatty acid derivatives against Staphylococcus aureus and MRSA. J Appl Microbiol. 2010;108:2152–2161. doi: 10.1111/j.1365-2672.2009.04622.x. [DOI] [PubMed] [Google Scholar]
- 19.Park K-M, Jo S-K, Yu H, Park J-Y, Choi SJ, Lee CJ, Chang P-S. Erythorbyl laurate as a potential food additive with multi-functionalities: antibacterial activity and mode of action. Food Control. 2018;86:138–145. doi: 10.1016/j.foodcont.2017.11.008. [DOI] [Google Scholar]
- 20.Park K-M, Lee MJ, Jo S-K, Choi SJ, Lee J, Chang P-S. Erythorbyl laurate as a potential food additive with multi-functionalities: interfacial characteristics and antioxidant activity. Food Chem. 2017;215:101–107. doi: 10.1016/j.foodchem.2016.07.174. [DOI] [PubMed] [Google Scholar]
- 21.Park K-M, Sung H, Lee J, Chang P-S. Lipase-catalysed synthesis of erythorbyl laurate in acetonitrile. Food Chem. 2011;129:59–63. doi: 10.1016/j.foodchem.2011.04.019. [DOI] [Google Scholar]
- 22.Pridmore A, Burch D, Lees P. Determination of minimum inhibitory and minimum bactericidal concentrations of tiamulin against field isolates of Actinobacillus pleuropneumoniae. Vet Microbiol. 2011;151:409–412. doi: 10.1016/j.vetmic.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Royer M, Nollet M, Catté M, Collinet M, Pierlot C. Towards a new universal way to describe the required hydrophilic lipophilic balance of oils using the phase inversion temperature of C10E4/n-octane/water emulsions. Colloids Surf Physicochem Eng Aspects. 2018;536:165–171. doi: 10.1016/j.colsurfa.2017.07.024. [DOI] [Google Scholar]
- 24.Sabljic A, Guesten H, Hermens J, Opperhuizen A. Modeling octanol/water partition coefficients by molecular topology: chlorinated benzenes and biphenyls. Environ Sci Technol. 1993;27:1394–1402. doi: 10.1021/es00044a015. [DOI] [Google Scholar]
- 25.Watanabe Y, Ishido E, Fang X, Adachi S, Matsuno R. Oxidation kinetics of linoleic acid in the presence of saturated acyl l-ascorbate. J Am Oil Chem Soc. 2005;82:389–392. doi: 10.1007/s11746-005-1082-5. [DOI] [Google Scholar]
- 26.Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, Bornscheuer UT, Schmid RD. Lipase-catalyzed synthesis of vitamin C fatty acid esters. Biotechnol Lett. 1999;21:1051–1054. doi: 10.1023/A:1005620125533. [DOI] [Google Scholar]
- 28.Yi B, Kim M-J, Lee J. Effects of emulsifier charges on the oxidative stability in oil-in-water emulsions under riboflavin photosensitization. Food Sci Biotechnol. 2016;25:1003–1009. doi: 10.1007/s10068-016-0162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao L, Zhang H, Hao T, Li S. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem. 2015;187:370–377. doi: 10.1016/j.foodchem.2015.04.108. [DOI] [PubMed] [Google Scholar]
- 30.Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579:5157–5162. doi: 10.1016/j.febslet.2005.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.