Abstract
Background
Factor Xa (FXa) inhibitors, used for stroke prevention in atrial fibrillation and venous thromboembolism treatment and prevention, are the dominant non-Vitamin K oral anticoagulants on the market. While major bleeding may be less common with these agents compared to warfarin, it is always a risk, and little has been published on the most serious bleeding scenarios. This study describes a cohort of patients with FXa inhibitor-associated life-threatening bleeding events, their clinical characteristics, interventions and outcomes.
Methods
We performed a retrospective, 5-center review of FXa inhibitor-treated major bleeding patients. Investigators identified potential cases by cross-referencing ICD-9/10 codes for hemorrhage with medication lists. Investigators selected cases they deemed to require immediate reversal of coagulopathy, and reviewed charts for characteristics, reversal strategies and other interventions, and outcomes.
Results
A total of 56 charts met the inclusion criteria for the retrospective cohort, including 29 (52%) gastrointestinal bleeds (GIB), 19 (34%) intracranial hemorrhages (ICH) and 8 (14%) others. Twenty-four (43%) patients received various factor or plasma products, and the remainder received supportive care. Thirty-day mortality was 21% (n = 12). Re-anticoagulation within 30-days occurred in 23 (41%) patients. Thromboembolic events (TEEs) occurred in 6 (11%) patients. No differences were observed in outcomes by treatment strategy.
Conclusions
This cohort of FXa inhibitor-associated major bleeding scenarios deemed appropriate for acute anticoagulant reversal illustrates the variable approaches in the absence of a specific reversal agent.
1. Introduction
Non-Vitamin K oral anticoagulants, including the direct thrombin inhibitor dabigatran (Pradaxa®) and the oral Factor Xa (FXa) inhibitors rivaroxaban (Xarelto®), apixaban (Eliquis®) and edoxaban (Savaysa®), are rapidly replacing Vitamin K antagonists (VKAs) as the mainstay for stroke prevention in atrial fibrillation and treatment of venous thromboembolism (VTE) [1]. These drugs are easier to manage than VKAs as they do not require routine monitoring of the international normalized ratio and are associated with less intracranial hemorrhage (ICH) [2–5]. Meta-analyses suggest superiority in both safety and efficacy of the newer drugs over VKAs; [6,7] however, serious bleeding still occurs, with an estimated 80,000 cases of major hemorrhage in the U.S. annually on FXa inhibitors alone [8].
There are no currently available agents for reversal of anticoagulation effects of FXa inhibitors outside of clinical trials. When trial enrollment is not an option, clinicians who manage patients with FXa inhibitor-associated major hemorrhage are left to use various factor products with even less safety data or supportive care alone [9]. Little is known about the clinical characteristics, interventions and outcomes of these critically ill bleeding patients outside clinical trials.
The primary aim of this study was to characterize the natural history of a multi-center cohort of critically ill FXa inhibitor-associated major hemorrhage patients, the clinical interventions used and their 30-day post-death/discharge outcomes. This cohort provides a historical control group that may no longer be obtainable in the near future when targeted FXa inhibitor reversal agents are likely to be approved and in use.
2. Methods
2.1. Data source
Emergency physicians and neurologists from 5 geographically dispersed U.S. medical centers participated in this retrospective chart review study. Investigators identified potential charts from electronic medical records by cross-referencing medication lists and hemorrhage International Classification of Diseases (ICD)-9/10 codes.
2.2. Eligibility criteria
Inclusion criteria included: 1) anticoagulation initiated via direct or indirect FXa inhibitor limited to apixaban, rivaroxaban or low molecular weight heparin; and 2) admitted to the hospital on or after January 1, 2014 for a serious/life-threatening acute major bleeding episode that would be indicated for immediate reversal of anticoagulation as determined by the investigator.
Acute major bleeding was defined using a modified version of the International Society on Thrombosis and Haemostasis (ISTH) bleeding criteria [10].
2.3. Outcomes
The primary objectives were to describe the patient characteristics, clinical approach, and outcomes in severe FXa inhibitor-associated major bleeding.
2.4. Quality control
We confirmed precision of the chart review through standardized quality control procedures involving re-abstraction of 10% of charts by a second provider at each site not participating in the initial review and calculated inter-rater reliability reporting a kappa statistic. Inter-rater reliability was conducted on six key variables in the study including patient diagnosis, exposure to concomitant antiplatelets, utilization of coagulation factor products or plasma to treat the bleeding event, 30-day mortality, 30-day re-anticoagulation and occurrence of thromboembolic events.
2.5. Statistical analysis
We analyzed patient demographics, clinical characteristics, resource utilization and outcomes descriptively. We summarized dichotomous and categorical variables using counts and proportions and tested for significance using Fisher’s exact test. We summarized continuous variables using means and standard deviations (SD) or medians and inter-quartile ranges (IQR), where appropriate, and tested for significance using the Kruskal-Wallis test. All analyses were conducted using IBM SPSS software, version 24.
3. Results
3.1. Physician and patient characteristics
Physician investigators from 5 medical centers (see Table S1 for overview of medical centers) submitted data from 56 patients with serious/life-threatening acute major bleeding. Index admission dates ranged from January 2014 to April 2016.
The mean (±SD) age of the enrolled patients was 76 ± 12 years. Of these patients, 77% (43/56) were non-Hispanic Caucasian and 93% (52/56) were government insured. These patients had significant comorbidities, including congestive heart failure (36%; n = 20), pulmonary disease/asthma (27%; n = 15), cancer (16%; n = 9) and renal disease (13%; n = 7), with a mean Charlson Comorbidity Index score of 2 (Tables 1 and S2) [11].
Table 1.
Characteristics of the patients at baseline.
| Characteristica | Retrospective chart review (n = 56) |
ANNEXA-4 safety population (n = 67) |
|---|---|---|
| Age –yr | 75.6 ± 11.5 | 77.1 ± 10.0 |
| Male sex –no. (%) | 33 (59) | 35 (52) |
| White race –no. (%) | 43 (77) | 54 (81) |
| Estimated creatinine clearance –no. (%) | ||
| <30 mL/min | 7 (13) | 6 (9) |
| 30 to <60 mL/min | 24 (43) | 31 (46) |
| ≥60 mL/min | 25 (45) | 26 (39) |
| Unknown | 0 (0) | 4 (6) |
| Indication for anticoagulation –no. (%) | ||
| Atrial fibrillation | 38 (68) | 47 (70) |
| Venous thromboembolism | 15 (27) | 15 (22) |
| Atrial fibrillation and venous thromboembolism | 1 (2) | 5 (7) |
| Valve repair | 1 (2) | 0 (0) |
| Unknown | 1 (2) | 0 (0) |
| Medical history –no. (%) | ||
| Myocardial infarction | 10 (18) | 13 (19) |
| Stroke | 10 (18) | 17 (25) |
| Deep-vein thrombosis | 9 (16) | 20 (30) |
| Pulmonary embolism | 7 (13) | 6 (9) |
| Atrial fibrillation | 38 (68) | 49 (73) |
| Congestive heart failure | 20 (36) | 23 (34) |
| Diabetes mellitus | 20 (36) | 23 (34) |
| Factor Xa inhibitor | ||
| Rivaroxaban | ||
| Patients –no. (%) | 38 (68) | 32 (48) |
| Median daily dose (IQR) –mg | 15 (15–20) | 20 (15–20) |
| Time from last dose to andexanet bolus or index hospitalization –hr | 15.1 ± 4.9 | 12.8 ± 4.2 |
| Apixaban | ||
| Patients –no. (%) | 12 (21) | 31 (46) |
| Median daily dose (IQR) –mg | 5 (5–10) | 5 (5–10) |
| Time from last dose to andexanet bolus or index hospitalization –hr | 16.7 ± 8.6 | 12.1 ± 4.7 |
| Enoxaparin | ||
| Patients –no. (%) | 6 (11) | 4 (6) |
| Median daily dose (IQR) –mg | 160 (130–180) | 90 (80–150) |
| Time from last dose to andexanet bolus or index hospitalization –hr | 12.4 ± 3.0 | 10.8 ± 3.5 |
| Concomitant antiplatelets with DOACsb –no. (%) | 22 (44) | N/A |
| Aspirin | 20 (40) | N/A |
| Clopidogrel | 5 (10) | N/A |
| Both antiplatelets | 3 (6) | N/A |
Plus-minus values are means ± standard deviations. Percentages may not total 100 because of rounding. IQR denotes interquartile range.
DOACs: Direct-acting oral anticoagulants, include rivaroxaban and apixaban.
3.2. Location of bleeding and presentation of bleeding
The index hemorrhage sites for acute major bleed patients were 29 (52%) gastrointestinal bleeds (GIB), 19 (34%) intracranial hemorrhages (34%) and 8 (14%) “others”. The “other” bleeding locations consisted of 2 intra-abdominal, 2 respiratory tract, 1 nasal, 1 pericardial, 1 skin and 1 urinary tract. Upper gastrointestinal tract bleeds were observed in 38% (11/29) of patients with GIBs, mostly in the stomach (n = 6); 10% (3/29) were lower gastrointestinal bleeds (in the remaining 15 GIBs the location of bleeding was not reported). At hospital presentation, 10% (3/29) of patients with GIBs had systolic blood pressure < 90 mm Hg and 21% (6/29) had poor skin perfusion. Laboratory scores for these patients generally showed low hemoglobin levels, with 86% (25/29) of patients having hemoglobin levels ≤10 g/dL (Table 2).
Table 2.
Characteristics of acute major bleeding episodes
| Characteristica | Retrospective chart review (n = 56) |
ANNEXA-4 safety population (n = 67) |
|---|---|---|
| Gastrointestinal bleeding –no./total no. (%) | 29/56 (52) | 33/67 (49) |
| Patients receiving rivaroxaban or apixaban | 28/29 (97) | 31/33 (94) |
| Site of bleeding | ||
| Upper gastrointestinal tract | 11/29 (38) | 9/33 (27) |
| Lower gastrointestinal tract | 3/29 (10) | 10/33 (30) |
| Unknown | 15/29 (52) | 14/33 (42) |
| Baseline hemoglobin ≤10 g/dl | 25/29 (86) | 20/33 (61) |
| Systolic blood pressure < 90 mm Hg | 3/29 (10) | N/A |
| Intracranial hemorrhage –no./total no. (%) | 19/56 (34) | 28/67 (42) |
| Patients receiving rivaroxaban or apixaban | 17/19 (89) | 27/28 (96) |
| Intracerebral site | 14/19 (74) | 14/28 (50) |
| Hematoma volume | ||
| ≤ 10 mL | 4/14 (29) | 8/14 (57) |
| 11–60 mL | 7/14 (50) | 6/14 (43) |
| > 60 mL | 3/14 (21) | 0/14 (0) |
| Subdural site | 2/19 (11) | 11/28 (39) |
| Subarachnoid site | 3/19 (16) | 3/28 (11) |
| Other bleeding site –no./total no. (%) | 8/56 (14) | 6/67 (9) |
| Patients receiving rivaroxaban or apixaban | 5/8 (63) | 6/6 (100) |
| Site of bleeding | ||
| Nasal | 1/8 (13) | 1/6 (17) |
| Pericardial, pleural or retroperitoneal | 3/8 (38) | 3/6 (50) |
| Genital or urinary | 1/8 (13) | 1/6 (17) |
| Other bleeding site | 3/8 (38) | 1/6 (17) |
Percentages may not total 100 because of rounding.
The type of ICH was reported in 84% (16/19) of patients. Of the known causes, 10 were non-traumatic and 6 were traumatic. Six of the 10 non-trauma ICHs were intraparenchymal, 3 of 10 were hemorrhagic transformations of ischemic stroke and 1 of 10 was an aneurysmal subarachnoid hemorrhage. The 6 traumatic ICHs were equally distributed between subdural hemorrhage, subarachnoid hemorrhage and intraparenchymal contusion (n = 2 each).
3.3. Treatment exposure and presentation of bleeding
Thirty-eight (68%) patients were on rivaroxaban, 12 (21%) were on apixaban and 6 (11%) were on enoxaparin. Of the 50 patients on direct-acting oral anticoagulants, 20 (40%) were on aspirin, 5 (10%) were on clopidogrel and 3 (6%) were on both antiplatelet agents. The last anticoagulant dose was administered at a mean (±SD) of 15 ± 5, 17 ± 9 and 12 ± 3 h before admission for patients on rivaroxaban, apixaban and enoxaparin, respectively (Table 1). Bleeding occurred after being on an anticoagulant for a median (IQR) of 97 (20, 210) days (Table S3).
3.4. Types of interventions to manage the bleeding event
Clinicians used coagulation factor or plasma products in 24 (43%) patients. Factor VIII inhibitor bypassing activity (FEIBA) and fresh frozen plasma (FFP) were the most commonly used coagulation factor and plasma products, respectively. FEIBA was used in 12 (21%) major bleeding patients, with a mean (±SD) 2193 ± 799 units, and FFP was used in 10 (18%) patients, with a mean (±SD) 3 ± 1 units. Three (5%) patients received 4-factor prothrombin complex concentrate. FEIBA was the most used agent for treating ICH (8/19, 42%), and FFP was the most used for GIB (8/29, 28%). Usage of FEIBA was driven by one center that used the product in 64% (9/14) of submitted acute bleeding patients.
Platelets were used in 4 (7%) patients, of which 3 of 4 were on concomitant antiplatelets at admission. Additionally, 2 (4%) patients received vitamin K and 2 (4%) received protamine sulfate (both patients were on enoxaparin; Table 3).
Table 3.
Interventions used to manage the retrospective study population and by bleeding location.
| Total (n = 56)
|
Gastrointestinal bleeding (n = 29)
|
Intracranial hemorrhage (n = 19)
|
Other bleeding site (n = 8)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Patients no. (%) | Units mean ± SDc median (IQR) | Patients no. (%) | Units mean ± SD median (IQR) | Patients no. (%) | Units mean ± SD median (IQR) | Patients no. (%) | Units mean ± SD median (IQR) | |
| Coagulation or plasma products | 24 (43) | – | 9 (31)‡ | – | 14 (74)‡ | – | 1 (13) | – |
| FEIBA –units | 12 (21) | 2192.8 ± 798.6 1803.0 (1690.0, 2719.5) |
3 (10) | 1675.0 ± 151.7 1755.0 (1627.5, 1762.5) |
8 (42) | 2431.5 ± 893.0 2290.0 (1690.0,3063.5) |
1 (13) | 1836.0 ± N/A 1836.0 (1836.0, 1836.0) |
| FFP –units | 10 (18) | 2.6 ± 1.4 2.0 (2.0,2.8) |
8 (28) | 2.3 ± 0.9 2.0 (2.0,2.3) |
1 (5) | 6.0 ± N/A 6.0 (6.0,6.0) |
1 (13) | 2.0 ± N/A 2.0 (2.0,2.0) |
| Platelets –pools | 4 (7) | 3.3 ± 3.2 2.0 (1.8,3.5) |
1 (3) | 2.0 ± N/A 2.0 (2.0,2.0) |
3 (16) | 3.7 ± 3.8 2.0 (1.5,5.0) |
0 (0) | – |
| PCC –units | 3 (5) | 1913.3 ± 522.0 1740.0 (1620.0, 2120.0) |
1 (3) | 1500.0 ± N/A 1500.0 (1500.0, 1500.0) |
2 (11) | 2120.0 ± 537.4 2120.0 (1930.0, 2310.0) |
0 (0) | – |
| Vitamin K –mg | 2 (4) | 7.5 ± 3.5 7.5 (6.3,8.8) |
2 (7) | 7.5 ± 3.5 7.5 (6.3,8.8) |
0 (0) | – | 0 (0) | – |
| Protamine sulfate –mg | 2 (4) | 92.5 ± 10.6 92.5 (88.8,96.3) |
0 (0) | – | 2 (11) | 92.5 ± 10.6 92.5 (88.8,96.3) |
0 (0) | – |
| Supportive therapiesa | 46 (82) | – | 29 (100)§ | – | 11 (58)§ | – | 6 (75) | – |
| Packed red blood cells –units | 28 (50) | 2.9 ± 1.3 2.5 (2.0,4.0) |
24 (83) | 2.8 ± 1.1 2.5 (2.0,4.0) |
1 (5) | 2.0 ± N/A 2.0 (2.0,2.0) |
3 (38) | 4.0 ± 2.5 4.0 (2.5,5.0) |
| Crystalloid –mL | 27 (48) | 1650.0 ± 1218.4 1050.0 (1000.0, 2000.0) |
17 (59) | 1923.5 ± 1441.4 1750.0 (1000.0, 2000.0) |
6 (32) | 966.7 ± 225.1 1000.0 (925.0, 1000.0) |
4 (50) | 1525.0 ± 563.3 1525.0 (1037.5, 2000.0) |
| Supportive interventionsb | 30 (54) | – | 21 (72)‖ | – | 5 (26)‖ | – | 4 (50) | – |
| Endoscopy –no. | 22 (39) | 1.3 ± 1.5 1.0 (1.0,1.8) |
21 (72) | 1.3 ± 1.5 1.0 (1.0,2.0) |
0 (0) | – | – | – |
Supportive therapies not listed include levetiracetam, mannitol, colloid, whole blood, norepinephrine infusion and ondansetron.
Supportive interventions not listed include burr hole/EVD placement, bronchoscopy, surgery, radiological embolization and capsule endoscopy.
Statistical difference: p = 0.007.
Statistical difference: p < 0.001.
Statistical difference: p = 0.003.
SD: Standard deviation; IQR: Interquartile range; GIB: Gastrointestinal bleed; ICH: Intracranial hemorrhage; FEIBA: Factor VIII inhibitor bypassing activity; FFP: Fresh frozen plasma; PCC: Prothrombin complex concentrate.
3.5. Hospital length of stay and resource utilization
The median (IQR) hospital LOS was 5 (3, 8) days. In 30 (54%) patients, the LOS was ≤5 days, while 17 (30%) had an LOS between 5 and 10 days, 6 (11%) between 10 and 20 days and 2 (4%) between 20 and 30 days. The LOS was reported as 47 days for 1 patient. The median (IQR) ICU LOS was 4 (2, 7) days (n = 35). In addition, the median (IQR) telemetry/stepdown unit LOS was 4 (3, 5) days (n = 20; Table S4).
3.6. Clinical outcomes
At 30-days post-death/discharge, the mortality was 21% (12/56) across all bleed types, with subgroup mortalities of 14% (4/29) for GIB and 37% (7/19) for ICH (Table 4). There was no association found between mortality or thromboembolic events (TEEs) based on the type of FXa inhibitor (data not shown).
Table 4.
Clinical outcomes of acute major bleeding episodes in retrospective chart review.
| Characteristic | Retrospective chart review (n = 56)a |
|---|---|
| Clinical outcomes at 30-days post-death/discharge –no./total no. (%) | |
| Death | 12/56 (21) |
| Gastrointestinal bleeding | 4/29 (14) |
| Intracranial hemorrhage | 7/19 (37) |
| Other bleeding site | 1/8 (13) |
| Death during index hospitalization | 7/56 (13) |
| Gastrointestinal bleeding | 2/29 (7) |
| Intracranial hemorrhage | 5/19 (26) |
| Other bleeding site | 0/8 (0) |
| Thromboembolic events | 6/56 (11) |
| Gastrointestinal bleeding | 5/29 (17) |
| Intracranial hemorrhage | 0/19 (0) |
| Other bleeding site | 1/8 (13) |
| Re-anticoagulation | 23/56 (41) |
| Gastrointestinal bleeding | 14/29 (48) |
| Intracranial hemorrhage | 4/19 (21) |
| Other bleeding site | 5/8 (63) |
| All-cause re-admissionb | 9/49 (18) |
| Gastrointestinal bleeding | 7/27 (26) |
| Intracranial hemorrhage | 0/14 (0) |
| Other bleeding site | 2/8 (25) |
Percentages may not total 100 because of rounding.
Excludes 2 patients with gastrointestinal bleeds and 5 with intracranial hemorrhages who died in the index hospitalization.
Re-anticoagulation within 30-days post-discharge occurred in 41% (23/56) of patients, with 17 of 23 restarting on the same FXa inhibitor (Table 4).
No differences were observed in resource utilization or clinical outcomes by treatment strategy (data not shown). However, patients on direct-acting oral anticoagulants with concomitant antiplatelets were associated with an occurrence of TEEs (p = 0.034), a trend toward a greater likelihood of mortality (p = 0.084) and a reduced likelihood of re-anticoagulation (p = 0.086).
3.7. Inter-rater reliability
To confirm accuracy of data abstraction, 10% of the charts were re-abstracted and analyzed on the clinical diagnosis, exposure with concomitant antiplatelets, overall use of coagulation factors and plasma to reverse the bleeding event and clinical outcomes (mortality, re-anticoagulation and TEEs). The kappa statistic for the re-abstracted data was 0.97 due to a differing interpretation on one patient’s site of bleed (retroperitoneal vs. intra-abdominal), although there was alignment in both abstractions on a previous survey question which indicated this was a spontaneous retroperitoneal bleed. Similarly, in the re-abstraction of another patient, there was a differing opinion on whether a clinically significant complication (infarction of kidneys) should be considered a TEE. Assuming both differences, an updated kappa statistic of 0.94 was calculated for the 6 characteristics.
4. Limitations
As this was a retrospective study, the quality and completeness of information were dependent on quality and accuracy of documented medical records. Patients were excluded from this study if they were enrolled in an interventional reversal agent study, which may have biased the sample. Although site monitoring was not required for this study, extensive data validation and quality checks were established for the electronic case report form, including re-abstraction of specific data points. Incomplete data may have resulted from information not being captured by routine physician documentation.
As only 5 sites were included in the study, generalizability of the findings may be limited. Institutional practices highlighted in this study may not be representative of the overall treatment practice, as patients were excluded from this study if they were enrolled in an interventional reversal agent study.
Furthermore, the relatively small sample size likely impacted the power of our study to detect statistically significant differences, particularly between subgroups. Subgroup analysis based on a 95% confidence level and 80% power would require >1000 patients. Given the relatively rare nature of FXa inhibitor-associated serious/life-threatening acute major bleeding, combined with the short study timeframe, a sample size of this magnitude was not feasible.
5. Discussion
In this retrospective chart review, patients in the cohort of critically ill FXa inhibitor-associated major bleeding had a high mortality rate despite aggressive management in the absence of a specific reversal agent. The mortality rate of 21% (12/56) is higher than the interim analysis of the ongoing, prospective, Phase 3b/4 trial of andexanet alfa (ANNEXA-4) in patients with acute major bleeding (10/67, 15%) [12]. Andexanet alfa is an investigational agent for targeted reversal of anticoagulation effects of FXa inhibitors. It is a modified FXa decoy molecule that binds all FXa inhibitors, including indirect FXa inhibitors such as enoxaparin, allowing native FXa to function in the clotting cascade [13,14]. Statistical comparisons of such small groups, such as our group and ANNEXA4, are underpowered and confounded by retrospective versus prospective designs, lack of blinding and investigator bias. However, the cohorts seem to be similar in terms of patient characteristics, co-morbidities, bleeding type and severity (Tables 1 and 2), and all were enrolled at active ANNEXA4 sites [12]. All trials of anticoagulant reversal agents, including prospective and randomized trials, are underpowered to detect differences in the relatively rare safety outcomes of death and TEEs [12,17,19].
Characteristics of these newer anticoagulants challenge major bleeding definitions and reversal paradigms established in the warfarin era. The widely used ISTH major bleeding criteria, which include a hemoglobin drop of 2 g/dL and do not require hemodynamic instability, may be an appropriate aggressive anticoagulant reversal trigger for patients on vitamin K antagonists (VKAs). VKAs’ exceedingly long half-lives might preclude watchful waiting as a reasonable reversal option. The FXa inhibitors, however, have short half-lives, and stable bleeding patients may not require aggressive reversal strategies, which is why we asked investigators to enroll only patients in need of immediate reversal for this cohort. This shift in acuity likely explains some of the differences between the first randomized, plasma-controlled trials reversing warfarin with 4-factor non-activated prothrombin complex concentrate (4F-PCC, Kcentra®) [17,18] and the recent and ongoing studies of direct-acting oral anticoagulant reversal agents idarucizumab (ReverseAD) and andexanet alfa (ANNEXA4) [12,19].
In the KCentra studies, patients were randomized to receive 4F–PCC or plasma to reverse acquired coagulation factor deficiency induced by VKAs, e.g. warfarin. Kcentra received FDA approval for reversal of VKA therapy in major bleeding in 2013 [17,18]. Idarucizumab (Praxbind®), a monoclonal antigen-binding antibody fragment studied in a single arm trial for reversing the anticoagulation effects of the direct thrombin inhibitor dabigatran, was FDA approved in 2015 [19].
The study investigating 4F-PCC adhered very closely to the standard ISTH criteria and enrolled a broader range of patients, with a larger percentage of GIBs (64%) and smaller percentage of ICHs (12%) compared to our retrospective cohort, ANNEXA4 and RE-VERSE AD [17]. The study had a low 30-day mortality (6%) and TEE rate (7%) [17]. Those studies also had the benefit of a two arm study design with a control group receiving plasma, which allowed the study to show any differences in mortality, safety and TEEs between the 4F-PCC and plasma groups [20,21]. Designing clinical trials to test the reversal agents for direct-acting oral anticoagulants presented new challenges. The newer anticoagulants’ shorter half-lives made a “wait and see” approach for patients in the milder end of the major bleeding spectrum reasonable. This led, by combination of design and investigator selection bias, to a much higher acuity of illness cohort of bleeding patients enrolled in the newer anticoagulant reversal trials. The 30-day mortality tripled (18%) in RE-VERSE AD (idarucizumab) and nearly so (15%) in the interim report of ANNEXA-4 (andexanet alfa) [12,19].
Much of the mortality differences in these reversal studies probably can be ascribed to the high percentage of ICH patients in RE-VERSE AD and ANNEXA-4. ICHs represented 35% of the major bleeding cohort of RE-VERSE AD [19], 42% in the interim ANNEXA-4 analysis [12], but only 12% in 4F-PCC [17]. Our cohort had 34% ICHs and a 30-day mortality of 21%. For comparison, of the 327 cases of major bleeding in the ARISTOTLE Trial of apixaban for atrial fibrillation, only 52 (16%) patients on apixaban experienced an ICH, and the overall mortality for all major bleeds was 11% [22].
Thromboembolic events, which include strokes and systemic emboli, myocardial infarctions, deep-vein thromboses and pulmonary emboli, may be related to the reversal strategy or the native prothrombotic state for which the patient was initially prescribed an anticoagulant. TEEs within 30-days were reported in 11% (6/56) of this chart review cohort compared to 7% (7/98) in the major bleeding 4F-PCC arm [17], 8% (8/104) in the plasma control [17], 6% (5/90) in RE-VERSE AD [19] and 18% (12/67) in the ANNEXA-4 interim analysis [12]. ANNEXA-4 investigators have since updated their analysis reporting a TEE rate of 12% (13/105) [23]. In this chart review, 5 of the TEEs occurred during the index admission, 4 within the first 72 h (7%). Early TEEs, defined in RE-VERSE AD as <72 h, occurred in 1 (1%) idarucizumab patient [19], 4 (6%) andexanet alfa patients based on the ANNEXA-4 interim analysis [12], 2 (2%) 4F-PCC major bleeding patients and 5 (5%) plasma controls (See Fig. 1 for TEEs in the retrospective chart review and Fig. 2 for comparison across studies) [17]. Late TEEs might be more related to the exposed native thrombotic state of these patients and whether they were eventually restarted on anticoagulation. There is also the interplay of illness acuity and mortality, i.e. sicker, bedridden patients, and particularly patients with ICH are at higher risk of TEEs. However, these patients are also more likely to die early, removing them from the TEE risk pool and creating a survival bias for patients less likely to have TEEs. Together, this data might suggest that early TEEs are inevitable regardless of the reversal strategy and even without active reversal, e.g. two of the early TEEs in our cohort were not given plasma or a factor product. In contrast, late TEEs might be a therapeutic target for more aggressive re-anticoagulation.
Fig. 1.
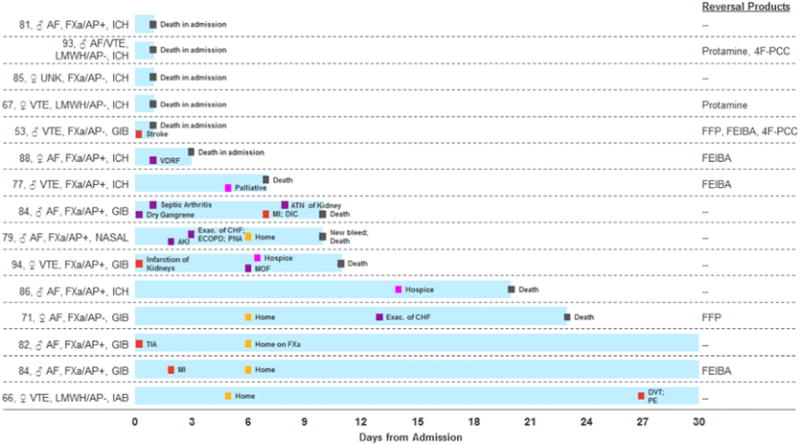
Thromboembolic events and deaths in the retrospective study population (n = 15). * ♂: Male; ♀: Female; FXa: Factor Xa inhibitor (rivaroxaban or apixaban); LMWH: Low molecular weight heparin (enoxaparin); AF: Atrial fibrillation; VTE: Venous thromboembolism; UNK: Unknown reason; AP: Concomitant antiplatelets; ICH: Intracranial hemorrhage; GIB: Gastrointestinal bleed; IAB: Intra-abdominal hemorrhage; VDRF: Ventilator-dependent respiratory failure; DVT: Deep-vein thrombosis; PE: Pulmonary embolism; TIA: Transient ischemic attack; MI: Myocardial infarction; Exac: Exacerbation; MOF: Multi-organ failure; AKI: Acute kidney injury; ECOPD: Exacerbation of chronic obstructive pulmonary disease; PNA: Pneumonia; DIC: Disseminated intravascular coagulation; ATN: Acute tubular necrosis; 4F–PCC: 4-factor prothrombin complex concentrate; FFP: Fresh frozen plasma; FEIBA: Factor VIII inhibitor bypassing activity.
Fig. 2.
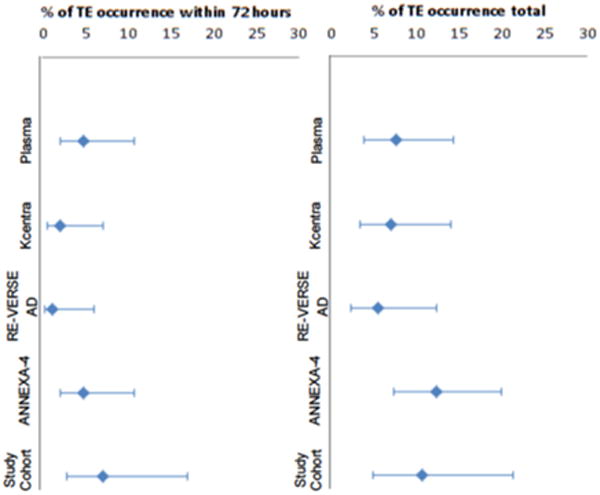
Thromboembolic events and timing across clinical trials and the retrospective cohort with 95% CI.
Restarting anticoagulation after major hemorrhage has become an area of interest in hopes of mitigating the later TEE disease burden after anticoagulant reversal or cessation for bleeding [24]. Most major hemorrhage patients, especially with GIBs but also many with ICHs, can be restarted in the weeks after the bleeding has stabilized, though guidelines differ on the timing [25,26]. Despite these recommendations, few patients actually are restarted. The 4F-PCC studies did not report percentages but noted restarting was rare [17,18]. The RE-VERSE AD study noted that none of the 5 patients with TEEs were restarted. The most recent ANNEXA-4 update reported a restart rate of 40% [23], and 41% was restarted in our cohort.
For the direct-acting oral anticoagulant reversal trials, there was no active control, such as plasma for VKA reversal, and using placebo arms in major bleeding patients was deemed unethical [27]. Also, any usual care control would suffer from heterogeneity of use of various factor products. This is illustrated in our data, which showed several approaches to these critically ill patients: plasma, prothrombin complex concentrates (PCCs), FEIBA or supportive care alone. It is unlikely that plasma would be an effective reversal agent for any of the newer direct-acting oral anticoagulants. Clinicians may have been using plasma as a volume expander in these bleeding scenarios rather than as a reversal agent. Factor product reversal strategies were common in our cohort, though this was driven by one center’s standard of rapid FEIBA administration. The evidence for FEIBA, an activated PCC used in 12 of our patients, and other PCCs is largely limited to small trials of healthy normal non-bleeding males [28,29]. The only human PCC bleeding data was from a trial of volunteers given edoxaban followed by thigh punch biopsies and reversal with the 4F-PCC [30]. Three patients in this chart review received 4F-PCC.
Finally, the data presented here provide a detailed look at the burden of disease of FXa inhibitor-associated major hemorrhage, which is not insignificant despite the better safety of the direct-acting oral anticoagulants. In this chart review, patients required significant health care resources and complex care in ICU settings with numerous physician specialty consultations. This burden is likely to grow as more and more patients are prescribed these drugs. According to data from the MarketScan databases, approximately 2.9 million people in the U.S. were treated with FXa inhibitors in 2015 [31]. There are currently > 80,000 annual U.S. FXa inhibitor-associated, ISTH-defined major bleeding events [8]. Reversal agents may only be required in a subset, perhaps a third to a half (see Note S1 for explanation), but that number is anticipated to grow, and data presented here contradicts the notion that FXa inhibitor-associated bleeding is mild and reversal agents serve a psychological more than a medical need [32]. As FXa inhibitor use increases so will major bleeding, as will the subset with the highest acuity. Reversal agents serve a self-reinforcing purpose in this framework. By addressing clinician and patient fears of lack of reversibility, they will likely increase uptake of the newer anticoagulants. By doing so, they increase the incidence of major bleeding and the need for reversal agents. This increase in major bleeding is, of course, offset by a much greater decrease in stroke and VTE.
This chart review of data from patients with FXa inhibitor-associated critical major bleeding, who would be deemed appropriate for reversal of anticoagulation, illustrates the variable approaches to the problem in the absence of a specific reversal agent and the high associated resource utilization and mortality.
Supplementary Material
Acknowledgments
Source of funding
Funding was provided by Portola Pharmaceuticals.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx. doi.org/10.1016/j.ajem.2017.08.042.
References
- 1.Institute for Safe Medication Practices. QuarterWatch monitoring FDA MedWatch reports: annual report issue. https://www.ismp.org/quarterwatch/pdfs/2015Q4.pdf; june 29, 2016 (Accessed November 10, 2016)
- 2.Bauer KA. Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464–70. doi: 10.1182/asheducation-2013.1.464. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 6.Chai-Adisaksopha C, Crowther M, Isayama T, et al. The impact of bleeding complications in patients receiving target-specific oral anticoagulants: a systematic review and meta-analysis. Blood. 2014;124(15):2450–8. doi: 10.1182/blood-2014-07-590323. [DOI] [PubMed] [Google Scholar]
- 7.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2013;383(9921):955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 8.Deitelzweig S, Neuman WR, Lingohr-Smith M, et al. Incremental economic burden associated with major bleeding among atrial fibrillation patients treated with factor Xa inhibitors. Ann Emerg Med. 2016;68(4):S18. doi: 10.1080/13696998.2017.1362412. (Abstract) [DOI] [PubMed] [Google Scholar]
- 9.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87:S141–5. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 10.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Couris CM, Fushimi K, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Milling TJ, Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–41. doi: 10.1056/NEJMoa1607887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19(4):446–51. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 14.Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–24. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 17.Sarode R, Milling TJ, Jr, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–43. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein JN, Refaai MA, Milling TJ, Jr, et al. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385(9982):2077–87. doi: 10.1016/S0140-6736(14)61685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack CV, Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6):511–20. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 20.Milling TJ, Jr, Refaai MA, Goldstein JN, et al. Thromboembolic events after vitamin K antagonist reversal with 4-factor prothrombin complex concentrate: exploratory analyses of two randomized, plasma-controlled studies. Ann Emerg Med. 2016;67(1):96–105 e5. doi: 10.1016/j.annemergmed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milling TJ, Jr, Refaai MA, Sarode R, et al. Safety of a four-factor prothrombin complex concentrate versus plasma for vitamin K antagonist reversal: an integrated analysis of two phase IIIb clinical trials. Acad Emerg Med. 2016;23(4):466–75. doi: 10.1111/acem.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE trial (Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol. 2014;63(20):2141–7. doi: 10.1016/j.jacc.2014.02.549. [DOI] [PubMed] [Google Scholar]
- 23.Connolly SJ, Gibson CM, Crowther M. Letter to the Editor, andexanet alfa for factor Xa inhibitor reversal. N Engl J Med. 2016;375(25):2499–500. doi: 10.1056/NEJMc1613270. [DOI] [PubMed] [Google Scholar]
- 24.Milling TJ, Jr, Spyropoulos AC. Re-initiation of dabigatran and direct factor Xa antagonists after a major bleed. Am J Med. 2016;129(11s):S54–63. doi: 10.1016/j.amjmed.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians Evidence-based Clinical Practice Guidelines. (9th) 2012;141(2 Suppl):7s–47s. doi: 10.1378/chest.1412S3. Chest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 27.Sarich TC, Seltzer JH, Berkowitz SD, et al. Novel oral anticoagulants and reversal agents: considerations for clinical development. Am Heart J. 2016;375(12):1185–6. doi: 10.1016/j.ahj.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Marlu R, Hodaj E, Paris A, et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108(2):217–24. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 29.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 30.Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4-factor prothrombin complex concentrate. Circulation. 2015;131(1):82–90. doi: 10.1161/CIRCULATIONAHA.114.013445. [DOI] [PubMed] [Google Scholar]
- 31.IMS Institute for Healthcare Informatics. The use of medicines in the United States: review of 2015. Available at: https://www.imshealth.com/files/web/IMSH%20Institute/Reports/The%20Use%20of%20Medicines%20in%20the%20United%20States%202011/IHII_Medicines_in_U.S_Report_2015.pdf (Accessed July 2016)
- 32.Hunt BJ, Levi M. Engineering reversal - finding an antidote for direct oral anticoagulants. N Engl J Med. 2016;375(12):1185–6. doi: 10.1056/NEJMe1610510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


