Abstract
Purpose
A thorough understanding of the natural history and consensus regarding the optimal management of pathologic lymph node-positive (pN1) prostate cancer are lacking. Our objective was to describe patterns of care and outcomes of a contemporary cohort of men with pN1 prostate cancer.
Materials and Methods
The National Cancer Data Base (NCDB) was used to identify 7,791 men who were found to have LN metastases at the time of radical prostatectomy (RP). Multinomial logistic regression and Cox proportional hazards regression were used to identify patient, tumor and facility characteristics associated with choice of post-RP management strategy and overall survival (OS), respectively.
Results
Sixty-three percent of men were initially managed with observation, 20% with ADT alone, 5% with RT alone and 13% with ADT and RT. Younger age, lower comorbidity burden, higher grade and stage and the presence of positive surgical margins were associated with a higher likelihood of receiving combination therapy. Grade group 4-5 disease, pT3b-T4 disease, positive surgical margins and a higher number of positive LN were independent predictors of worse OS, with adjusted ten-year OS probabilities decreasing from 84% to 32% with the presence of an increasing number of adverse prognostic factors. Treatment with combined ADT and RT was associated with better OS (multivariable HR 0.69 for combination therapy vs. observation, 95% CI 0.52, 0.92, p=0.010).
Conclusions
Patient and tumor characteristics are associated with both choice of post-RP management strategy and survival in men with pN1 prostate cancer. Multimodal therapy may be of benefit in this patient population.
Keywords: Prostatic neoplasms, retropubic prostatectomy, lymph node dissection, lymphatic metastasis
Introduction
Lymph node (LN) metastases in men with adenocarcinoma of the prostate undergoing radical prostatectomy (RP) have traditionally been thought to be a manifestation of widely disseminated disease and to consequently portend a poor prognosis. This paradigm was the basis for an Eastern Cooperative Oncology Group (ECOG) randomized trial comparing immediate versus delayed androgen deprivation therapy (ADT), which showed higher overall survival (OS) among men receiving immediate as opposed to delayed ADT.1 However, recent observational studies have shown that even in the absence of any treatment, ten-year cancer-specific survival (CSS) among men with pN1 disease can be as high as 70%,2 suggesting that immediate and lifelong ADT constitutes overtreatment in the majority of such men. Furthermore, there is a lack of data pertaining to the role of radiation therapy (RT) after RP in men with pN1 disease as prior randomized trials of adjuvant RT excluded men with LN metastases.3–5 The uncertainty regarding the optimal management of these patients is reflected in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines, which list observation, adjuvant ADT and adjuvant ADT and RT as acceptable management options for men with pN1 disease.6
Although the issues outlined above make it likely that immediate treatment with ADT and/or RT has not been widely adopted in contemporary clinical practice, studies examining patterns of care after RP in this patient population are lacking. Additionally, it is unknown whether the favorable outcomes reported by high-volume academic centers apply to all men with pN1 disease. To address these knowledge gaps, we set out to describe the management and outcomes of a large, diverse and contemporary cohort of men with pN1 prostate cancer.
Materials and Methods
The National Cancer Data Base (NCDB) was used to identify 283,802 men without a prior history of malignancy who were diagnosed with non-metastatic adenocarcinoma of the prostate between 2004 and 2014 and were treated with RP. Of these patients, 9,673 (3.4%) were found to have LN metastases. We excluded 227 men who received chemotherapy, 1,117 men who were treated with ADT or RT prior to RP and 537 men with missing data pertaining to whether ADT or RT were administered or the timing of these treatments. We also excluded one patient who was an extreme outlier with respect to the number of LN removed (88). This left 7,791 men for inclusion in the analysis pertaining to choice of post-RP management strategy. Only men diagnosed between 2004 and 2010 were included in the survival analyses so as to allow for sufficient follow-up. Of the 3,988 men diagnosed during this time period, 104 were excluded because of missing data pertaining to follow-up time and an additional 204 were excluded because they either died or were lost to follow-up within one year of RP. The remaining 3,680 men were included in the survival analyses.
The primary objectives of our study were to identify patient, tumor and facility characteristics associated with choice of post-RP management strategy and survival. Management strategies were categorized as (1) observation; and treatment with (2) ADT alone; (3) RT alone; or (4) both ADT and RT within 12 months of RP. Multinomial regression was used to model the relationship between patient, facility and tumor characteristics and post-RP management strategy. Survival analyses were performed using Cox proportional hazards regression. To limit “time-to-treatment” bias, we performed a landmark analysis7 in which the start of follow-up was defined as occurring 12 months after RP. All models included age, Charlson Comorbidity Index, race, insurance status, income, urbanicity, facility location, facility designation, annual facility pN1 prostate cancer case volume, year of diagnosis, grade group, pathologic T stage, margin status, number of positive LN removed and number of negative LN removed.
Robust standard errors were used to account for clustering of outcomes of patients treated at the same facility. Multiple imputation by chained equations was used to account for missing covariate data, the frequency of which varied from a high of 7% for PSA to <3% for all other covariates. All statistical tests were two-sided and p-values less than 0.05 were deemed to be significant. Statistical analyses were performed using Stata 14 (StataCorp LP, College Station, TX).
Results
Fifty-one percent of men had grade group 4-5 disease and 81% had non-organ-confined (pT3-T4) disease (see Table 1). The incidence of positive surgical margins was 47%. The median LN yield was nine (IQR 5, 14) and the median number of positive LN was one (IQR 1, 2).
Table 1.
Patient, tumor and facility characteristics by post-RP management strategy
| All (n = 7,791) |
Observation (n = 4,889) |
ADT Alone (n = 1,571) |
RT Alone (n = 355) |
ADT & RT (n = 976) |
|
|---|---|---|---|---|---|
| Age (years) | 62 (57, 67) | 63 (57, 67) | 63 (57, 67) | 61 (55, 66) | 61 (55, 65) |
| CCI | |||||
| 0 | 6,375 (82%) | 3,958 (81%) | 1,291 (82%) | 306 (86%) | 820 (84%) |
| ≥1 | 1,416 (18%) | 931 (19%) | 280 (18%) | 49 (14%) | 156 (16%) |
| Race | |||||
| White | 6,365 (82%) | 3,973 (81%) | 1,296 (83%) | 280 (79%) | 816 (84%) |
| Other | 1,310 (17%) | 838 (17%) | 250 (16%) | 69 (19%) | 153 (16%) |
| Unknown | 116 (1%) | 78 (2%) | 25 (2%) | 6 (2%) | 7 (<1%) |
| Insurance | |||||
| Private | 4,501 (58%) | 2,805 (57%) | 853 (54%) | 220 (62%) | 623 (64%) |
| Medicare | 2,677 (34%) | 1,747 (36%) | 559 (36%) | 94 (26%) | 277 (28%) |
| Medicaid/other | 329 (4%) | 177 (4%) | 89 (6%) | 17 (5%) | 46 (5%) |
| Uninsured | 205 (3%) | 111 (2%) | 52 (3%) | 18 (5%) | 24 (2%) |
| Unknown | 79 (1%) | 49 (1%) | 18 (1%) | 6 (2%) | 6 (<1%) |
| Median income | |||||
| <$38,000 | 1,207 (15%) | 728 (15%) | 256 (16%) | 73 (21%) | 150 (15%) |
| $38,000–$47,999 | 1,713 (22%) | 1,043 (21%) | 392 (25%) | 75 (21%) | 203 (21%) |
| $48,000–$62,999 | 2,081 (27%) | 1,311 (27%) | 399 (25%) | 98 (28%) | 273 (28%) |
| ≥$63,000 | 2,727 (35%) | 1,767 (36%) | 514 (33%) | 105 (30%) | 341 (35%) |
| Unknown | 63 (<1%) | 40 (<1%) | 10 (<1%) | 4 (1%) | 9 (<1%) |
| Urbanicity | |||||
| Metropolitan | 6,260 (80%) | 3,955 (81%) | 1,228 (78%) | 293 (83%) | 784 (80%) |
| Non-metropolitan | 1,320 (17%) | 795 (16%) | 302 (19%) | 55 (15%) | 168 (17%) |
| Missing | 211 (3%) | 139 (3%) | 41 (3%) | 7 (2%) | 24 (2%) |
| Facility location | |||||
| Northeast | 1,426 (18%) | 973 (20%) | 230 (15%) | 50 (14%) | 173 (18%) |
| Midwest | 2,303 (30%) | 1,295 (26%) | 593 (38%) | 91 (26%) | 324 (33%) |
| South | 2,434 (31%) | 1,581 (32%) | 424 (27%) | 156 (44%) | 273 (28%) |
| West | 1,623 (21%) | 1,038 (21%) | 323 (21%) | 57 (16%) | 205 (21%) |
| Unknown | 5 (<1%) | 2 (<1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Facility designation | |||||
| Academic | 3,930 (50%) | 2,457 (50%) | 903 (57%) | 128 (36%) | 442 (45%) |
| Non-academic | 3,856 (49%) | 2,430 (50%) | 667 (42%) | 226 (64%) | 533 (55%) |
| Unknown | 5 (<1%) | 2 (<1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Facility case volume (cases per year) | 4 (2, 9) | 4 (2, 10) | 4 (2, 10) | 2 (1, 4) | 3 (1, 6) |
| Year of diagnosis | |||||
| 2004 | 393 (5%) | 258 (5%) | 75 (5%) | 27 (8%) | 33 (3%) |
| 2005 | 456 (6%) | 301 (6%) | 79 (5%) | 29 (8%) | 47 (5%) |
| 2006 | 478 (6%) | 309 (6%) | 110 (7%) | 18 (5%) | 41 (4%) |
| 2007 | 609 (8%) | 361 (7%) | 150 (10%) | 26 (7%) | 72 (7%) |
| 2008 | 607 (8%) | 383 (8%) | 124 (8%) | 27 (8%) | 73 (7%) |
| 2009 | 680 (9%) | 453 (9%) | 114 (7%) | 27 (8%) | 86 (9%) |
| 2010 | 765 (10%) | 463 (9%) | 160 (10%) | 40 (11%) | 102 (10%) |
| 2011 | 827 (11%) | 514 (11%) | 169 (11%) | 36 (10%) | 108 (11%) |
| 2012 | 887 (11%) | 563 (12%) | 161 (10%) | 41 (12%) | 122 (13%) |
| 2013 | 996 (13%) | 633 (13%) | 195 (12%) | 44 (12%) | 124 (13%) |
| 2014 | 1,093 (14%) | 651 (13%) | 234 (15%) | 40 (11%) | 168 (17%) |
| PSA (ng/mL) | 9 (6, 18) | 9 (5, 16) | 11 (7, 21) | 10 (6, 20) | 10 (6, 20) |
| Grade group | |||||
| 1-2 | 1,618 (21%) | 1,176 (24%) | 203 (13%) | 77 (22%) | 162 (17%) |
| 3 | 2,095 (27%) | 1,360 (28%) | 384 (24%) | 105 (30%) | 246 (25%) |
| 4 | 1,187 (15%) | 742 (15%) | 256 (16%) | 42 (12%) | 147 (15%) |
| 5 | 2,749 (35%) | 1,527 (31%) | 700 (45%) | 116 (33%) | 406 (42%) |
| Unknown | 142 (2%) | 84 (2%) | 28 (2%) | 15 (4%) | 15 (2%) |
| Pathologic stage | |||||
| T2 | 1,401 (18%) | 1,077 (22%) | 181 (12%) | 47 (13%) | 96 (10%) |
| T3a | 2,294 (29%) | 1,511 (31%) | 437 (28%) | 110 (31%) | 236 (24%) |
| T3b | 3,747 (48%) | 2,116 (43%) | 861 (55%) | 181 (51%) | 589 (60%) |
| T4 | 280 (4%) | 138 (3%) | 78 (5%) | 12 (3%) | 52 (5%) |
| Unknown | 69 (<1%) | 47 (<1%) | 14 (<1%) | 5 (1%) | 3 (<1%) |
| Surgical margins | |||||
| Negative | 4,068 (52%) | 2,898 (59%) | 680 (43%) | 147 (41%) | 343 (35%) |
| Positive | 3,652 (47%) | 1,956 (40%) | 873 (56%) | 204 (57%) | 619 (63%) |
| Unknown | 71 (<1%) | 35 (<1%) | 18 (1%) | 4 (1%) | 14 (1%) |
| Positive LN count | 1 (1, 2) | 1 (1, 2) | 1 (1, 3) | 1 (1, 2) | 1 (1, 2) |
| LN yield | 9 (5, 14) | 9 (5, 14) | 9 (5, 14) | 7 (4, 12) | 8 (4, 13) |
Categorical and continuous variables reported as counts (percentages) and medians (interquartile range), respectively; CCI, race and insurance categories collapsed to comply with NCDB stipulation against reporting counts <10; percentages may not add up to 100% because of rounding; ADT, androgen deprivation therapy; LN, lymph node; PSA, prostate-specific antigen; RT, radiation therapy
Sixty-three percent of patients received no ADT or RT within 12 months of RP, whereas 20% were managed with ADT alone, 5% with RT alone and 13% with both ADT and RT. Utilization of combination therapy increased over time, with 15% of men diagnosed in 2014 receiving ADT and RT compared to 8% of those diagnosed in 2004 (see Figure 1). This modest rise was statistically significant (ptrend <0.001) even after accounting for all other covariates in a multivariable analysis, the results of which are shown in Table 2. Treatment with ADT alone was more common at academic facilities. Older patients and those with more comorbidities were less likely to receive either combination therapy or RT alone compared to observation, with black patients likewise being less likely to receive combination therapy than observation.
Figure 1.
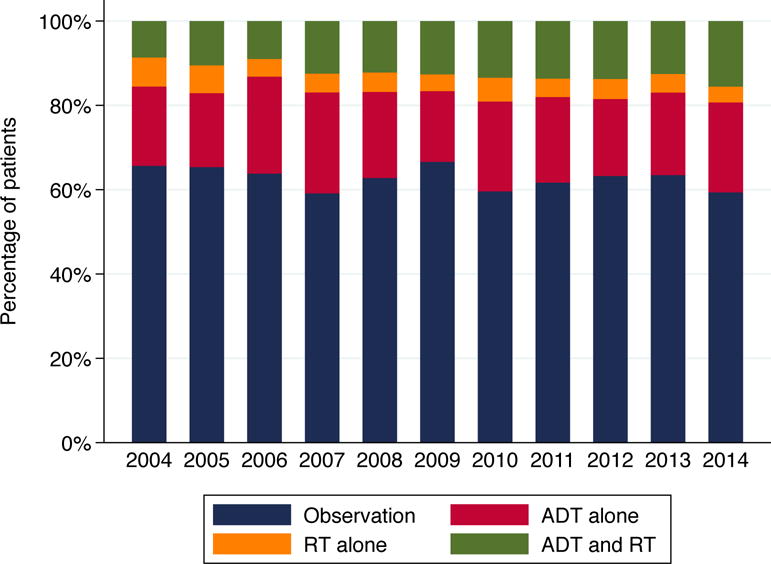
Distribution of post-RP management strategies by year of diagnosis
Table 2.
Predictors of post-RP management strategy on multivariable analysis (observation as referent)
| ADT Alone
|
RT Alone
|
ADT & RT
|
||||
|---|---|---|---|---|---|---|
| RRR (95% CI) | p-value | RRR (95% CI) | p-value | RRR (95% CI) | p-value | |
| Age (per five years) | 0.97 (0.92, 1.03) | 0.33 | 0.88 (0.79, 0.98) | 0.017 | 0.80 (0.75, 0.85) | <0.001‡ |
| CCI | ||||||
| 0 | Ref. | Ref. | Ref. | |||
| 1 | 0.85 (0.71, 1.03) | 0.29 | 0.66 (0.48, 0.90) | 0.024 | 0.79 (0.63, 0.99) | 0.024 |
| ≥2 | 1.14 (0.78, 1.66) | 0.81 (0.36, 1.85) | 0.73 (0.42, 1.26) | |||
| Race | ||||||
| White | Ref. | Ref. | Ref. | |||
| Black | 0.88 (0.72, 1.07) | 0.20 | 0.88 (0.64, 1.22) | 0.44 | 0.76 (0.59, 0.98) | 0.033 |
| Other | 1.11 (0.77, 1.60) | 0.56 | 1.02 (0.45, 2.29) | 0.97 | 1.47 (0.97, 2.21) | 0.068 |
| Insurance | ||||||
| Private | Ref. | Ref. | Ref. | |||
| Medicare | 1.10 (0.94, 1.30) | 0.24 | 0.83 (0.60, 1.14) | 0.26 | 1.03 (0.84, 1.28) | 0.76 |
| Medicaid | 1.89 (1.36, 2.62) | <0.001 | 1.19 (0.63, 2.25) | 0.59 | 1.02 (0.66, 1.58) | 0.92‡ |
| Other | 1.21 (0.67, 2.16) | 0.53 | 1.00 (0.38, 2.65) | 1.00 | 1.38 (0.79, 2.41) | 0.27 |
| Uninsured | 1.34 (0.83, 2.14) | 0.23 | 1.63 (0.91, 2.92) | 0.10 | 0.89 (0.53, 1.51) | 0.67 |
| Median income | ||||||
| <$38,000 | Ref. | Ref. | Ref. | |||
| $38,000–$47,999 | 0.99 (0.82, 1.20) | 0.062 | 0.74 (0.52, 1.06) | 0.13 | 0.90 (0.69, 1.17) | 0.94 |
| $48,000–$62,999 | 0.84 (0.69, 1.02) | 0.78 (0.55, 1.11) | 0.93 (0.72, 1.21) | |||
| ≥$63,000 | 0.85 (0.68, 1.06) | 0.71 (0.50, 1.02) | 0.96 (0.72, 1.26) | |||
| Urbanicity | ||||||
| Metropolitan | Ref. | Ref. | Ref. | |||
| Non-metropolitan | 1.06 (0.86, 1.30) | 0.61 | 0.76 (0.56, 1.05) | 0.093 | 1.01 (0.80, 1.27) | 0.95 |
| Facility location | ||||||
| Northeast | Ref. | Ref. | Ref. | |||
| Midwest | 1.82 (0.92, 3.58) | 0.084 | 1.05 (0.64, 1.70) | 0.86 | 1.13 (0.77, 1.68) | 0.53 |
| South | 1.07 (0.63, 1.84) | 0.79 | 1.22 (0.76, 1.95) | 0.40 | 0.71 (0.47, 1.08) | 0.11§ |
| West | 1.28 (0.68, 2.39) | 0.45 | 0.75 (0.46, 1.23) | 0.25 | 0.87 (0.56, 1.36) | 0.54 |
| Facility designation | ||||||
| Academic | Ref. | Ref. | Ref. | |||
| Non-academic | 0.65 (0.51, 0.84) | 0.001 | 1.29 (0.95, 1.76) | 0.10† | 0.93 (0.70, 1.23) | 0.60‡ |
| Facility case volume (per ten cases per year) | 0.91 (0.68, 1.22) | 0.53 | 0.58 (0.45, 0.75) | <0.001† | 0.68 (0.60, 0.76) | <0.001 |
| Year of diagnosis | ||||||
| 2004 | Ref. | Ref. | Ref. | |||
| 2005 | 0.92 (0.63, 1.33) | 0.95 | 0.98 (0.57, 1.67) | 0.74 | 1.25 (0.75, 2.09) | <0.001ठ|
| 2006 | 1.18 (0.82, 1.70) | 0.61 (0.33, 1.15) | 1.04 (0.65, 1.67) | |||
| 2007 | 1.40 (0.98, 1.99) | 0.78 (0.43, 1.42) | 1.63 (1.05, 2.54) | |||
| 2008 | 1.08 (0.75, 1.57) | 0.78 (0.45, 1.32) | 1.60 (1.03, 2.48) | |||
| 2009 | 0.84 (0.58, 1.23) | 0.65 (0.37, 1.15) | 1.60 (1.03, 2.47) | |||
| 2010 | 1.18 (0.81, 1.73) | 0.99 (0.58, 1.68) | 1.99 (1.27, 3.13) | |||
| 2011 | 1.15 (0.80, 1.65) | 0.81 (0.48, 1.38) | 1.92 (1.19, 3.08) | |||
| 2012 | 0.94 (0.64, 1.39) | 0.86 (0.52, 1.43) | 1.96 (1.26, 3.04) | |||
| 2013 | 1.00 (0.67, 1.49) | 0.80 (0.46, 1.38) | 1.75 (1.11, 2.76) | |||
| 2014 | 1.21 (0.81, 1.78) | 0.78 (0.46, 1.31) | 2.49 (1.60, 3.87) | |||
| PSA (per 5 ng/mL) | 1.03 (1.02, 1.05) | <0.001 | 1.00 (0.97, 1.04) | 0.89 | 1.01 (0.99, 1.04) | 0.15 |
| Grade group | ||||||
| 1-2 | Ref. | Ref. | Ref. | |||
| 3 | 1.47 (1.21, 1.79) | <0.001 | 1.26 (0.92, 1.72) | 0.97† | 1.18 (0.94, 1.49) | 0.003‡ |
| 4 | 1.74 (1.41, 2.15) | 0.84 (0.56, 1.27) | 1.22 (0.96, 1.57) | |||
| 5 | 1.99 (1.62, 2.44) | 1.11 (0.79, 1.54) | 1.39 (1.12, 1.72) | |||
| Pathologic stage | ||||||
| T2 | Ref. | Ref. | Ref. | |||
| T3a | 1.40 (1.14, 1.72) | <0.001 | 1.67 (1.15, 2.42) | 0.001 | 1.45 (1.14, 1.86) | <0.001ठ|
| T3b | 1.69 (1.41, 2.02) | 1.87 (1.33, 2.65) | 2.29 (1.80, 2.91) | |||
| T4 | 1.99 (1.39, 2.85) | 1.78 (0.84, 3.76) | 2.73 (1.84, 4.04) | |||
| Surgical margins | ||||||
| Negative | Ref. | Ref. | Ref. | |||
| Positive | 1.53 (1.34, 1.75) | <0.001 | 1.74 (1.37, 2.19) | <0.001 | 2.09 (1.74, 2.51) | <0.001‡ |
| Number of positive LN (per one LN) | 1.11 (1.07, 1.15) | <0.001 | 0.91 (0.80, 1.03) | 0.14† | 1.04 (0.99, 1.09) | 0.097‡§ |
| Number of negative LN (per five LN) | 0.96 (0.91, 1.00) | 0.052 | 0.99 (0.90, 1.08) | 0.78 | 0.94 (0.89, 1.01) | 0.078 |
p-values reported for CCI, income, year, grade group and stage derived from test for trend;
significant predictor of receipt of RT alone vs. ADT alone (p<0.05);
significant predictor of receipt of ADT and RT vs. ADT alone (p<0.05);
significant predictor of receipt of ADT and RT vs. RT alone (p<0.05); ADT, androgen deprivation therapy; CCI, Charlson Comorbidity Index; CI, confidence interval; LN, lymph node; PSA, prostate-specific antigen; RRR, relative risk ratio; RT, radiation therapy
Men with higher grade tumors were more likely to be managed with ADT alone than either observation, RT alone or combination therapy. Men with higher stage tumors and those with positive surgical margins were more likely to receive any treatment than undergo observation and were more also likely to be managed with combination therapy than ADT alone. Men with higher positive LN counts were more likely to receive ADT alone compared to all other management strategies and more likely to receive combination therapy compared to RT alone. Higher preoperative PSA levels were also associated with a higher likelihood of treatment with ADT alone compared to observation.
Of the 3,680 patients included in the survival analyses, 641 died during follow-up. The median follow-up time from the date of RP among survivors was 5.9 years (interquartile range, IQR, 4.7, 7.7). Probabilities of being alive at five and ten years after RP conditional on surviving at least one year after RP were 89% (95% confidence interval, CI, 87%, 90%) and 66% (95% CI 63%, 69%), respectively.
Associations between patient, tumor and facility characteristics and survival are described in Table 3. Grade group 4-5 disease, pathologic stage T3b-T4 disease and positive surgical margins were found to be independent predictors of worse OS. Survival was also found to be associated with nodal disease burden, with the optimal cut-point being determined to be three LN. Adjusted ten-year OS probabilities for patients with zero, one, two, three and four of the adverse pathologic features listed above were 84%, 75%, 65%, 51% and 32%, respectively (see Table 4).
Table 3.
Predictors of overall survival on multivariable analysis
| Multivariable HR (95% CI) | p-value | |
|---|---|---|
| Age (per five years) | 1.14 (1.05, 1.23) | 0.001 |
| CCI | ||
| 0 | Ref. | |
| 1 | 1.32 (1.07, 1.62) | 0.001 |
| ≥2 | 2.35 (1.25, 4.41) | |
| Race | ||
| White | Ref. | |
| Black | 1.22 (0.96, 1.56) | 0.11 |
| Other | 0.53 (0.27, 1.04) | 0.063 |
| Insurance | ||
| Private | Ref. | |
| Medicare | 0.96 (0.77, 1.21) | 0.74 |
| Medicaid | 1.28 (0.76, 2.13) | 0.35 |
| Other | 1.35 (0.70, 2.59) | 0.36 |
| Uninsured | 0.78 (0.44, 1.38) | 0.40 |
| Median income | ||
| <$38,000 | Ref. | |
| $38,000–$47,999 | 1.31 (1.02, 1.67) | 0.17 |
| $48,000–$62,999 | 0.99 (0.76, 1.28) | |
| ≥$63,000 | 0.95 (0.73, 1.23) | |
| Urbanicity | ||
| Metropolitan | Ref. | |
| Non-metropolitan | 0.95 (0.73, 1.23) | 0.67 |
| Facility location | ||
| Northeast | Ref. | |
| Midwest | 0.90 (0.69, 1.16) | 0.41 |
| South | 1.22 (0.92, 1.62) | 0.17 |
| West | 0.98 (0.75, 1.28) | 0.90 |
| Facility designation | ||
| Academic | Ref. | |
| Non-academic | 1.18 (0.97, 1.45) | 0.10 |
| Facility case volume (per ten cases per year) | 0.91 (0.82, 1.02) | 0.10 |
| Year of diagnosis | ||
| 2004 | Ref. | |
| 2005 | 0.75 (0.55, 1.01) | 0.49 |
| 2006 | 0.85 (0.62, 1.17) | |
| 2007 | 0.96 (0.71, 1.30) | |
| 2008 | 0.91 (0.65, 1.28) | |
| 2009 | 1.11 (0.79, 1.57) | |
| 2010 | 0.87 (0.61, 1.24) | |
| PSA (per 5 ng/mL) | 1.01 (0.99, 1.03) | 0.41 |
| Grade group | ||
| 1-2 | Ref. | |
| 3 | 1.00 (0.75, 1.33) | <0.001 |
| 4 | 1.48 (1.10, 1.99) | |
| 5 | 2.44 (1.91, 3.12) | |
| Pathologic stage | ||
| T2 | Ref. | |
| T3a | 1.11 (0.86, 1.44) | 0.002 |
| T3b | 1.42 (1.12, 1.80) | |
| T4 | 1.46 (0.97, 2.20) | |
| Surgical margins | ||
| Negative | Ref. | |
| Positive | 1.30 (1.10, 1.54) | 0.002 |
| Number of positive LN (per one LN) | 1.06 (1.04, 1.09) | <0.001 |
| Number of negative LN (per five LN) | 0.90 (0.83, 0.96) | 0.003 |
| Management | ||
| Observation | Ref. | |
| ADT alone | 1.06 (0.87, 1.29) | 0.56 |
| RT alone | 0.75 (0.50, 1.10) | 0.14 |
| ADT and RT | 0.69 (0.52, 0.92) | 0.010 |
p-values grade group and stage derived from for CCI, income, year, test for trend; ADT, androgen deprivation therapy; CCI, Charlson Comorbidity Index; CI, confidence interval; HR, hazard ratio; LN, lymph node; PSA, prostate-specific antigen; RT, radiation therapy
Table 4.
Adjusted ten-year overall survival probabilities according to grade group, pathologic stage, surgical margin status and number of positive LN
| Negative margins, 1-2 positive LN
|
Positive margins, 1-2 positive LN
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| GG 1-2 | GG 3 | GG 4 | GG 5 | GG 1-2 | GG 3 | GG 4 | GG 5 | ||
| pT2 | 85% | 85% | 78% | 67% | pT2 | 81% | 81% | 73% | 59% |
| pT3a | 83% | 83% | 76% | 63% | pT3a | 78% | 79% | 70% | 56% |
| pT3b | 79% | 79% | 71% | 56% | pT3b | 74% | 74% | 64% | 48% |
| pT4 | 78% | 78% | 70% | 55% | pT4 | 73% | 73% | 63% | 46% |
|
| |||||||||
|
Negative margins, ≥3 positive LN
|
Positive margins, ≥3 positive LN
|
||||||||
| GG 1-2 | GG 3 | GG 4 | GG 5 | GG 1-2 | GG 3 | GG 4 | GG 5 | ||
|
| |||||||||
| pT2 | 76% | 76% | 67% | 52% | pT2 | 71% | 71% | 60% | 43% |
| pT3a | 74% | 74% | 64% | 48% | pT3a | 68% | 68% | 56% | 39% |
| pT3b | 68% | 68% | 57% | 40% | pT3b | 61% | 61% | 49% | 30% |
| pT4 | 67% | 67% | 56% | 38% | pT4 | 60% | 60% | 47% | 29% |
Adjusted survival probabilities derived from multivariable Cox regression model; GG, grade group; LN, lymph nodes
On multivariable analysis, the combination of ADT and RT was found to be associated with significantly lower all-cause mortality compared to both observation (multivariate HR 0.69, 95% CI 0.52, 0.92, p=0.010) and ADT alone (HR 0.65, 95% CI 0.48, 0.89, p=0.008; see Figure 2). Treatment with either ADT or RT alone was not associated with either better or worse OS compared to observation. Adjusted ten-year OS probabilities conditional on surviving at least one year after RP for patients managed with observation, ADT alone, RT alone and ADT and RT were 69%, 67%, 75% and 77%, respectively.
Figure 2.
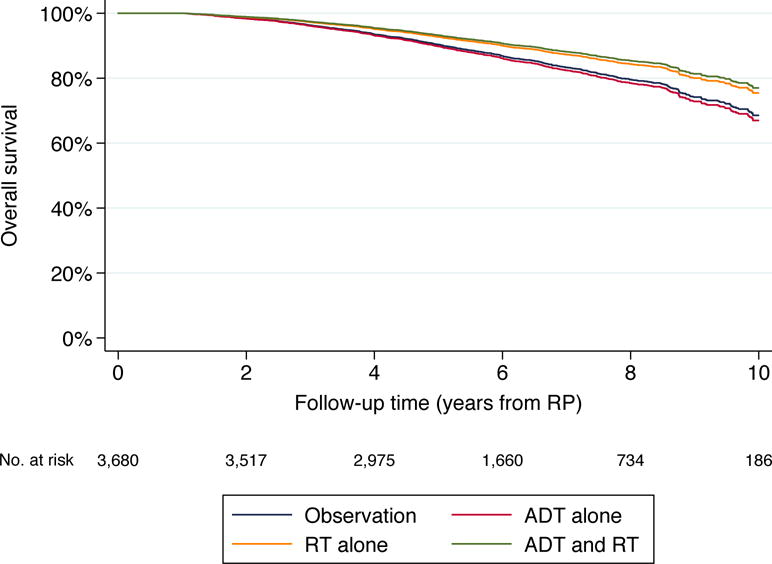
Adjusted overall survival by post-RP management strategy
Discussion
Using a large and nationally-representative cohort, we showed that the contemporary management of men with pN1 prostate cancer varies according to clinical and institutional characteristics. Although the use of combined ADT and RT appears to be increasing at a modest rate, multimodal therapy continues to be used much less frequently than either observation or ADT alone despite accumulating evidence that it is associated with better outcomes.
Although the prevalence of LN metastases among men undergoing RP has historically been reported to be low,8 it is strongly correlated with both the thoroughness of LN dissection (LND) and the pathologic characteristics of the primary tumor.9,10 Given the expected reverse stage migration precipitated by the US Preventive Services Task Force recommendations against prostate cancer screening, the shift towards performing RP on more high-risk patients and the increasing utilization of extended LND, the number of men with pN1 prostate cancer is expected to increase. These temporal trends, along with the unequivocally detrimental impact of LN metastases on survival,11 make understanding the natural history of and defining the optimal management strategy for pN1 prostate cancer issues of increasing importance.
To our knowledge, ours is the largest study examining patterns of care of men with pN1 prostate cancer. A prior study that used the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database to describe the management of 731 men with pN1 prostate cancer diagnosed between 1991 and 1999 reported that 61% of men were managed with observation and only 2% with both ADT and RT.12 In a subsequent SEER-Medicare analysis of 577 men diagnosed between 1995 and 2007, the reported proportion of men receiving combination therapy was 8%,13 further supporting the notion that the use of combination therapy has increased modestly over time. Neither of these studies assessed factors influencing the choice of post-RP management strategy. Importantly, our findings suggest that clinicians consider the pathologic characteristics of the primary tumor as well as the burden of LN metastases when deciding on management. Specifically, we found that patients with higher grade tumors and a greater number of positive LN were more likely to be managed with ADT alone than either observation, RT alone or combination therapy. This is in spite of the general lack of evidence that men with higher grade disease and higher metastatic LN burden are less likely to benefit from additional local therapy. The finding that black men with pN1 disease are less likely than white men to receive combination therapy is consistent with that of prior studies showing lower usage of definitive local therapies among black men with clinically localized prostate cancer14,15 and suggests that improving access to multimodal therapy may help address racial disparities in outcomes among men with this disease.
The finding of LN metastases at the time of RP has traditionally been thought to portend the presence of synchronous extra-nodal metastatic disease and therefore a uniformly poor prognosis, a paradigm whose validity is being increasingly challenged.16 Many men with pN1 disease do not experience disease recurrence on long-term follow-up, as shown by a study from our institution that reported a ten-year metastasis-free survival probability of 65% among a cohort of men managed without adjuvant ADT or RT.2 Although the present study confirms that the majority of men with pN1 prostate cancer have favorable outcomes (89% and 66% of men in our cohort were alive five and ten years after RP, respectively), it also draws attention to substantial variation in the prognosis of individual patients. As reported by several prior single-institution series, we found grade group 4-5 disease, pT3b-T4 disease, positive surgical margins and an increasing number of positive LN to be independent predictors of worse OS,17,18 which highlights the fact that the natural history of pN1 prostate cancer is driven as much by the characteristics of the primary tumor as the burden of LN metastases.
We also found that while combination therapy was associated with a significant reduction in all-cause mortality compared to observation alone, monotherapy with either ADT or RT was not. These findings stand in contrast to those of ECOG EST-3886, a randomized trial that showed immediate ADT to be superior to observation with delayed treatment with respect to OS among pN1 patients.1 The discrepant results pertaining to the benefit of ADT monotherapy may be explained by differences between the two study populations. Specifically, patients who were enrolled in the ECOG trial had a higher incidence of positive surgical margins and seminal vesicle invasion and, on average, a higher number of positive LN. These differences likely explain the significantly higher all-cause mortality in the observation arm of the ECOG trial, which approached 50% at ten years, compared to our cohort, in which the adjusted ten-year all-cause mortality was only 31%. Additionally, treatment of men in the observation arm of the ECOG trial was deferred until the development of clinical recurrence, which does not reflect contemporary practice. In contrast, our finding of improved OS with multimodal therapy is in line with that of recent observational studies. In the largest such study published to date, which included 1,107 patients at two tertiary care centers, both cancer-specific and all-cause mortality were found to be significantly lower among men who received both ADT and RT compared to those who received ADT alone, with subgroup analyses suggesting that the beneficial effect of combination therapy was limited to patients with 1-2 positive LN and either pT3b-pT4 disease or positive surgical margins and those with 3-4 positive LN irrespective of other tumor characteristics.19
The limitations of our analysis with respect to determining whether the association between post-RP management and survival is a causal one must be acknowledged. Most importantly, the NCDB does not contain information pertaining to post-RP PSA levels, an important limitation given that an undetectable PSA post-RP has been shown to be an independent predictor of survival among pN1 patients.20 It is possible that men whose PSA levels do not become undetectable after surgery or rise rapidly after becoming undetectable are less likely to receive combination therapy as opposed to ADT alone or observation as they are regarded as having systemic disease and therefore less likely to benefit from local therapy. Furthermore, because of a lack of data pertaining to recurrence, we could not address the question of whether combination therapy given in an adjuvant setting is superior to early salvage therapy at the time of PSA recurrence. Lastly, the benefit of ADT and RT with respect to potentially prolonging survival must be balanced against the significant toxicities associated with these treatments, which are not captured by the NCDB.
Strengths of our study include a large sample size and a follow-up period of sufficient length to capture the majority of cancer-related deaths in this high-risk population. Its main limitation is that the observational nature of the study means that the results are susceptible to bias due to unmeasured or residual confounding. The study was also limited by the absence of information pertaining to surgical approach and the administration of novel hormonal therapies and chemotherapy among those men who eventually developed castrate-resistant disease.
Conclusions
In summary, we describe significant heterogeneity in the post-RP management and outcomes of men with pN1 prostate cancer. Our data suggest that combined ADT and RT may be associated with a survival benefit in this setting, which is in line with the findings of prior observational studies and suggests that a randomized trial of multimodal therapy in this population is warranted.
Acknowledgments
The data used in this study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
This work was supported by NIH/NCI Cancer Center Support Grant P30 CA008748 and the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations
- ADT
androgen deprivation therapy
- CCI
Charlson Comorbidity Index
- CSS
cancer-specific survival
- HR
hazard ratio
- LN
lymph node
- LND
lymph node dissection
- NCDB
National Cancer Data Base
- OS
overall survival
- pN1
pathologic lymph node-positive
- PSA
prostate-specific antigen
- RP
radical prostatectomy
- RT
radiation therapy
- SEER
Surveillance, Epidemiology and End Results
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Peter T. Scardino is a consultant for Steba Biotech. The remaining authors have no conflicts of interest to disclose.
References
- 1.Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 2.Touijer KA, Mazzola CR, Sjoberg DD, et al. Long-term outcomes of patients with lymph node metastasis treated with radical prostatectomy without adjuvant androgen-deprivation therapy. Eur Urol. 2014;65:20. doi: 10.1016/j.eururo.2013.03.053. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27:2924. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 6.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 7.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 8.Abdollah F, Sun M, Thuret R, et al. Decreasing rate and extent of lymph node staging in patients undergoing radical prostatectomy may undermine the rate of diagnosis of lymph node metastases in prostate cancer. Eur Urol. 2010;58:882. doi: 10.1016/j.eururo.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 9.Kluth LA, Abdollah F, Xylinas E, et al. Clinical nodal staging scores for prostate cancer: a proposal for preoperative risk assessment. Br J Cancer. 2014;111:213. doi: 10.1038/bjc.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdollah F, Suardi N, Gallina A, et al. Extended pelvic lymph node dissection in prostate cancer: a 20-year audit in a single center. Ann Oncol. 2013;24:1459. doi: 10.1093/annonc/mdt120. [DOI] [PubMed] [Google Scholar]
- 11.Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185:869. doi: 10.1016/j.juro.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong YN, Freedland S, Egleston B, et al. Role of androgen deprivation therapy for node-positive prostate cancer. J Clin Oncol. 2009;27:100. doi: 10.1200/JCO.2007.14.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan JR, Kowalczyk KJ, Borza T, et al. Patterns of care and outcomes of radiotherapy for lymph node positivity after radical prostatectomy. BJU Int. 2013;111:1208. doi: 10.1111/bju.12079. [DOI] [PubMed] [Google Scholar]
- 14.Schmid M, Meyer CP, Reznor G, et al. Racial differences in the surgical care of Medicare beneficiaries with localized prostate cancer. JAMA Oncol. 2016;2:85. doi: 10.1001/jamaoncol.2015.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziehr DR, Mahal BA, Aizer AA, et al. Income inequality and treatment of African American men with high-risk prostate cancer. Urol Oncol. 2015;33:18 e7. doi: 10.1016/j.urolonc.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Briganti A, Passoni NM, Abdollah F, et al. Treatment of lymph node-positive prostate cancer: teaching old dogmas new tricks. Eur Urol. 2014;65:26. doi: 10.1016/j.eururo.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Carlsson SV, Tafe LJ, Chade DC, et al. Pathological features of lymph node metastasis for predicting biochemical recurrence after radical prostatectomy for prostate cancer. J Urol. 2013;189:1314. doi: 10.1016/j.juro.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moschini M, Sharma V, Zattoni F, et al. Risk stratification of pN+ prostate cancer after radical prostatectomy from a large single institutional series with long-term follow-up. J Urol. 2016;195:1773. doi: 10.1016/j.juro.2015.12.074. [DOI] [PubMed] [Google Scholar]
- 19.Abdollah F, Karnes RJ, Suardi N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. 2014;32:3939. doi: 10.1200/JCO.2013.54.7893. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi L, Nini A, Bianchi M, et al. The role of prostate-specific antigen persistence after radical prostatectomy for the prediction of clinical progression and cancer-specific mortality in node-positive prostate cancer patients. Eur Urol. 2016;69:1142. doi: 10.1016/j.eururo.2015.12.010. [DOI] [PubMed] [Google Scholar]


