Abstract
The objectives of this study were to develop a symbiotic oat-based beverage (SOB) and to analyze its physiochemical properties and the probiotic survivability. The beverage base was prepared by fermenting a mix containing oat flour (10%, w/w), sugar (4%, w/w), and inulin (1%, w/w) with a commercial Lactobacillus plantarum (0.003%, w/w) at 30 °C for 12 h. The SOB was formulated using the fermented oat base, sugar, stabilizers (pectin and λ-carrageenan), vitamin C, and citric acid. The beverage was analyzed for total solids (11.65 ± 0.22%), protein (0.58 ± 0.02%), fat (0.37 ± 0.02%), carbohydrate (10.70 ± 0.33%), ash (0.14 ± 0.01%), and dietary fiber (0.70 ± 0.05%). The pH value of the beverage was stable at about 3.60 during 7-week storage. Lactobacillus plantarum population in the beverage remained above 107 CFU/g throughout the storage. Oat-based beverage is a low fat and high dietary fiber symbiotic food.
Electronic supplementary material
The online version of this article (10.1007/s10068-017-0290-0) contains supplementary material, which is available to authorized users.
Keywords: Oat, Symbiotic, Fermentation, Probiotic surviviability
Introduction
Oat, the sixth most important cereal in the world, has been recorded for at least 2000 years. It is reported to be good source of proteins, dietary fibers, vitamins, unsaturated fatty acids, and phytochemicals. Proteins of oat range from 12 to 20% with chemical score of 72–75 and contain high level of lysine [1]. Dietary fibers account for about 3.1% of oat and were composed of 55% soluble fiber and 45% insoluble fiber [2]. β-Glucan, well known for its “function claims” and “claims connected with the reduction of disease”, constitutes the major water-soluble fraction of oat [3]. It was reported to be responsible for many biological activities of oat [3], such as reducing blood glucose and blood cholesterol levels [4], antiproliferative properties [5], anticancer properties [6]. However, oat-based food products are limited to oat meal, flakes, and cereal breakfast [7].
Probiotic is a kind of microorganisms, which can colonize in the colon and then exert beneficial health effects on the host by altering microflora. Lactic acid bacteria (LAB) are representative live food ingredients of probiotic. Among LABs, Lactobacillus plantarum is a prominent species which has been proved to be able to enhancing the immune system and promoting nutrient absorption by colonizing in human colon [8]. Lactobacillus plantarum is widely used in the fermentation of vegetables and fruits. Prebiotic are a group of carbohydrates which cannot be decomposed or absorbed in the upper digestive tract but selectively enhance the growth of one or more probiotic [9]. In recent years, β-glucan derived from oat or other cereals has been intensively studied for its prebiotic effects. Barley β-glucan can improve the growth rate of Lactobacillus acidophilus LA5, L. plantarum WCFS1, and Lactobacillus fermentum CECT8448. It was reported that oat-based products stimulate the bifidobacteria in humans [10]. Symbiotic foods are foods combined probiotic with prebiotic and might be more efficient than the individual components in the colon.
Symbiotic foods is a popular topic in food industry due to consumers’ demands. Dairy products are the common carrier of symbiotic food and have been commercialized. However, considerable proportion of consumers are suffering milk protein allergy and lactose intolerance [11]. Seeking for alternatives to dairy food is necessary and oat-based symbiotic foods might be the good choice. The objectives of the present study are to develop a symbiotic oat-based beverage containing probiotic (L. plantarum) and prebiotic (inulin) and analyze the physiochemical properties and probiotic survivability of the product.
Materials and methods
Materials
Whole oat flour was provided by Seamild Co., Ltd. (Guilin, China). The raw material was analyzed for (w/w): protein 11.97 ± 0.16%, fat 7.54 ± 0.15%, carbohydrate 65.04 ± 1.33%, and dietary fiber 10.57 ± 0.10%. Starter cultures ABY-3 (Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus, with Bifidobacterium BB-12 and Lactobacillus acidophilus LA-5), Lactobacillus helveticus and L. plantarum Vege-Start 60, GIN696265 were purchased from Chr. Hansen Co., Ltd. (Milwaukee, WI, USA). Inulin was purchased from local Zhangye Co., Ltd. (Gansu, China). Pectin and λ-carrageenan were donated by CPKELCO (Lille Skensved, Denmark). Sugar was purchased from a local market. Citric acid and vitamin C were provided by Qiaofu Co., Ltd. (Beijing, China). Fortification mix containing iron gluconate, zinc gluconate, calcium gluconate, and vitamin E was provided by DSM Co., Ltd. (Heerlen, Netherlands).
Optimization of fermentation conditions for oat base and formulation of SOB
SOB was formulated with proper amount of fermented oat base and stabilizer. The fermentation conditions for oat base including starter cultures, inoculation level, fermentation temperature and time were optimized as shown in Table 1. The factors were studied under the following conditions: (a) all samples were inoculated with 0.0005–0.005% L. plantarum and incubated at 30 °C for 12 h; (b) all samples were inoculated with 0.003% L. plantarum and then incubated at 30 °C for 6–36 h; (c) all samples were inoculated with 0.003% L. plantarum and then incubated at 15–50 °C for 12 h. Sugar and vitamin C, oat base level ranged from 5 to 30% and levels of stabilizers were optimized during the formulation of trials (Table 1).
Table 1.
Variables for optimization of fermentation conditions for oat base and formulation of SOB
| Level | |
|---|---|
| Fermentation conditions | |
| Starter culture | ABY-3, L. helveticus, L. plantarum |
| Inoculation level (%, w/v) | 0.0005, 0.001, 0.002, 0.003, 0.004, 0.005 |
| Fermentation temperature (oC) | 15, 21, 30, 37, 42, 50 |
| Fermentation time (h) | 6, 12, 18, 24, 30, 36 |
| Production of SOB | |
| Sugar (%, w/v) | 0, 1, 2, 3, 4, 5, 6 |
| Vitamin C (%, w/v) | 0, 0.02, 0.04, 0.06, 0.08, 0.1 |
| Oat base (%, w/v) | 5, 10, 15, 20, 25, 30 |
| Pectin (%, w/v) | 0.1, 0.2, 0.3, 0.4, 0.5 |
| λ-Carrageenan (%, w/v) | 0.1, 0.2, 0.3, 0.4, 0.5 |
Preparation of SOB
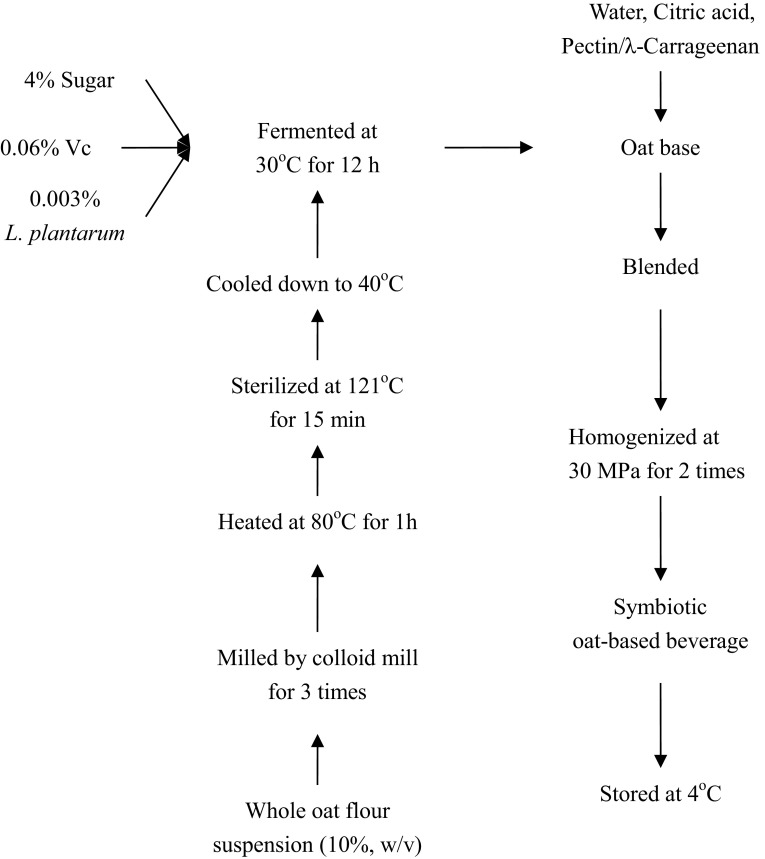
The SOB was prepared as described in Fig. 1. Whole oat flour mix (10%, w/w) was blended for 3 times, gelatinized at 80 °C for 1 h, sterilized at 121 °C for 15 min, and then cooled down to 40 °C, incubated with L. plantarum (0.003%, w/w). The inoculated oat mix was fermented at 30 °C for 12 h, and then the oat base with water, stabilizers, prebiotic and citric acid was homogenized at 30 MPa twice. The formulation of the final product was as follows (w/w): whole oat flour 5% (oat base 10%), inulin 1%, sugar 6%, vitamin C 0.06%, pectin 0.4%, λ-carrageenan 0.2%, and citrate acid 0.15%. Three batches of SOB were prepared on different days and stored at 4 °C for the subsequent test.
Fig. 1.
Flow chart of preparation of the symbiotic oat-based beverage (SOB)
Physiochemical analysis
The pH was determined with a pH-meter (PHS-3C, Jingke, Shanghai, China) calibrated at 25 °C. Total solids content was determined by a moisture meter (MJ33, Mettler Toledo, Zurich, Switzerland). Protein content was measured by Kieldahl Azotometer (UDK-159, VELP Scientific Co., Ltd., Brianza, Italy) using a conversion factor of 6.25. Fat content was measured by Gabriel centrifugation method (5 min, 110×g). Ash content was measured by dry-ashing using a muffle furnace (SX-2.5-12, Jingke, Shanghai, China). Carbohydrate content was calculated by total solids minus protein content, fat content or ash content. Measurable dietary fiber was determined according to previous study [12]. All measurements were performed in triplicates for the three trials.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of oat protein isolate
SOB samples (50 g) were frozen at − 80 °C for until further usage. Frozen samples were thawed and left immersed in hexane solution (1:4, w/v) for 6 h with the constant agitation for complete contact. After extraction, the upper hexane liquid was dumped and the oat base extract was left in the ventilation for drying. Dried oat base extract was added with 100 mL of NaOH solution (15 mM), and then agitated for 1 h, centrifuged at 4000×g at 10 °C for 10 min. The process was repeated twice and the two suspensions were collected and combined. The combined suspension was adjusted to pH 4.5 with HCl (2 M), and then centrifuged at 10,000×g for 20 min, the precipitate obtained after centrifugation was the oat protein isolate (OPI). The SDS-PAGE analysis for OPI was conducted using gel imaging system (GelDoc 2000, Bio-Rad, CA, USA) according to the method of Angelov et al. [13].
Viscosity determination
Viscosity was measured using a Brookfield viscometer (DV-3, Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA). Samples were loaded to the viscometer with constant revolution of 200 rpm with a LV4 spindle.
Sensory evaluation
Thirty graduate students at the Department of Food Science, Jilin University were selected and trained as the primary evaluators. And then a panel including 10 persons was screened based on the ability of describing and sensitivity to sensory attributes. The panel was trained according to international standards [14]. An overall acceptability was rated based on the aspects of color, texture, flavor and taste. Before tasting each sample, the panelists were required to rinse their mouth thoroughly with deionized water. Then, all the panelists were required to assess the samples individually and gave the overall acceptability score with a full score of 10 (from dislike to like).
Storage stability determination
Samples added with different level of stabilizers were prepared and then stored in graduated tubes at 4 °C for 2 weeks. The height of upper liquid layer was measured and recorded as the index of the separation. It would be 0 if there was no separation, or it would be “gelation” if the beverage gelled when stabilizers were added.
Shelf-life tests and survivability of probiotics
During storage, overall acceptability, survivability of probiotics, pH, and viscosity of the beverage samples were conducted weekly for 7 weeks. Enumeration of Bifidobacterium and L. acidophilus were conducted according to the procedure of Chr. Hansen [15]. Count of L. helveticu was conducted as the method described by Shah et al. [5]. The enumeration of L. plantarum was measured according to China National Food Safety Standard [16] and the probiotic result was expressed as CFU/g. SDS-PAGE of stored samples was conducted according to the instruction of the SDS-PAGE Gel Kit (Kangwei, Beijing, China) using an electrophoresis unit (Bio-Rad Laboratories, USA) at 130 V.
Yeast, mold, and coliform counts
Coliform, mold, and yeast counts were determined using Petrifilm plates (3M™ Petrifilm™, St. Paul, MN, USA). The coliform plates were incubated at 35 °C for 24 h. Mold and yeast plates were incubated at 21 °C for 5 d. Coliform count was expressed as MPN/100 mL and mold/yeast counts was expressed as CFU/g.
Statistical analysis
All data obtained were expressed as mean ± standard deviation (SD). The significant differences of data between samples and control were calculated using Version SPSS 11.5 (SPSS Inc. Chicago, IL). The significance level was set at p < 0.05 and p < 0.01. Data were checked for homogeneity by Leveneǐs test. When the data were homogeneous, one-way analysis of variance (ANOVA) and then a least squared differences (LSD) model was used. When the data were heterogeneous, the Dunnett’s test was applied. All the figures were drawn by Origin 8.0 (OriginLab Corporation, Northampton. USA).
Results and discussion
Preliminary results
Preparation of oat base
Whole oat flour suspension was mixed, gelatinized and sterilized before fermentation. Oat flour at high levels would form into paste during heat treatment while too low level would be insufficient to provide enough oat to the final SOB. Therefore, choosing an appropriate whole flour oat level for the preparation of base was essential. Oat flour ranged from 5 to 30% (w/v) was studied and results indicated that 10% level of oat flour gave preferable texture and sensory properties to the base and final product (data was not shown). Walsh et al. [17] reported that a 5% level of whole oat flour was used for the production of yogurt-like symbiotic oats-based product. However, it should be noticed that the authors used a gelation agent before fermentation and the starter cultures were different. Angelov et al. [13] stated that 5.5% oat flour was the appropriate level for fermentation of oat using L. plantarum B28 as starter culture with consideration of viable cell counts and β-glucan content.
Starter culture, inoculant level, incubation time and temperature
To achieve required level of probiotic population and sensory attributes, the oat mix was inoculated with ABY-3, L. plantarum or L. helveticus. Results (Supplementary File Fig. S1) indicated that both ABY-3 and L. plantarum give a final pH value below 5, while L. helveticus about 6.13. Compared with ABY-3, L. plantarum had higher acid producing ability and a final pH below 4.5. The survival rate of the different starter cultures on the oat was measured. Compared with ABY-3 or L. helveticus, oat inoculated with L. plantarum showed the highest population of probiotic (~ 8.26 Log CFU/g) under the optimized conditions, while the other two was 7.58 and 6.86 Log CFU/g, respectively. It was documented that the most suitable pH for the growth of L. plantarum was about 6.5, while the original pH value of 10% oat is about 6.29. This proved the feasibility of L. plantarum for the following fermentation. Similar results were also reported by other scholars who used same starter culture but different strains. Angelov et al. [13] developed an oat-based probiotic drink using L. plantarum B28. Russo et al. [18] developed an oat-based fermented beverage using L. plantarum Lp90. Magala et al. [19] also formulated an oat beverage using L. planatarum CCM 7039.
Effects of inoculant level, incubation time and temperature of L. plantarum on overall acceptability, pH, and probiotic population of SOB were shown in Fig. 2. With the inoculant level increased, the overall acceptability increased and the highest score occurred at 0.003 or 0.004% [Fig. 2(A)]. As regarding of viable probiotic, there was no significant difference between oat with 0.003 or 0.004% (p > 0.05). The pH value of oat fermented with 0.004% starter culture was significantly lower than other levels (p < 0.05). Effects of incubation time ranging from 0 to 36 h on properties of SOB were studied [Fig. 2(B)]. Beverage prepared with oat base fermented for 12 h showed highest overall acceptability score. With prolonged incubation time, pH of the SOB decreased from 6.29 to 3.80. Probiotic population started to decrease from 30 h, which might be the beginning of decline period. Effects of incubation temperature on overall acceptability and probiotic cell were shown in Fig. 2(C). It can be seen that oat incubated at 30 °C showed the highest overall acceptability score (7.86 ± 0.06) and viable probiotic population (8.53 ± 0.09 Log CFU/g). Combined all the results discussed above, oat base was fermented at 30 °C for 12 h with inoculant level of 0.003% of L. plantarum. The temperature was lower and time was shorter than other studies which reported to be 37 °C for 16–48 h [13, 18, 19]. However, another study reported the similar results where a yogurt-like beverage made by fermenting a mixture of cereals, soy, and grape used various levels of L. plantarum at 30 °C for 8 h [20]. The difference should be attributed to the different starter cultures, the concentration of the oat or the type of the substrate.
Fig. 2.
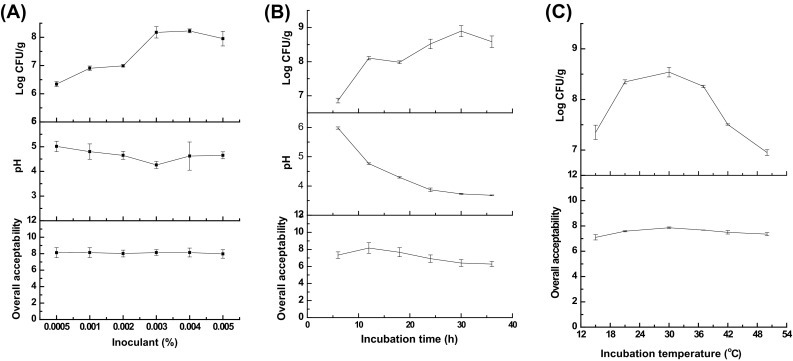
Effects of inoculant level (A), incubation time (B) and temperature (C) on overall acceptability, pH and probiotic population in the beverage
SOB formulation
Sugar, as the main carbon source of microorganism and a sweetener, was added into the product to facilitate the production of acidity in fermented oat product [17]. Sugar level ranged from 0 to 6% was studied. Compared with the control, 4% level increased the population of L. plantarum by almost 2 logs and there was no significant difference between SOB with 4% or with 6% (p > 0.05). Combined with the sensory properties of the final product, 6% addition level was selected.
To increase the acidity of the final product, effects of vitamin C ranged from 0 to 0.1% on the population of probiotic were investigated (data were not shown here). Vitamin C addition gave the product a final pH value about 3.45. Furthermore, compared with the control, 0.06% addition can improve the population of L. plantarum by almost 1 log. Therefore, 0.06% vitamin C addition was selected.
Flavor is one of the major characteristics of non-dairy symbiotic drink [11] and it is important to balance the oat flavor and the texture of the final product. SOB prepared with 50% base exhibited preferable sensory properties and uniform texture after 24 h storage. Similar result was reported by Coda et al. [20], who developed yogurt-like beverages using 50% oat and other cereals as the base.
Gums are often used as stabilizers to improve the texture and stability of beverages [21]. Effectiveness of high methoxyl pectin in stabilizing fermented dairy drinks has been known for 50 years [22]. Pectin and λ-carrageenan are anion polysaccharides, which can interact with the positive site of the protein, forming a mixture, preventing flocculation of the protein by steric hindrance [23, 24]. Combination of pectin and λ-carrageenan was evaluated as the stabilizer of SOB and the results were listed in Supplementary File (Table S1). SOB added with 0.3% pectin/0.5% λ-carrageenan, 0.4% pectin/0.2–0.5% λ-carrageenan remained homogeneous after 2 weeks’ storage. Combined with the consideration of cost, 0.4% pectin and 0.2% λ-carrageenan was selected as the compound stabilizers.
Chemical composition of SOB
Chemical composition of the SOB was shown in Table 2. The total solids of the product was 11.65 ± 0.22% and was similar to that of cow’ milk (~ 11.5%) [25]. It was higher than those reported by Walsh et al. [17].
Table 2.
Chemical composition of SOB
| Composition | Content |
|---|---|
| Total solids (%, w/w) | 11.65 ± 0.22 |
| Protein (%, w/w) | 0.58 ± 0.02 |
| Fat (%, w/w) | 0.37 ± 0.02 |
| Carbohydrate (%, w/w) | 10.70 ± 0.33 |
| Ash (%, w/w) | 0.14 ± 0.01 |
| Dietary fiber (%, w/w) | 0.70 ± 0.05 |
| Calcium (mg/kg) | 743.33 ± 13.15 |
| Iron (mg/kg) | 17.56 ± 0.44 |
| Zinc (mg/kg) | 12.13 ± 0.49 |
| Vitamin E (mg/kg) | 0.41 ± 0.12 |
Oat protein, mainly composed of oat globulins (~ 50%), is superior source of plant protein [1]. The protein content of the new product was 0.58 ± 0.02%. Whole grains and cereal brans are the rich source of insoluble fiber. It should be notable from the chemical composition analysis that the soluble dietary fibers in the new product was as high as 0.70 ± 0.05%. β-Glucan, inulin, and pectin existing in the product were responsible. β-Glucan, which was typically 3–5%, was the main dietary fiber in oat [26]. Arena et al. found that barley β-glucans could improve the growth of L. acidophilus LA5, L. plantarum WCFS1, L. plantarum CETC 8328, and L. fermentum CECT 8448 [27]. Inulin is well known for enhancing probiotic growth and has been widely reported [28]. The SOB is fortified for calcium, iron, zinc, and vitamin E. The contents of above nutrients for fortification were: 700 mg/kg calcium, 15 mg/kg iron, 12 mg/kg zinc, 0.40 mg/kg vitamin. The final nutrients were 743.33 ± 13.15 mg/kg calcium, 17.56 ± 0.44 mg/kg iron, 12.13 ± 0.49 mg/kg zinc, 0.41 ± 0.12 mg/kg vitamin, which was 1024, 506, 1254, and 655% of the SOB without fortification, respectively.
Shelf-life tests
Sensory evaluation is related with consumers’ acceptance and satisfaction of a new product. The sensory score of SOB was about 9 at the beginning and kept up to 7 within the first 3 weeks, which means the beverage has a good sensory in 21 days [Fig. 3(A)]. Although there was some decrease in overall acceptability in the following 4 weeks, the score was still around 5.
Fig. 3.
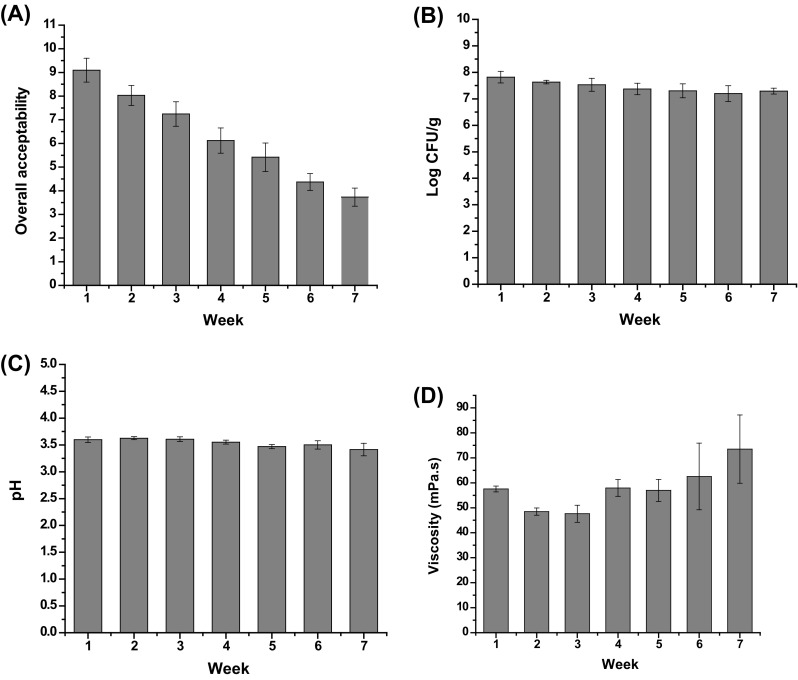
Changes of SOB in overall acceptability (A), probiotic population (B), pH (C) and viscosity (D) during 7-week storage
The counts of L. plantarum stayed above 5 × 107 CFU/g which far exceeds the national requirement 1 × 106 CFU/g for symbiotic beverage [Fig. 3(B)]. It has been recommended that the viability of probiotics should be at least 107 CFU/g of product at the time of consumption to provide various health benefits [29]. Yeast, mold, or coliform determination was conducted each week and there was no yeast, mold, or coliform detected during 7 weeks’ storage.
The initial pH of the SOB was about 3.5, which were a desirable sensory property and a favorable condition for oat protein to be effective. Acidic protein beverage usually had a pH value ranging from 3.6 to 4.5. Previous study has revealed that oat protein isolate has poor functional properties at slightly acidic and neutral pH levels [30]. During the storage period, pH values did not change significantly (p > 0.05) [Fig. 3(C)].
Viscosity of the SOB was 57.53 ± 1.19 mPa s. Similar result was reported by Nakamura et al. [31] who developed a dairy drink with pectin as stabilizer with viscosity of ~ 60.5 mPa s. The viscosity of the drink was reduced significantly in the first 3 weeks (p < 0.05) and then increased significantly with the highest value at the end of the study at 73.55 mPa s (p < 0.05) [Fig. 3(D)].
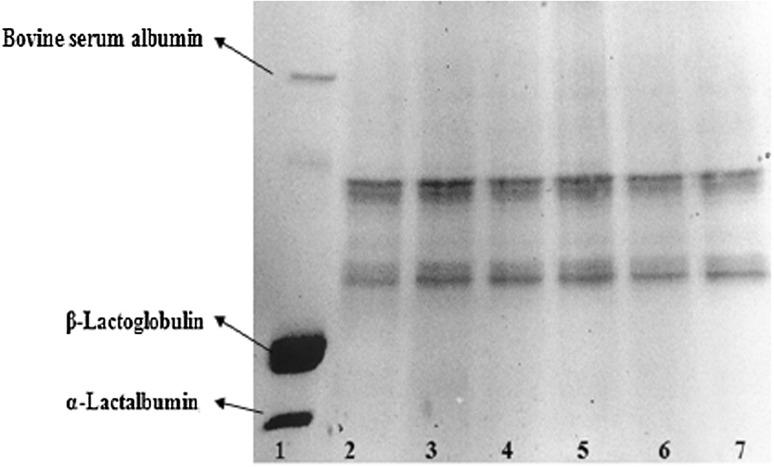
Oat proteins include globulins, albumins, prolamins and glutelin [32]. The protein profiles of the beverage were shown in Fig. 4. The molecular weight of the standards was about 14, 17, and 64 kDa for α-lactalbumin, β-lactoglobulin, and bovine serum albumin, respectively [33]. The two main bands in the lane of beverage samples may be that of globulin for the upper band, the main fraction of oat protein with molecular weight of about 45 kDa [34]. The lower band can be distributed to albumins with molecular weight about 36 and 22 kDa [35]. There were no considerable changes in the intensity of the major protein bands during storage.
Fig. 4.
SDS-PAGE of oat protein in the beverage during 6-week storage. Lane 1: whey protein; Lane 2–7: SOB samples from week 1 to 6
In summary, the optimal processing conditions for the symbiotic oat beverage with stable texture and good sensory attributes using L. plantarum as starter culture were at 30 °C and 12 h for fermentation temperature and time. Population of probiotic in the beverage remained above 107 CFU/g during the shelf life tests. This beverage is a low fat and high dietary fiber beverage. This new product maybe a good alternative to dairy beverages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank The Ministry of Science and Technology of China (Project# 2013BAD18B07) for financial support for this project.
Compliance with ethical standards
Conflict of interest
The authors’ declare that they have no conflicts of interest.
References
- 1.Zhang B, Guo X, Zhu K, Peng W, Zhou H. Improvement of emulsifying properties of oat protein isolate-dextran conjugates by glycation. Carbohyd. Polym. 2015;127:168–175. doi: 10.1016/j.carbpol.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 2.Fuller S, Beck E, Salman H, Tapsell L. New horizons for the study of dietary fiber and health: a review. Plant Food Hum. Nutr. 2016;71:1–12. doi: 10.1007/s11130-016-0529-6. [DOI] [PubMed] [Google Scholar]
- 3.Bevilacqua A, Casanova FP, Petruzzi L, Sinigaglia M, Corbo MR. Using physical approaches for the attenuation of lactic acid bacteria in an organic rice beverage. Food Microbiol. 2016;53:1–8. doi: 10.1016/j.fm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. Jama. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 5.Shah A, Masoodi FA, Gani A, Ashwar BA. Effect of γ-irradiation on antioxidant and antiproliferative properties of oat β-glucan. Radiat Phys. Chem. 2015;117:120–127. doi: 10.1016/j.radphyschem.2015.06.022. [DOI] [Google Scholar]
- 6.Choromanska A, Kulbacka J, Rembialkowska N, Pilat J, Oledzki R, Harasym J, Saczko J. Anticancer properties of low molecular weight oat beta-glucan - An in vitro study. Int. J. Biol. Macromol. 2015;80:23–28. doi: 10.1016/j.ijbiomac.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 7.Londono DM, Smulders MJM, Visser RGF, Gilissen LJWJ, Hamer RJ. Effect of kilning and milling on the dough-making properties of oat flour. LWT-Food Sci. Technol. 2015;63:960–965. doi: 10.1016/j.lwt.2015.04.033. [DOI] [Google Scholar]
- 8.Gbassi GK, Vandamme T, Ennahar S, Marchioni E. Microencapsulation of Lactobacillus plantarum spp. in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 2009;129:103–105. doi: 10.1016/j.ijfoodmicro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Ogueke CC, Owuamanam CI, Ihediohanma NC, Iwouno JO. Probiotics and prebiotics: unfolding prospects for better human health. Pakistan J. Nutr. 2010;9:833–843. doi: 10.3923/pjn.2010.833.843. [DOI] [Google Scholar]
- 10.Mårtensson O, Biörklund M, Lambo AM, Dueñas-Chasco M, Irastorza A, Holst O, Norin E, Welling G, Öste R, Önning G. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr. Res. 2005;25:429–442. doi: 10.1016/j.nutres.2005.03.004. [DOI] [Google Scholar]
- 11.Kumar BV, Vijayendra SVN, Reddy OVS. Trends in dairy and non-dairy probiotic products-a review. J. Food Sci. Tech. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Standardization Administration of the People’s Republic of China. Determination of dietary fiber in foods. Available from: http://bz.cfsa.net.cn/staticPages/78E7C3F0-F79D-42D1-B870-89839A74E392.html. Accessed Mar. 21, 2016
- 13.Angelov A, Gotcheva V, Kuncheva R, Hristozova T. Development of a new oat-based probiotic drink. Int. J. Food Microbiol. 2006;112:75–80. doi: 10.1016/j.ijfoodmicro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 14.International Organization for Standardization. Sensory analysis–General guidelines for the selection, training and monitoring of selected assessors and expert sensory assessors. Available from: https://www.iso.org/standard/45352.html. Accessed May.12, 2012.
- 15.Hansen C. Lactobacillus acidophilus, Lactobacillus casei and Bifidobacteria in fermented milk products-guidelines. Method for counting probiotic bacteria. Technical Bulletin F-6: 1–8 (2005)
- 16.Standardization Administration of the People’s Republic of China. National food safety standard food microbiological examination: Lactic Acid Bacteria.. Available from:http://bz.cfsa.net.cn/staticPages/5DA9E166-7962-4A92-B4EF-F30F7252010D.html. Accessed Dec.23, 2016.
- 17.Walsh H, Ross J, Hendricks G, Guo M. Physico-chemical properties, probiotic survivability, microstructure, and acceptability of a yogurt-like symbiotic oats-based product using pre-polymerized whey protein as a gelation agent. J. Food Sci. 2010;75:327–337. doi: 10.1111/j.1750-3841.2010.01637.x. [DOI] [PubMed] [Google Scholar]
- 18.Russo P, Chiara MLVD, Capozzi V, Arena MP, Amodio ML, Rascón A, Dueñas MT, López P, Spano G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT-Food Sci. Technol. 2016;68:288–294. doi: 10.1016/j.lwt.2015.12.040. [DOI] [Google Scholar]
- 19.Magala M, Kohajdová Z, Karovičová J, Greifová M, Greif G. Utilization of oat flour for preparation of Lactic Acid fermented beverages. Chem. Listy. 2015;109:210–213. [Google Scholar]
- 20.Coda R, Lanera A, Trani A, Gobbetti M, Cagno RD. Yogurt-like beverages made of a mixture of cereals, soy and grape must: microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012;155:120–127. doi: 10.1016/j.ijfoodmicro.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Sun C, Xue Y, Gao Y. Impact of pH, freeze-thaw and thermal sterilization on physicochemical stability of walnut beverage emulsion. Food Chem. 2016;196:475–485. doi: 10.1016/j.foodchem.2015.09.061. [DOI] [PubMed] [Google Scholar]
- 22.Kiani H, Mousavi ME, Razavi H, Morris ER. Effect of gellan, alone and in combination with high-methoxy pectin, on the structure and stability of doogh, a yogurt-based Iranian drink. Food Hydrocolloids. 2010;24:744–754. doi: 10.1016/j.foodhyd.2010.03.016. [DOI] [Google Scholar]
- 23.Beaulieu M, Turgeon SL, Doublier JL. Rheology, texture and microstructure of whey proteins/low methoxyl pectins mixed gels with added calcium. Int. Dairy J. 2001;11:961–967. doi: 10.1016/S0958-6946(01)00127-3. [DOI] [Google Scholar]
- 24.Lizarraga MS, Dde PV, GonzáLez R, Rubiolo A, Santiago LG. Rheological behaviour of whey protein concentrate and λ-carrageenan aqueous mixtures. Food Hydrocolloids. 2006;20:740–748. doi: 10.1016/j.foodhyd.2005.07.007. [DOI] [Google Scholar]
- 25.Routray W, Mishra HN. Scientific and technical aspects of yogurt aroma and taste: a review. Compr. Rev. Food Sci. 2011;10:208–220. doi: 10.1111/j.1541-4337.2011.00151.x. [DOI] [Google Scholar]
- 26.Rebello CJ, O’Neil CE, Greenway FL. Dietary fiber and satiety: the effects of oats on satiety. Nutr. Rev. 2016;74:131–147. doi: 10.1093/nutrit/nuv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arena MP, Caggianiello G, Fiocco D, Russo P, Torelli M, Spano G, Capozzi V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014;15:3025–3039. doi: 10.3390/ijms15023025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 29.Rajam R, Karthik P, Parthasarathi S, Joseph GS, Anandharamakrishnan C. Effect of whey protein–alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods. 2012;4:891–898. doi: 10.1016/j.jff.2012.06.006. [DOI] [Google Scholar]
- 30.Ma CY. Chemical characterization and functionality assessment of protein concentrates from oats. Cereal Chem. 1983;60:36–42. [Google Scholar]
- 31.Nakamura A, Furuta H, Kato M, Maeda H, Nagamatsu Y. Effect of soybean soluble polysaccharides on the stability of milk protein under acidic conditions. Food Hydrocolloids. 2003;17:333–343. doi: 10.1016/S0268-005X(02)00095-4. [DOI] [Google Scholar]
- 32.Runyon JR, Sunilkumar BA, Nilsson L, Rascon A, Bergenståhl B. The effect of heat treatment on the soluble protein content of oats. J. Cereal Sci. 2015;65:119–124. doi: 10.1016/j.jcs.2015.06.008. [DOI] [Google Scholar]
- 33.O’Loughlin IB, Murray BA, Kelly PM, Fitzgerald RJ, Brodkorb A. Enzymatic hydrolysis of heat-induced aggregates of whey protein isolate. J. Agric. Food Chem. 2012;60:4895–4904. doi: 10.1021/jf205213n. [DOI] [PubMed] [Google Scholar]
- 34.Yang C, Wang Y, Chen L. Fabrication, characterization and controlled release properties of oat protein gels with percolating structure induced by cold gelation. Food Hydrocolloids. 2017;62:21–34. doi: 10.1016/j.foodhyd.2016.07.023. [DOI] [Google Scholar]
- 35.Mirmoghtadaie L, Kadivar M, Shahedi M. Effects of succinylation and deamidation on functional properties of oat protein isolate. Food Chem. 2009;114:127–131. doi: 10.1016/j.foodchem.2008.09.025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.