Abstract
Physicochemical properties and oxidative stability of refined coconut oil (RCO), refined soybean oil (SBO), pure olive oil (POO), and vegetable shortening (VST) during repeated frying of potato chips were determined. Polyunsaturated fatty acids of all the oils significantly decreased after frying. Tocopherols in SBO, POO and VST, and DPPH radical scavenging activities of POO and VST significantly decreased after frying. L* values of the oils significantly decreased, and a* and b* values significantly increased after 80 times repeated frying. Conjugated dienes and p-anisidine value of SBO after 80 times repeated frying were 21.8 mmol/L and 47.7, respectively, the highest among the oils. Levels of total polar compounds of all the oils after 80 times repeated frying were between 8.1 and 9.5%, not exceeding rejection limit after frying. Compositions and contents of alkanals, 2-alkenals, and 2,4-alkadienals in the oils during frying were largely affected by their fatty acid compositions.
Keywords: Deep-fat frying, Fatty acid composition, Physicochemical property, Oxidative stability, Volatile compound
Introduction
Deep-fat frying is one of the best cooking techniques to make palatable foods with golden color, savory flavor, and desirable texture through a complete immersion of food materials in a frying oil. During frying, heat is transferred from oil to food materials, and water in fried products evaporates simultaneously with the products absorbing the oil [1]. In commercial frying process, frying oils are usually repeatedly used, consequently changing quality of fried foods with formation of non-volatiles and volatiles, some of which are potentially harmful to human health [2]. Therefore, it is important to monitor changes in quality of frying oils. Physical parameters such as color and viscosity, and chemical parameters such as acid value (AV), total polar compound (TPC), and p-anisidine value (p-AN) have been generally considered to assess the quality of frying oils [3]. As TPC have been considered to be a reasonable factor to evaluate quality of frying oils, acceptable limits of TPC have been suggested to be 24–27% (w/w) [4]. In Korea, AV has been used to monitor quality of oils with legal rejection limit of frying oil being 3.0 AV [5]. Volatile compounds, especially aldehydes, formed during frying process of oils are a decisive factor for flavor of fried products even at low concentrations. Thus, determination of volatile compounds in frying oils would be crucial to monitor their quality [6].
In frying process, hydrolysis, oxidation, and polymerization of the oils occur. These chemical reactions of frying oils mostly depend on their fatty acid compositions and antioxidant contents [7]. It is known that the higher unsaturated fatty acids (UFA) exist in the oils, the faster thermal oxidation occurs during frying. It has been suggested that fats and oils with high amounts of saturated fatty acids (SFA), such as coconut oil and palm oil, be used in frying in substitution for conventional frying oils with high amounts of UFA [4]. Compared to conventional frying oils, coconut oil and vegetable shortening with a mixture of palm oil and palm stearin are expected to be most stable during frying. Man and Hussin [8] determined physicochemical properties of refined, bleached, and deodorized palm olein and coconut oil during intermittent frying of potato chips at 180 °C for 5 h/day for 5 consecutive days. Srivastava and Semwal [9] monitored frying performance and oxidative stability of virgin coconut oil during frying of chick pea at 180 °C for 8 h (48 repeated frying). These studies indicated that coconut oil can be stable during intermittent frying conditions. Meanwhile, comparison between the oils with high amounts of SFA and UFA in terms of physicochemical properties and oxidative stability during repeated frying has been little studied.
In this study, physicochemical properties and oxidative stability of refined coconut oil (RCO), refined soybean oil (SBO), pure olive oil (POO), and vegetable shortening (VST), which are widely used in frying and have quite different fatty acid compositions one another, were determined during repeated frying of potato chips.
Materials and methods
Materials
RCO, SBO, POO (a mixture of 85% refined and 15% virgin olive oils), VST (a mixture of palm oil and palm stearin; mixed ratio unknown), and fresh potatoes were purchased from local markets in Seoul, Korea. The oils were stored at 4 °C until used for frying. A mixture of 37 fatty acid methyl esters (FAME), α-, γ-, and δ-tocopherols, pentanal, hexanal, octanal, decanal, 2-hexenal, 2-octenal, 2,4-heptadienal, 2,4-decadienal, p-anisidine, boron trifluoride (BF3)-methanol solution, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, acetonitrile, ethanol, ether, hexane, isooctane, methanol, phenolphthalein, potassium hydroxide, 2-propanol, and sodium hydroxide were purchased from Samchun Chemical (Seoul, Korea). All the chemicals were of analytical reagent grades.
Frying procedure and sampling
To prepare potato chips, fresh potatoes were washed, peeled, and cut into slices of 4 mm thickness using a potato slicer. They were immersed in cold water and then pat dried with paper towels. Each oil (4 L) was placed in an electric fryer with 6 L capacity (Delki, Goyang, Korea) and heated to 180 ± 5 °C. The potato chips were fried for 4 min. After the chips were taken out, the oil was heated again for 2 min before the next frying. This process was repeated with 80 cycles. At every 20th cycle, 80 mL of the oil was collected and stored at − 20 °C until analyzed.
Analysis of fatty acid composition
The oils were methylated using BF3–methanol solution according to AOCS Official Method Ce 2-66 [10]. Fatty acid composition was determined using an Agilent 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with a capillary column (DB-23, 30 m × 0.25 mm × 0.25 μm, J and W Scientific, Folsom, CA, USA) and a flame ionization detector. Oven temperature was programmed as follows: from 50 to 160 °C at 25 °C/min, to 220 °C at 4 °C/min held for 8 min, and to 250 °C at 25 °C/min held for 5 min. Split ratio was 1:50. Injector and detector temperatures were kept at 220 and 260 °C, respectively. Fatty acids were identified with retention times of the standards and expressed as % (relative area).
Analysis of tocopherols and DPPH radical scavenging activity
Tocopherols of the oils were analyzed using an HPLC (Ultimate 3000; Thermo Scientific Dionex, Waltham, MA, USA) equipped with a silica-based column (ZORBAX Eclipse Plus C18, Agilent Technologies, Santa Clara, CA, USA) [11]. Each oil was dissolved in 1 mL 2-propanol, followed by filtering the solution using a 0.2 μm hydrophobic syringe filter (Advantec MFS Inc., Dublin, CA, USA). Mobile phase was a mixture of methanol and acetonitrile (1:1) at 1.5 mL/min. A fluorescence detector was set at an emission wavelength of 325 nm and an excitation wavelength of 295 nm. Tocopherols were identified by comparing their retention times with those of corresponding standards.
DPPH radical scavenging activity of the oils were determined according to a method by Brand-Williams et al. [12]. Fifty μL of each oil was added with 950 μL 0.2 mM DPPH in methanol. The mixture was vortexed and held at room temperature for 5 min in the dark. Absorbance was measured at 515 nm using a UV–vis spectrometer (Spectramax 190, Molecular Devices, Sunnyvale, CA, USA) against a blank sample. DPPH radical scavenging activity (%) was calculated as follows: DPPH radical scavenging activity (%) = (1 − absorbance of the sample/absorbance of the DPPH solution) × 100.
Physicochemical properties
Hunter L* (lightness: black (0) to white (100)), a* (greenness (−) to redness (+)), and b* (blueness (−) to yellowness (+)) values of the oils were measured using a spectrophotometer (CM-5, Konica Minolta Co., Tokyo, Japan). VST was melted at 60 °C and the other oils were placed in a water bath at 30 °C. Twelve mL of each oil was placed in a glass cuvette (10 mm path length). The cuvette was washed with hexane before the next measurement. AV and p-AN of the oils were determined according to AOCS Official Method Ca 5a-40 and Cd 18-90, respectively [10]. Conjugated dienes (CD) of the oils were determined spectrophotometrically at 234 nm and read against hexane as blank. An extinction coefficient of 29,000 mol/L was used to quantify CD [13]. TPC of the oils were spectrophotometrically determined at 490 nm using a method based on a correlation between TPC contents and absorbances of the oils proposed by Xu [14]. The equation used for a conversion of the absorbance to TPC (%) was: y = − 2.7865x 2 + 23.782x + 1.039, where y is TPC (%) of an oil and x is its absorbance.
Analysis of volatile compounds
Volatile compounds of the oils were determined using HS-SPME-GC/MS. Fiber type, extraction condition, and operating conditions followed a method by Lee et al. [15] with a slight modification. One g of each oil was weighed in a headspace vial and placed into a shaking water bath at 65 °C for 15 min for equilibrium. Extraction temperature was 65 °C because VST melts above 60 °C. After that, a fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (50/30 μm film thickness, Supelco, Bellefonte, PA, USA) was inserted into the headspace of the vial and maintained for 60 min at 65 °C to extract volatile compounds. The fiber was desorbed for 10 min in injection port of a Shimazdu QP2010 Plus gas chromatography (Shimazdu Co., Kyoto, Japan) equipped with a 0.75 mm ID glass injection liner, a capillary column (DB-5, 30 m × 0.25 mm × 0.25 μm, J and W Scientific, Folsom, CA, USA), and a mass selective detector. Oven temperature was programmed as follows: 40 °C held for 2 min, to 160 °C at 6 °C/min, and to 280 °C at 10 °C/min held for 2 min. Interface and ion source temperatures were kept at 260 °C. Helium was used as carrier gas at 25 cm/s. Injector temperature was kept at 250 °C and splitless mode was used for injection. Scan mode was used for data analysis. Mass peaks of volatile compounds were identified by matching with mass spectrum and similarity indices of the National Institute of Standards and Technology (NIST) library or retention times of chemical standards spiked with RCO at cycle 0, which was free of these compounds under the same conditions.
Statistical analysis
All experiments were carried out in triplicate. The results were expressed as means ± standard deviations. Independent t test, one-way analysis of variance (ANOVA) with Duncan’s multiple range test, Pearson correlation test, and principal component analysis (PCA) combined with VARIMAX rotation were performed with SPSS program (version 21.0, SPSS Chicago, IL, USA).
Results and discussion
Fatty acid compositions of the frying oils
Fatty acid compositions of the frying oils used 80 times repeatedly are shown in Table 1. Palmitic (C16:0) and stearic acids (C18:0) in RCO and VST significantly increased after 80 times repeated frying, and only C18:0 in POO significantly increased (p < 0.05). Oleic acid (C18:1) in RCO significantly decreased from 6.5 to 4.8% (p < 0.05). Linoleic acid (C18:2) in the oils except SBO significantly decreased, and linolenic acid (C18:3) in SBO and POO significantly decreased from 7.3 to 7.1% and from 0.2 to 0% (p < 0.05), respectively. These results were similar to previous studies: C18:1 and C18:2 in Picual and Arbequina olive oils and high oleic sunflower oil significantly decreased after 70 times repeated frying at 180 °C [16]. C18:1 and C18:2 in canola oil significantly decreased and C16:0 and C18:0 relatively increased after frying at 185 °C for 49 h [17]. The increase in SFA and decrease in UFA in the oils fried repeatedly might result from more oxidation of UFA than that of SFA in the oils during frying.
Table 1.
Fatty acid composition of frying oils used 80 times repeatedly (%, relative area)
| Fatty acid | Refined coconut oil | Refined soybean oil | Pure olive oil | Vegetable shortening | ||||
|---|---|---|---|---|---|---|---|---|
| Fresh | 80 times repeated use | Fresh | 80 times repeated use | Fresh | 80 times repeated use | Fresh | 80 times repeated use | |
| C16:0 | 10.6 ± 0.4 | 11.7 ± 0.1* | 11.3 ± 0.1 | 11.6 ± 0.3 | 12.0 ± 0.2 | 12.6 ± 0.3 | 50.1 ± 0.4 | 51.2 ± 0.1* |
| C18:0 | 3.6 ± 0.2 | 4.0 ± 0.1* | 4.7 ± 0.03 | 4.9 ± 0.1 | 3.2 ± 0.03 | 3.3 ± 0.1* | 4.7 ± 0.1 | 4.8 ± 0.01* |
| C18:1 | 6.5 ± 0.2 | 4.8 ± 0.4* | 21.5 ± 0.1 | 21.7 ± 0.1 | 72.9 ± 0.6 | 73.3 ± 1.4 | 35.9 ± 0.4 | 35.6 ± 0.3 |
| C18:2 | 1.6 ± 0.1 | 0.8 ± 0.1* | 53.7 ± 0.2 | 53.3 ± 0.3 | 9.4 ± 0.1 | 8.7 ± 0.2* | 7.9 ± 0.1 | 7.1 ± 0.1* |
| C18:3 | ND | ND | 7.3 ± 0.04 | 7.1 ± 0.2* | 0.2 ± 0.4 | ND | ND | ND |
| SFA | 92.0 ± 0.3 | 94.4 ± 0.5* | 16.1 ± 0.2 | 16.7 ± 0.3 | 15.2 ± 0.2 | 15.9 ± 0.4* | 56.0 ± 0.4 | 57.1 ± 0.1* |
| MUFA | 6.5 ± 0.2 | 4.8 ± 0.4* | 22.9 ± 0.1 | 23.0 ± 0.2 | 75.2 ± 0.7 | 74.9 ± 0.6 | 36.1 ± 0.3 | 35.8 ± 0.1 |
| PUFA | 1.6 ± 0.8 | 0.8 ± 0.1* | 61.0 ± 0.2 | 60.3 ± 0.2* | 9.6 ± 0.4 | 8.7 ± 0.2* | 7.9 ± 0.1 | 7.1 ± 0.1* |
Values are means and standard deviations (n = 3)
ND not detected, SFA saturated fatty acids, MUFA monounsaturated fatty acids and PUFA polyunsaturated fatty acids
*Significantly different within the same oil (p < 0.05; t-test)
Tocopherols and DPPH radical scavenging activities of the frying oils
Contents of tocopherols and antioxidants present in an oil are significant factors on its oxidative stability besides its fatty acid composition [17]. Changes in tocopherols in the frying oils used repeatedly are shown in Table 2. Tocopherols were not detected in RCO. During frying, α-, (β + γ)-, and δ-tocopherols of SBO, POO, and VST significantly decreased (p < 0.05). Kamal-Eldin [18] reported that in frying process α-tocopherol can be degraded to several oxidation products (7,8-epoxy- α-tocopherolquinone, 4a,5-epoxy- α-tocopherolquinone, and α-tocopherolquinone). Total tocopherols in SBO, POO, and VST after 80 times repeated frying decreased from 89.9 to 82.9 mg/100 g oil, from 14.9 to 2.3 mg/100 g oil, and from 8.7 to 0.6 mg/100 g oil, respectively, and their degradation rates in POO (− 84.8%) and VST (− 93.5%) were remarkably higher than in SBO (− 7.8%).
Table 2.
Changes in tocopherols (mg/100 g oil) and DPPH radical scavenging activity (%) in frying oils used repeatedly
| Repetition | α-Tocopherol | (β + γ)-Tocopherol | δ-Tocopherol | Total tocopherols | DPPH radical scavenging activity | |
|---|---|---|---|---|---|---|
| Refined coconut oil | 0 | ND | ND | ND | ND | 20.3 ± 1.8NS |
| 20 | ND | ND | ND | ND | 15.9 ± 3.7 | |
| 40 | ND | ND | ND | ND | 15.5 ± 2.8 | |
| 60 | ND | ND | ND | ND | 16.0 ± 2.3 | |
| 80 | ND | ND | ND | ND | 16.4 ± 2.6 | |
| Refined soybean oil | 0 | 9.6 ± 0.3a | 57.3 ± 0.4a | 23.0 ± 0.3a | 89.9 ± 0.9a | 86.5 ± 4.1NS |
| 20 | 9.5 ± 0.2ab | 56.1 ± 0.8a | 22.7 ± 0.3a | 88.3 ± 1.3a | 85.8 ± 2.9 | |
| 40 | 9.1 ± 0.3b | 53.1 ± 1.3b | 22.0 ± 0.3b | 84.2 ± 1.6b | 85.9 ± 3.9 | |
| 60 | 9.4 ± 0.4ab | 53.1 ± 0.6b | 22.0 ± 0.2b | 84.5 ± 1.1b | 82.3 ± 7.1 | |
| 80 | 9.2 ± 0.1b | 51.7 ± 0.5b | 22.1 ± 0.2b | 82.9 ± 0.4b | 78.8 ± 8.5 | |
| Pure olive oil | 0 | 14.0 ± 0.2a | 0.8 ± 0.0a | 0.06 ± 0.00a | 14.9 ± 0.2a | 31.3 ± 2.9a |
| 20 | 10.4 ± 1.1b | 0.6 ± 0.1b | 0.05 ± 0.01a | 11.0 ± 1.2b | 26.7 ± 4.4ab | |
| 40 | 7.6 ± 1.0c | 0.4 ± 0.04c | 0.04 ± 0.01b | 8.0 ± 1.0c | 23.2 ± 3.9bc | |
| 60 | 4.6 ± 1.0d | 0.2 ± 0.03d | 0.04 ± 0.01bc | 4.9 ± 1.0d | 22.1 ± 3.2bc | |
| 80 | 2.1 ± 0.7e | 0.1 ± 0.01e | 0.03 ± 0.01c | 2.3 ± 0.7e | 19.3 ± 2.9c | |
| Vegetable shortening | 0 | 6.9 ± 0.2a | 1.1 ± 0.3a | 0.7 ± 0.1a | 8.7 ± 0.1a | 39.8 ± 9.5a |
| 20 | 3.2 ± 0.6b | 0.6 ± 0.1b | 0.5 ± 0.1b | 4.3 ± 0.8b | 23.6 ± 1.8b | |
| 40 | 1.0 ± 0.8c | 0.3 ± 0.04c | 0.3 ± 0.1c | 1.6 ± 0.9c | 19.4 ± 4.5bc | |
| 60 | 0.3 ± 0.03c | 0.1 ± 0.03d | 0.2 ± 0.04 cd | 0.6 ± 0.1d | 15.1 ± 3.2bc | |
| 80 | 0.4 ± 0.1c | 0.02 ± 0.01d | 0.2 ± 0.03d | 0.6 ± 0.1d | 12.5 ± 2.3c |
Values are means and standard deviations (n = 3)
ND not detected, NS not significant
a–eValues with different superscripts represent significant differences within the same columns in each oil (p < 0.05; one-way ANOVA and Duncan’s multiple range test)
DPPH radical scavenging activities of POO and VST after 80 times repeated frying significantly decreased from 31.3 to 19.3% and from 39.8 to 12.5%, respectively (p < 0.05) (Table 2). Lee et al. [19] reported that DPPH absorbance of soybean oil, canola oil, olive oil significantly increased during thermal oxidation at 180 °C for 8 h. They suggest that radical scavenging compounds such as α-tocopherol in edible oil be degraded during thermal oxidation in accordance with the decrease in DPPH radical scavenging activity of the oil. Likewise, in the present study, significantly strong correlations between total tocopherols and DPPH radical scavenging activities of POO (r 2 = 0.87) and VST (r 2 = 0.90) were observed (p < 0.01).
Physicochemical properties of the frying oils
Changes in L*, a*, b*, AV, CD, TPC, and p-AN of frying oils used repeatedly are shown in Table 3. Color is a useful parameter often used in food industry for prompt monitoring of oil quality. Color of an oil during frying may be changed by brown pigments eluted from fried foods to the oil and degradation products derived from the oil during hydrolysis, oxidation, and polymerization [1]. In general, L* values of the oils decreased, and a* and b* values increased during frying. These results were similar to a previous study, reporting that L* value of virgin coconut oil decreased, and a* and b* values increased during 8 h frying [9]. However, b* value of the fresh POO was higher than the oil used 80 times repeatedly in our study, probably because the fresh POO might contain a high amount of chlorophylls and carotenoids, although their levels were not measured in this study, which in turn might be rapidly degraded during frying [16]. It was found that carotenoid content and b* value of an olive oil had a significant correlation: the more carotenoids were in the oil, the higher its b* value was [20].
Table 3.
Changes in L*, a*, b*, acid value, conjugated dienes (mmol/L), total polar compounds (%), and p-anisidine value of frying oils used repeatedly
| Repetition | L* | a* | b* | Acid value | Conjugated dienes | Total polar compounds | p- Anisidine value | |
|---|---|---|---|---|---|---|---|---|
| Refined coconut oil | 0 | 99.6 ± 0.1a | − 1.1 ± 0.0c | 4.4 ± 0.02e | 0.02 ± 0.00d | 4.5 ± 0.1d | 2.8 ± 0.3e | 1.0 ± 0.03e |
| 20 | 97.7 ± 0.1b | − 0.9 ± 0.1bc | 7.3 ± 0.5d | 0.1 ± 0.00c | 5.6 ± 0.5c | 4.6 ± 0.9d | 4.7 ± 1.3d | |
| 40 | 96.1 ± 0.4c | − 0.7 ± 0.2ab | 8.9 ± 0.3c | 0.2 ± 0.07c | 6.8 ± 0.9b | 5.8 ± 0.4c | 7.3 ± 1.4c | |
| 60 | 94.4 ± 0.1d | − 0.5 ± 0.2a | 10.7 ± 0.4b | 0.3 ± 0.02b | 8.3 ± 0.4a | 7.1 ± 0.1b | 9.8 ± 1.0b | |
| 80 | 92.5 ± 0.1e | − 0.4 ± 0.2a | 12.6 ± 0.8a | 0.3 ± 0.06a | 9.0 ± 0.3a | 8.1 ± 0.5a | 12.0 ± 0.8a | |
| Refined soybean oil | 0 | 99.0 ± 0.1a | − 2.8 ± 0.2b | 9.8 ± 1.0e | 0.1 ± 0.03a | 10.5 ± 1.5d | 2.9 ± 0.04d | 3.0 ± 0.3e |
| 20 | 97.6 ± 0.4a | − 2.8 ± 0.1b | 12.2 ± 0.7d | 0.1 ± 0.03a | 13.7 ± 2.0 cd | 4.6 ± 0.3c | 17.8 ± 1.2d | |
| 40 | 95.9 ± 0.7b | − 3.0 ± 0.1b | 15.8 ± 0.9c | 0.1 ± 0.04a | 16.6 ± 1.7bc | 5.9 ± 0.3c | 26.3 ± 1.1c | |
| 60 | 93.5 ± 1.0c | − 2.8 ± 0.3ab | 20.1 ± 1.6b | 0.2 ± 0.02ab | 19.8 ± 1.5ab | 7.8 ± 0.9b | 36.0 ± 1.6b | |
| 80 | 90.9 ± 1.5d | − 2.2 ± 0.5a | 23.9 ± 1.7a | 0.2 ± 0.04b | 21.8 ± 2.0a | 9.5 ± 1.0a | 47.7 ± 1.5a | |
| Pure olive oil | 0 | 95.2 ± 0.05a | − 4.1 ± 0.0d | 27.0 ± 0.1a | 0.2 ± 0.01d | 4.9 ± 0.8d | 6.9 ± 0.03d | 4.3 ± 0.1e |
| 20 | 95.0 ± 0.3a | − 3.8 ± 0.1 cd | 18.3 ± 0.2d | 0.4 ± 0.01c | 8.0 ± 1.1c | 5.5 ± 0.2c | 16.7 ± 1.4d | |
| 40 | 93.5 ± 0.4b | − 3.5 ± 0.2bc | 19.9 ± 0.6d | 0.4 ± 0.03bc | 9.8 ± 0.9b | 6.7 ± 0.2c | 24.5 ± 0.8c | |
| 60 | 91.8 ± 1.0c | − 3.1 ± 0.4ab | 22.9 ± 1.3c | 0.4 ± 0.04b | 11.3 ± 1.0b | 8.2 ± 0.6b | 31.6 ± 0.7b | |
| 80 | 90.5 ± 1.2c | − 2.9 ± 0.5a | 25.1 ± 1.6b | 0.6 ± 0.05a | 14.3 ± 0.2a | 9.2 ± 0.7a | 37.8 ± 0.5a | |
| Vegetable shortening | 0 | 97.6 ± 0.1a | − 4.4 ± 0.1c | 17.0 ± 0.5d | 0.1 ± 0.02d | 8.2 ± 0.1d | 3.6 ± 0.1c | 8.68 ± 0.2e |
| 20 | 94.4 ± 0.4b | − 4.2 ± 0.1c | 22.5 ± 0.2c | 0.2 ± 0.03c | 9.5 ± 0.4c | 6.5 ± 1.1b | 28.4 ± 2.7d | |
| 40 | 92.6 ± 0.6c | − 3.9 ± 0.2b | 25.9 ± 1.2b | 0.2 ± 0.03b | 10.5 ± 0.2c | 7.4 ± 0.5b | 34.9 ± 1.8c | |
| 60 | 91.4 ± 0.7d | − 3.6 ± 0.2a | 27.4 ± 1.5ab | 0.3 ± 0.03b | 11.7 ± 0.2b | 8.5 ± 0.4a | 38.7 ± 1.0b | |
| 80 | 90.6 ± 0.4d | − 3.4 ± 0.2a | 28.2 ± 1.4a | 0.4 ± 0.01a | 14.7 ± 1.3a | 9.1 ± 0.3a | 41.9 ± 1.3a |
Values are means and standard deviations (n = 3)
a–eValues with different superscripts represent significant differences within the same columns in each oil (p < 0.05; one-way ANOVA and Duncan’s multiple range test)
AV is a quality parameter to determine free fatty acids in an oil [1]. AV of RCO remarkably increased from 0.02 in the fresh oil to 0.3 in the oil used 80 times repeatedly (p < 0.05). AV of SBO slightly increased from 0.1 to 0.2. Nawar [21] reported that when oil is heated in presence of moisture, hydrolysis of triacylglycerols (TG) with butyric acid (C4:0), caprylic acid (C8:0), and lauric acid (C12:0) is faster than that of TG with myristic acid (C14:0), C16:0, and C18:0, explaining why RCO, which has relatively high content of C8:0 (5.6%) and C12:0 (47%), was more hydrolyzed than the other oils during frying. All of the tested frying oils had less free fatty acids than the acceptable limits regulated by the Food Code in Korea [5].
When PUFA in an oil are oxidized during frying, their double bond positions are shifted, consequently forming conjugated double bonds. Thus, measuring CD can be a reliable indicator on oxidation products in an oil during frying [22]. CD in all the tested oils significantly increased during the repeated frying (p < 0.05). CD in POO increased from 4.9 to 14.3 mmol/L, the highest increment rate among the oils. CD in SBO increased from 10.5 to 21.8 mmol/L, the highest among the oils. On the other hand, CD in RCO marginally increased with the lowest levels. These results suggest that RCO, which was the least in PUFA, may have less oxidation products with conjugated double bonds than the other oils.
Contents of TPC in an oil, such as TG dimers and polymers, oxidized TG monomers, and diacylglycerols, increased by thermal oxidation, resulting in lowering its quality [7]. Regardless of type of frying oil, TPC significantly increased after 80 times repeated frying (p < 0.05). TPC in RCO and SBO were 8.1 and 9.5%, respectively. Arsian et al. [23] reported that TPC in cottonseed oil and palm oil used for frying for 8 h were 8.5 and 9.0%, respectively. Srivastava and Semwal [9] also reported that TPC in virgin coconut oil increased from 2.8 to 8.1% after used for frying for 8 h, similar with the results of the present study. All the tested oils did not exceed the rejection limit of TPC (24–27%) suggested by European countries [4].
p-AN is a quality parameter to determine contents of aldehydes such as 2-alkenals and 2,4-alkadienals in frying oil [24]. p-AN of all the oils significantly increased during frying (p < 0.05). After frying, p-AN of SBO increased from 3.0 to 47.7 with the highest increment rate and level. On the other hand, p-AN of RCO marginally increased with the lowest level. Tompkins and Perkins [25] determined relationship of p-AN with sensory evaluation, reporting p-AN was significantly correlated with overall odor intensity (r = 0.82), fried food odor score (r = 0.53), and burn odor score (r = 0.43). Therefore, the repeatedly used SBO may have more oxidized odor and fried food flavor than the RCO.
RCO seemed to be the most stable among the oils during frying considering CD and p-AN, which were significantly lower than those of the other oils. On the other hand, although oxidation of SBO, which had the highest level of tocopherols (Table 2), might be suppressed by tocopherols in the oil during frying [7], CD and p-AN of SBO were the highest among the oils. Nayak et al. [1] reported that C18:3 showed the highest oxidation rate, followed by C18:2 and C18:1 among C18:0, C18:1, C18:2, and C18:3. It implies that oil with more USF produces more CD and aldehydes during frying.
Volatile compounds in the frying oils
Changes in total volatile compounds, alkanals, 2-alkenals, and 2,4-alkadienals in the repeatedly used frying oils are shown in Fig. 1. Six alkanals (pentanal, hexanal, heptanal, octanal, nonanal, and decanal), eight 2-alkenals (2-propenal, 2-butenal, 2-pentenal, 2-hexenal, 2-heptenal, 2-octenal, 2-nonenal, and 2-decenal), and three 2,4-alkadienals (2,4-heptadienal, 2,4-nonadienal, and 2,4-decadienal) were identified. Total volatile compounds in all the oils significantly increased until 20 times repeated frying, and then little changed until the end of frying (p < 0.05). Patterns of the changes in alkanals, 2-alkenals, and 2,4-alkadienals were almost the same as in the total volatile compounds. These results were similar to a previous study, reporting that changes in volatile compounds in oils did not show a linear increase during heating at 170 °C [26]. These results imply that although volatile compounds derived from oxidation of an oil are constantly generated during frying, contents of volatile compounds in the oil could depend on thermal degradation and emission of the volatile compounds into the atmosphere and reactions with food materials [7, 23].
Fig. 1.
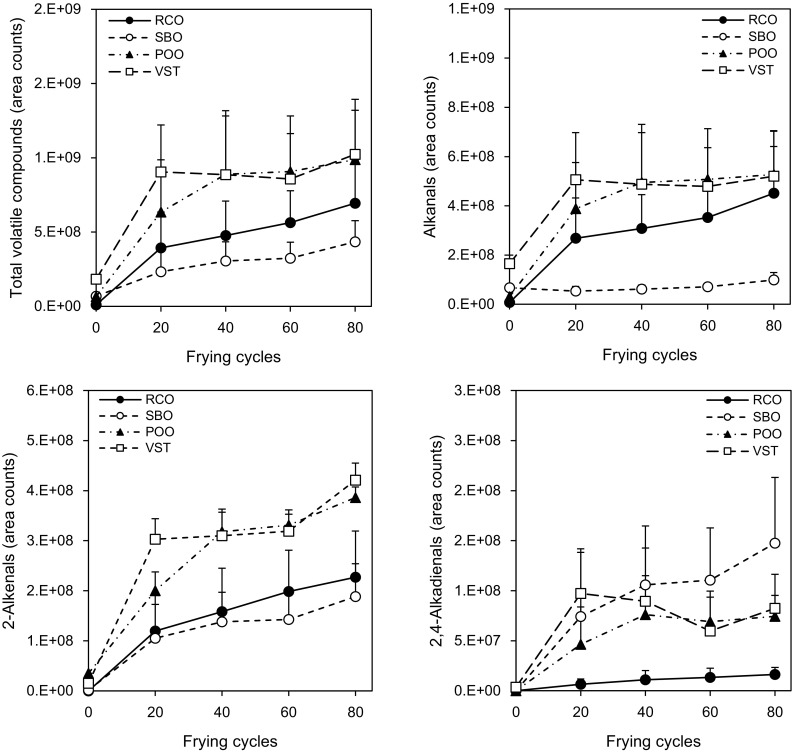
Changes in total volatile compounds, alkanals, 2-alkenals, and 2,4-alkadienals in frying oils used repeatedly. Values are means and standard deviations (n = 3). RCO: refined coconut oil; SBO: refined soybean oil; POO: pure olive oil; and VST: vegetable shortening
Levels of pentanal, nonanal, decanal, 2-nonenal, 2-decenal, 2,4-heptadienal, and 2,4-nonadienal were significantly different among the oils used 80 times repeatedly (p < 0.05) (Fig. 2). Pentanal, nonanal, and decanal were more in the RCO, POO, and VST used repeatedly than in the SBO. Especially, nonanal was predominantly detected in the RCO, POO, and VST. 2-Nonenal and 2-decenal were more in the POO and VST than in the RCO and SBO. 2,4-Nonadienal was more in the RCO and VST than in the SBO and POO. On the other hand, 2,4-heptadienal was more in the SBO than in the RCO, POO, and VST. These results were similar to previous studies: Guillén and Uriarte [27] reported that predominant volatile compounds in extra virgin olive oil and sunflower oil after heating at 180 °C were nonanal and 2-decenal, and 2,4-heptadienal in virgin linseed oil. Wang et al. [28] reported that generated volatile compounds, especially aldehydes, of soybean oil, corn oil, and canola oil during heating at 185 °C were categorized by proportions of fatty acids (C18:1, C18:2, and C18:3) of the frying oils into three clusters: 2-decenal and 2-undecenal (cluster 1 derived from C18:1), pentanal, hexanal, 2-octenal, and 2,4-decadienal (cluster 2 derived from C18:2), and 2-propenal, 2-hetepanl, and 2,4-heptadienal (cluster 3 derived from C18:3).
Fig. 2.
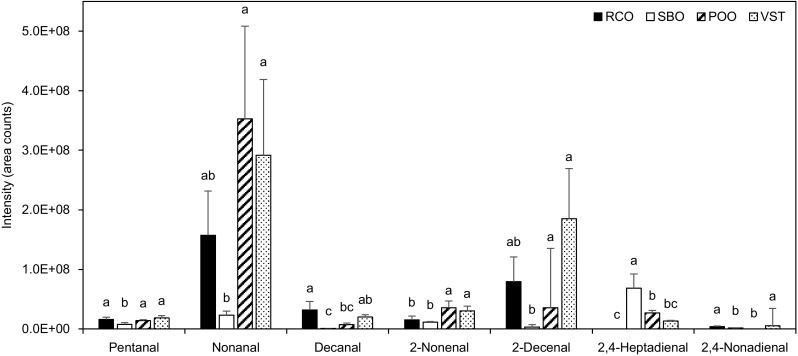
Volatile compounds in frying oils used 80 times repeatedly. Values are means and standard deviations (n = 3). a–cValues with different superscripts represent significant differences within the same compounds (p < 0.05; one-way ANOVA and Duncan’s multiple range test). RCO: refined coconut oil; SBO: refined soybean oil; POO: pure olive oil; and VST: vegetable shortening
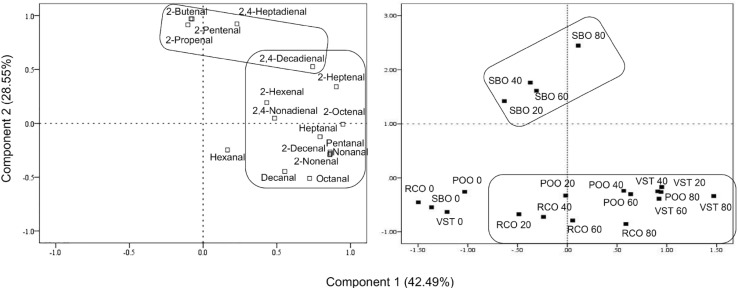
PCA for the volatile compounds in the frying oils used repeatedly are shown in Fig. 3. Component 1 was characterized by pentanal, heptanal, octanal, nonanal, decanal, 2-hexenal, 2-heptenal, 2-octenal, 2-nonenal, 2-decenal, 2,4-nonadienal, and 2,4-decadienal. PCA scores of RCO, POO, and VST moved into positive scores of component 1 with an increase of frying repetition. Component 2 was characterized by 2-propenal, 2-butenal, 2-pentenal, and 2,4-heptadienal. PCA scores of SBO moved into positive scores of component 2 with an increase of frying repetition. Especially, 2-propenal, 2-butenal, and 2-pentenal detected only from SBO during frying contributed to statistical classification between SBO and the other oils. SBO had 7.3% C18:3, higher than the other oils. In accordance with C18:3 in SBO significantly decreasing after frying (Table 1), 2-propenal derived from C18:3 significantly increased during frying (p < 0.05). 2-Propenal (acrolein) has been considered to be a possible carcinogen and can be generated during frying and transferred to fried products. [29] This result agrees with a previous study, reporting that the more proportions of PUFA were in an oil, the more 2-propenal formed during heating at 180 °C [22, 27].
Fig. 3.
PCA for volatile compounds in frying oils used repeatedly. RCO: refined coconut oil; SBO: refined soybean oil; POO: pure olive oil; and VST: vegetable shortening
In conclusion, the more fried repeatedly, the more oxidation of the oils without exceeding rejection limits of AV and TPC in all the oils used 80 times repeatedly. Although RCO did not contain tocopherols, its oxidation after frying was relatively slower than the other oils probably because of its higher level of SFA. Also, the volatile compositions in the repeatedly used frying oils were considerably affected by their fatty acid compositions.
Acknowledgements
This research was not funded.
References
- 1.Nayak PK, Dash U, Rayaguru K, Krishnan KR. Physio-chemical changes during repeated frying of cooked oil: a review. J. Food Biochem. 2016;40:371–390. doi: 10.1111/jfbc.12215. [DOI] [Google Scholar]
- 2.Takeoka GR, Full GH, Dao LT. Effect of heating on the characteristics and chemical composition of selected frying oils and fats. J. Agr. Food Chem. 1997;45:3244–3249. doi: 10.1021/jf970111q. [DOI] [Google Scholar]
- 3.Mba OI, Dumont MJ, Ngadi M. Deterioration kinetics of crude palm oil, canola oil and blend during repeated deep-fat frying. J. Am. Oil Chem. Soc. 2016;93:1243–1253. doi: 10.1007/s11746-016-2872-z. [DOI] [Google Scholar]
- 4.Hosseini H, Ghorbani M, Meshginfar N, Mahoonak AS. A review on frying: procedure, fat, deterioration progress and health hazards. J. Am. Oil Chem. Soc. 2016;93:445–466. doi: 10.1007/s11746-016-2791-z. [DOI] [Google Scholar]
- 5.Ministry of Food and Drug Safety. Food Code. Available from: http://www.foodsafetykorea.go.kr/foodcode/index.jsp. Accessed Sep 20 2016
- 6.Thomsen BR, Yesiltas B, Sørensen ADM, Hermund DB, Glastrup J, Jacobsen C. Comparison of three methods for extraction of volatile lipid oxidation products from food matrices for GC–MS analysis. J. Am. Oil Chem. Soc. 2016;93:929–942. doi: 10.1007/s11746-016-2837-2. [DOI] [Google Scholar]
- 7.Choe E, Min D. Chemistry of deep-fat frying oils. J. Food Sci. 2007;72:R77–R86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 8.Man YC, Hussin WW. Comparison of the frying performance of refined, bleached and deodorized palm olein and coconut oil. J. Food Lipids. 1998;5:197–210. doi: 10.1111/j.1745-4522.1998.tb00120.x. [DOI] [Google Scholar]
- 9.Srivastava Y, Semwal AD. A study on monitoring of frying performance and oxidative stability of virgin coconut oil (VCO) during continuous/prolonged deep fat frying process using chemical and FTIR spectroscopy. J. Food Sci. Technol. 2015;52:984–991. doi: 10.1007/s13197-013-1078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Oil Chemists’ Society . Official methods and recommended practices of the AOCS. IL: Champaign; 2009. [Google Scholar]
- 11.Gliszczyńska-Świgło A, Sikorska E. Simple reversed-phase liquid chromatography method for determination of tocopherols in edible plant oils. J. Chromatogr. A. 2004;1048:195–198. doi: 10.1016/j.chroma.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 13.Saguy IS, Shani A, Weinberg P, Garti N. Utilization of jojoba oil for deep-fat frying of foods. LWT-Food Sci. Technol. 1996;29:573–577. doi: 10.1006/fstl.1996.0088. [DOI] [Google Scholar]
- 14.Xu XQ. A new spectrophotometric method for the rapid assessment of deep frying oil quality. J. Am. Oil Chem. Soc. 2000;77:1083–1086. doi: 10.1007/s11746-000-0170-x. [DOI] [Google Scholar]
- 15.Lee J, Kim DH, Chang PS, Lee J. Headspace-solid phase microextraction (HS-SPME) analysis of oxidized volatiles from free fatty acids (FFA) and application for measuring hydrogen donating antioxidant activity. Food Chem. 2007;105:414–420. doi: 10.1016/j.foodchem.2006.12.059. [DOI] [Google Scholar]
- 16.Abenoza M, De Las Heras P, Benito M, Oria R, Sánchez-Gimeno AC. Changes in the physicochemical and nutritional parameters of Picual and Arbequina olive oils during frying. J. Food Process. Preserv. 2015;40:353–361. doi: 10.1111/jfpp.12612. [DOI] [Google Scholar]
- 17.Aladedunye FA, Przybylski R. Degradation and nutritional quality changes of oil during frying. J. Am. Oil Chem. Soc. 2009;86:149–156. doi: 10.1007/s11746-008-1328-5. [DOI] [Google Scholar]
- 18.Kamal-Eldin A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006;108:1051–1061. doi: 10.1002/ejlt.200600090. [DOI] [Google Scholar]
- 19.Lee J, Chung H, Chang PS, Lee J. Development of a method predicting the oxidative stability of edible oils using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) Food Chem. 2007;103:662–669. doi: 10.1016/j.foodchem.2006.07.052. [DOI] [Google Scholar]
- 20.Minguez-Mosquera MI, Rejano-Navarro L, Gandul-Rojas B, SanchezGomez AH, Garrido-Fernandez J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- 21.Nawar WW. Thermal degradation of lipids. J. Agr. Food Chem. 1969;17:18–21. doi: 10.1021/jf60161a012. [DOI] [Google Scholar]
- 22.Farhoosh R, Khodaparast MHH, Sharif A, Rafiee SA. Olive oil oxidation: rejection points in terms of polar, conjugated diene, and carbonyl values. Food Chem. 2012;131:1385–1390. doi: 10.1016/j.foodchem.2011.10.004. [DOI] [Google Scholar]
- 23.Arsian FN, Şapçı AN, Duru F, Kara H. A study on monitoring of frying performance and oxidative stability of cottonseed and palm oil blends in comparison with original oils. Int. J. Food Prop. 2017;20:704–717. doi: 10.1080/10942912.2016.1177544. [DOI] [Google Scholar]
- 24.Xu TT, Li J, Fan YW, Zheng TW, Deng ZY. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015;18:1478–1490. doi: 10.1080/10942912.2014.913181. [DOI] [Google Scholar]
- 25.Tompkins C, Perkins EG. The evaluation of frying oils with the p-anisidine value. J. Am. Oil Chem. Soc. 1999;76:945–947. doi: 10.1007/s11746-999-0111-6. [DOI] [Google Scholar]
- 26.Petersen KD, Jahreis G, Busch-Stockfisch M, Fritsche J. Chemical and sensory assessment of deep-frying oil alternatives for the processing of French fries. Eur. J Lipid Sci. Technol. 2013;115:935–945. doi: 10.1002/ejlt.201200375. [DOI] [Google Scholar]
- 27.Guillén MD, Uriarte PS. Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: presence of toxic oxygenated α, β unsaturated aldehydes. Food Chem. 2012;131:915–926. doi: 10.1016/j.foodchem.2011.09.079. [DOI] [Google Scholar]
- 28.Wang L, Csallany AS, Kerr BJ, Shurson GC, Chen C. Kinetics of forming aldehydes in frying oils and their distribution in French fries revealed by LC-MS-based chemometrics. J. Agr. Food Chem. 2016;64:3881–3889. doi: 10.1021/acs.jafc.6b01127. [DOI] [PubMed] [Google Scholar]
- 29.Abraham K, Andres S, Palavinskas R, Berg K, Appel KE, Lampen A. Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 2011;55:1277–1290. doi: 10.1002/mnfr.201100481. [DOI] [PubMed] [Google Scholar]