Abstract
Alcohol-induced liver disease progresses due to increased reactive oxygen species (ROS) and cellular lipid peroxidation. Quercetin is a flavonoid with strong antioxidant and hepatoprotective effects. We investigated whether 3′-O-methyl quercetin (3′MQ) and quercetin-3-O-glucuronide (Q3GA), two metabolites of quercetin, have protective effects against ethanol-induced hepatotoxicity. Cell viability was increased by quercetin, 3′MQ, and Q3GA in HepG2 hepatocarcinoma cells exposed to ethanol. Our results show that this effect was mediated by diminished ROS generation, decreased lipid peroxidation and up-regulation of antioxidant capacity, including glutathione, superoxide dismutase and catalase. Moreover, down-regulated heme oxygenase-1 (HO-1) expression by ethanol was restored by quercetin, 3′MQ, and Q3GA through the activation of nuclear factor E2-related factor 2 and activator protein-1, but not nuclear factor-kappa B. Overall results suggest that 3′MQ, Q3GA, and quercetin attenuate oxidative stress in hepatocytes exposed to ethanol by up-regulating HO-1 expression and can be used as therapeutic agents for ameliorating alcohol-induced liver disease.
Keywords: Quercetin, Metabolites, HepG2, Oxidative stress, Heme oxygenase-1
Introduction
Alcohol-induced oxidative stress contributes to hepatocellular damage by targeting various macromolecules such as proteins, lipids and DNA [1, 2]. Excess alcohol ingestion increases reactive oxygen species (ROS) or cellular lipid peroxidation, which plays a major role in the pathogenesis and progression of alcohol-induced liver disease [3]. According to several previous investigations on cytoprotective molecules against alcohol-induced oxidative stress, chemical and biological properties of polyphenols are involved in hepato-protection against alcohol-induced oxidative damages [4, 5]. In addition to free radical scavenging activity, several phenolic compounds have indirect antioxidative actions by up-regulating heme oxygenase-1 (HO-1), an inducible enzyme in response to oxidative stress [6]. HO-1 catalyzes degradation of heme to bilirubin and biliverdin which mediate protective role of HO-1 against alcohol-induced liver damage [7, 8].
Quercetin is a major polyphenol and flavonoid widely distributed in edible vegetables, fruits and wine. The flavonol quercetin has been known to possess a broad range of pharmacological properties, including strong antioxidant activity and hepatoprotective effects against oxidative stress [9, 10]. It is extensively metabolized to O-methylated, glucuronidated or sulfated flavonol during absorption in the small intestine and liver [11, 12]. While the significant free radical scavenging activity of quercetin was retained regardless of its conjugating substituent, these quercetin metabolites appeared to have different biological properties from the parent compound [13].
Previous reports indicated that the hepatoprotective effect of quercetin is mainly mediated by activation of MAPK signaling pathway, which stimulates translocation of nuclear factor E2-related factor 2 (Nrf2) and subsequent induction of HO-1. Nrf2 is an important transcription factor that induces expression of detoxifying genes including HO-1 by occupying the antioxidant responsible element (ARE) promoter sequence [14]. Nrf2 usually resides in the cytoplasm as a complex with Kelch-like ECH associated protein-1 (Keap-1) and translocates to the nucleus after dissociation from Keap-1 by several stimuli following binding to the ARE promoter [15, 16]. In addition to Nrf2, transactivation of HO-1 is regulated by other transcription factors including activator protein-1 (AP-1) or nuclear factor-κB (NF-κB) which binds to different cis-acting element of HO-1 promoter region from the ARE [17, 18]. Growing evidences indicate that AP-1 and NF-κB are important for the induction of HO-1, but distinguished from Nrf2, in response to various oxidative stimuli [19–22].
Protective effects of quercetin against alcohol-induced oxidative damage have been well established in various cell lines including rat and human hepatocytes [17, 23–25], but the effect of its conjugated metabolites or the mechanisms involved in HO-1 induction have not been fully elucidated. The major pathway participating in signal transduction required for regulating HO-1 has been identified well, but little is known for the mechanisms underlying which transcription factor contributes to the activation of HO-1 in response to flavonoids under the ethanol-induced oxidative stress. Moreover, in spite of its significant effect on hepatocytes, little is known about the molecular mechanism about the effect of major metabolites of quercetin.
Herein, we investigated the effect of quercetin and its metabolites, 3′-O-methyl quercetin (3′MQ) and quercetin-3-O-glucuronide (Q3GA), on HepG2 cells exposed to ethanol. Our results demonstrated that quercetin, 3′MQ, and Q3GA have cytoprotective effects against ethanol-induced oxidative stress. Antioxidative effects of quercetin and its metabolites were mediated by diminished ROS generation, decreased lipid peroxidation and up-regulation of activity on antioxidant enzymes such as superoxide dismutase (SOD) or catalase (CAT). This study also showed molecular mechanisms on antioxidant effects of quercetin, 3′MQ, and Q3GA via activation of transcription factors such as Nrf2 and AP-1, but not NF-κB, which are leading to the expression of HO-1 protein that can active antioxidant genes. Together, the present findings suggest that quercetin, 3′MQ, and Q3GA attenuate oxidative stress in hepatocytes exposed to ethanol by up-regulating HO-1 expression and provide support for the development of them as therapeutic agents for ameliorating alcohol-induced liver disease.
Materials and methods
Chemicals and reagents
Quercetin and Q3GA were purchased from Cayman Chemical (Ann Arbor, MI, USA) and Indofine Chemical (Hillsborough, NJ, USA), respectively. Penicillin–streptomycin was purchased from PAA Laboratories (Marlborough, MA, USA). Fetal bovine serum (FBS), minimum essential medium (MEM), phosphate buffered saline (PBS), Dulbecco′s Phosphate-Buffered Saline and sodium pyruvate were purchased from Welgene (Daejeon, Korea). 3′MQ, 2′,7′-dichlorofluorescein diacetate (DCF-DA), 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and Tween 20 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-histone H3 antibody was purchased from Millipore (Milford, MA, USA). All other primary and secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and sample treatment
The HepG2 human hepatocarcinoma cell line was purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in MEM medium with 10% FBS, 5% penicillin streptomycin, and 5% sodium pyruvate and grown in an atmosphere containing 5% CO2 at 37 °C. HepG2 cells were incubated with vehicle (0.2% DMSO or 0.2% ethanol), quercetin (1–10 µM, dissolved in DMSO), 3′MQ (1–10 µM, dissolved in DMSO), or Q3GA (1–10 µM, dissolved in ethanol) for 6 h and were treated with ethanol (2.5%) for additional 18 h. This condition was optimized by repeated experiments to have the cytoprotective effect on flavonols against ethanol (2.5%) treatment. We proceeded with subsequent experiments under this condition.
Cell viability assay
Cell viability was measured using the MTT assay as described previously [26]. Briefly, HepG2 cells (5 × 103 cells/well) were seeded in 96-well plates (SPL Inc., Seoul, Korea) and treated with various concentrations of quercetin, 3′MQ, or Q3GA for 24 h. Then, cells were washed with PBS and 10 µL MTT solution (5 mg/mL) was added to each well following incubation at 37 °C for 3 h. After incubation, the MTT solution was removed and DMSO was added to dissolve the formazan crystals. Absorbance was measured using an enzyme-linked immunosorbent assay reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 570 nm and percentage of cell viability was calculated.
Measurement of intracellular reactive oxygen species (ROS)
Intracellular ROS generation was evaluated with the fluorescence marker DCF-DA. DCFH-DA diffuses into cells and is deacetylated by cellular esterases to non-fluorescent 2′,7′-dichlorodihydrofluorescein, which is rapidly oxidized to the highly fluorescent 2′,7′-dichlorodihydrofluorescein (DCF) by ROS [27]. HepG2 cells (5 × 103 cells/well) were seeded in 96-well plates and pre-incubated with various concentrations of quercetin, 3′MQ, or Q3GA for 6 h, and the cells were stimulated with ethanol for 18 h. After the incubation, the cells were washed with PBS and incubated with 10 µM DCF-DA in PBS for 30 min. Stained cells were examined using a fluorescence microplate reader (Tecan Group Ltd., Basel, Switzerland) at 485/535 nm. Intracellular ROS generation was also observed using fluorescence microscopy (Leica Microsystem, Zena, Germany). Percentage of DCF fluorescence was compared with that of the control.
Measurement of lipid peroxidation and reduced glutathione (GSH)
Intracellular lipid peroxidation was measured by detecting the level of malondialdehyde (MDA). In brief, HepG2 cells (9 × 105 cells/dish) were cultured and pre-treated with various concentrations of quercetin, 3′MQ, or Q3GA for 6 h, and then cells were stimulated with ethanol for 18 h. Lipid peroxidation was determined using TBARS assay kit (Cell Biolabs, San Diego, USA) according to manufacturer’s instructions. In a parallel assay, cellular GSH level was measured in HepG2 cell lysate using total glutathione (GSSG/GSH) assay kit (Cell Biolabs).
Measurement of antioxidant enzymes activities
HepG2 cells (9 × 105 cells/dish) were cultured and pre-treated with various concentrations of quercetin, 3′MQ, or Q3GA for 6 h, and then cells were stimulated with ethanol for 18 h. The activity of SOD or CAT was measured using SOD or CAT activity assay kit (Cell Biolabs) according to the manufacturer’s instructions.
Protein extraction and Western blot analysis
HepG2 cells (9 × 105 cells/dish) were seeded in 100 mm dishes and pre-treated with the indicated concentrations of quercetin, 3′MQ, or Q3GA for 6 h, and then cells were stimulated with ethanol for 18 h. Cell lysates were prepared using RIPA buffer (Biosolution, Suwon, Korea) containing protease and phosphatase inhibitors (Sigma). Cell extracts were quantified using the BCA Protein Assay kit (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. 30–40 µg of proteins were separated on 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (GE Healthcare, Cambridge, UK) for Western blot analyses. At first, transferred membrane was blocked with blocking buffer containing 5% skim milk (BD biosciences, San Jose, CA, USA) in 1X Tris-buffered saline with Tween 20 (sigma) (TBST) for 1 h and then incubated with primary antibodies (HO-1, Nrf2, β-actin, and Histone H3) at appropriate dilutions in 1X TBST containing 1% skim milk for 2 h. The membrane was washed three times with 1X TBST for 10 min, and incubated with secondary antibody in 1X TBST containing 1% skim milk for 45 min. The membrane was also washed three times for 15 min, and the images were visualized with enhanced chemiluminescence detection reagent (Amersham Pharmacia Biotech, Parsippany, NJ, USA) and Chemi-Doc (Bio-Rad Laboratories, Hercules, CA, USA) imaging device. Primary and secondary antibodies were purchased from santa cruz.
Luciferase reporter assay
HepG2 cells (1.5 × 105 cells/dish) were seeded in 6 well plates and transfected with the luciferase plasmid reporting the activity of NF-κB (NF-κB-Luc) or the activity of AP-1 (AP-1-Luc) (kindly provided by Dr. TG Seo, Dongguk University, Seoul, Korea) using polyExpress™ (Excellgen, Rockville, MD, USA) according to the manufacturer’s instructions. After incubation for 24 h, the cells were treated with the indicated concentrations of quercetin, 3′MQ, or Q3GA for 6 h, and then the cells were stimulated with ethanol for 18 h. NF-kB activation can be evaluated in 24 h as well as short time (1–2 h) after in vitro treatment of chemical [28, 29]. Luciferase expression was measured using an assay kit (Promega, Madison, WI, USA) and a luminometer (Berthold Technologies, Bad Wildbad, Germany) according to the manufacturer’s instructions. Luciferase activity was normalized by comparison with β-galactosidase activity.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Results were expressed as mean ± standard error of three independent experiments. One-way analysis of variance and Bonferroni’s Multiple Comparison test were used to detect differences. Values of *P < 0.05, **P < 0.01, ***P < 0.001 and # P < 0.05, ## P < 0.01, ### P < 0.001 were established to be statistically significant when compared with control and ethanol, respectively.
Results and discussion
Cytoprotective effects of quercetin and its metabolites on human HepG2 cells
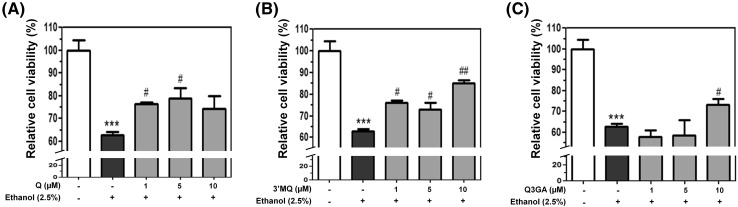
In this study, we focused on the molecular effects of quercetin or its metabolites, 3′MQ and Q3GA (Fig. 1), and evaluated whether quercetin metabolites also have cytoprotective efficacy using HepG2 cell, a hepatocarcinoma cell line, which is usually used for the study on in vitro metabolism of hepatocytes and toxicity to the liver [30]. Quercetin is converted to circulating forms, 3′MQ or Q3GA, during absorption in the liver [13, 31]. Although the protective role of quercetin from ethanol-induced oxidative stress has been reported well [12, 23, 25], few studies have reported the effect of quercetin metabolites. Cell viability was not affected by quercetin, 3′MQ, or Q3GA up to 50 µM (data not shown). Then, we investigated whether quercetin or its metabolites could protect cells from ethanol-induced oxidative stress. Cell viability was decreased to 60% of the control group by ethanol (2.5%) treatment, which was restored by adding quercetin or its metabolites (Fig. 2). In particular, quercetin and 3′MQ significantly recovered cell viability up to 80% at 1–5 and 1–10 µM respectively, indicating that cell viability increased 10–20% after exposure to quercetin or 3′MQ compared to that of cells treated with ethanol alone (Fig. 2A, B). Q3GA showed protective effect against ethanol-induced cytotoxicity at a higher concentration (10 µM) than quercetin or 3′MQ (Fig. 2C). Although cell viability of HepG2 cells treated ethanol (2.5%) was recovered with pre-treatment with quercetin at 10 µM by ~ 1.3-fold, the increased level of cell viability did not reach statistical significance to the contrary to our expectation. Similarly to our observation, the compound at lower concentration has antioxidant effect than concentration at 50 µM; therefore, they used the compound at 20 µM in all subsequent experiments [32]. It suggests that higher concentration of compound might be saturated to have protective effect against oxidative stress. Therefore, we used the one concentration at 5, 5 and 10 µM for quercetin, 3′MQ, and Q3GA, respectively, which has higher hepatoprotective effect against ethanol-induced oxidative stress to precede subsequent experiments. Taken together, these data indicate that quercetin and its metabolites have cytoprotective effects on hepatocytes against ethanol-induced oxidative stress.
Fig. 1.
Chemical structures of quercetin and its metabolites. (A) Quercetin (Q), (B) 3′-O-methyl quercetin (3′MQ) and (C) Quercetin-3-O-glucuronide (Q3GA)
Fig. 2.
Cytoprotective effects of quercetin and its metabolites in HepG2 cells. Cell viability was measured by MTT assay. Cells were pre-treated with (A) Q, (B) 3′MQ, or (C) Q3GA at various concentrations (1, 5, 10 µM) for 6 h and then oxidative stress was stimulated by addition of ethanol to be 2.5% for 18 h. Percentage of viable cells was calculated by comparison with control cells. ***p < 0.001 was considered statistically significant compared with control cells. # p < 0.05 and ## p < 0.01 were considered statistically significant compared with ethanol-treated cells
Quercetin and its metabolites suppress ethanol-induced ROS generation
The suppressive effect of quercetin or its metabolites on ethanol-induced ROS generation in HepG2 cells was investigated by ROS measurement. DCF diacetate was used to identify intracellular ROS generation. Compared to control cells, DCF fluorescence intensity was increased markedly to 30–40% in ethanol-treated cells. Notably, pre-treatment of quercetin, 3′MQ, or Q3GA decreased ROS generation dramatically in ethanol-treated HepG2 cells to a level lower than that of control cells (Fig. 3A). Microscopic analysis showed that less bright DCF green fluorescence was observed in flavonol-treated cells compared to that in ethanol-treated cells (Fig. 3B). These results indicate that quercetin and its metabolites have suppressive effects against ethanol-induced ROS generation.
Fig. 3.
Hepatoprotective effect of quercetin and its metabolites on ethanol-induced ROS generation in HepG2 cells. (A) Intracellular ROS accumulation was monitored by DCFH-DA fluorescence method. *p < 0.05 was considered statistically significant compared with control cells. # p < 0.05 and ## p < 0.01 were considered statistically significant compared with ethanol cells. (B) Intracellular ROS accumulation was monitored using fluorescence microscope (40 x)
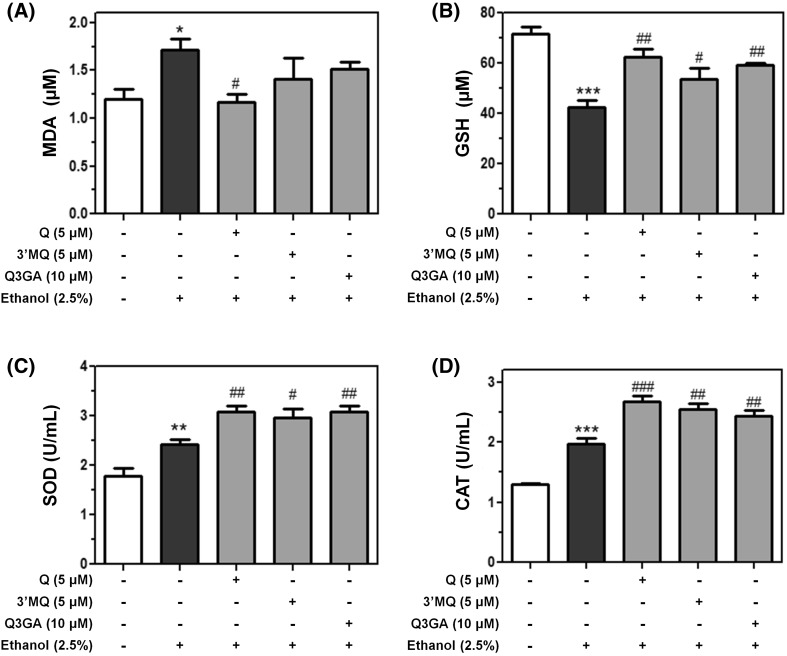
Suppression of lipid peroxidation, recovery of GSH, and increase of antioxidant enzyme activities by quercetin and its metabolites
For determination of whether flavonols affect the antioxidant activity in ethanol-treated HepG2 cells, intracellular level of lipid peroxidation and the reduced form of GSH was evaluated. Intracellular MDA, one of the most frequently used indicators for detecting lipid peroxidation [16], increased up to 1.75 µM by ethanol treatment was decreased to the control level by adding quercetin (Fig. 4A). Although 3′MQ or Q3GA showed inhibitory effect against lipid peroxidation, only quercetin showed statistically significant activity. In addition, quercetin and Q3GA recovered intracellular GSH level or protected against GSH depletion in ethanol-treated HepG2 cells (Fig. 4B). During oxidative stress, GSH, an important cellular component for preventing oxidative stress, is consumed, resulting in rapid depletion [33]. These results show that quercetin and its metabolites help hepatocytes attenuate lipid peroxidation and maintain the reduced form of GSH. Our study next focused on the activity of SOD or CAT. Interestingly, we found that ethanol treatment itself increased SOD or CAT activity in HepG2 cells. This is disagreed with a previous report showing that SOD or CAT activity in rat hepatocytes was decreased after ethanol exposure [17]. However, another study has reported that SOD or CAT activity can be up-regulated by ethanol treatment alone [34]. This result may have been derived from discrepancy in treatment time or ethanol concentration between experiments. As shown Fig. 4C and D, these cytoprotective responses were further increased by pre-treatment of quercetin, 3′MQ, or Q3GA. These data show that quercetin and its metabolites increase antioxidant action in hepatocytes by promoting SOD and CAT activities. Together, our results revealed that cytoprotective effects of flavonols are mediated by diminishing lipid peroxidation, increasing the reduced form of GSH, and increasing the activity of anti-oxidant enzyme such as SOD or CAT.
Fig. 4.
Hepatoprotective effects of quercetin or its metabolites against ethanol-induced oxidative stress by enhancement of MDA, GSH, SOD and CAT levels. The level of (A) hepatocellular MDA, (B) GSH depletion and activities of antioxidant enzymes, (C) SOD and (D) CAT, were assessed from HepG2 cell lysates pre-treated with flavonols for 6 h against ethanol-induced oxidative stress. *p < 0.05, **p < 0.01 and ***p < 0.001 were considered statistically significant compared with control cells. # p < 0.05, ## p < 0.01 and ### p < 0.001 were considered statistically significant compared with ethanol-treated cells
Up-regulation of HO-1 and Nrf2 expression by quercetin and its metabolites in ethanol-treated HepG2 cells
After confirming the antioxidant effects of quercetin and its metabolites, the effect of HO-1 and Nrf2 expression was evaluated, because previous studies reported that HO-1 is an antioxidant enzyme playing an important role in ethanol-induced oxidative stress in hepatic cells and its expression is up-regulated by Nrf2, a major transcription factor regulating ARE-driven phase II gene expression [18, 25]. Induction of the HO-1 results in cellular protection from oxidative stress [23], and its expression in hepatocytes is negatively affected by ethanol exposure [16]. The expressions of HO-1 and Nrf2 were decreased slightly in HepG2 cells treated with ethanol (second bars in graphs of Fig. 5A, B). Pre-treatment of quercetin, 3′MQ, or Q3GA induced the expression of HO-1 protein in spite of ethanol-induced cytotoxicity (Fig. 5A). Furthermore, quercetin and its metabolites increased the Nrf2 protein level specifically in the nuclear fraction, suggesting that Nrf2 translocates and plays a role in nucleus by treatment of quercetin or its metabolites (Fig. 5B). These results show that the hepatoprotective effects of quercetin and its metabolites are mediated by activating Nrf2 following up-regulation of HO-1 expression. It suggests that quercetin and its metabolites have cytoprotective ability against ethanol-induced oxidative stress by inducing HO-1 expression in HepG2 cells.
Fig. 5.
HO-1 and Nrf2 expression on quercetin and its metabolites against ethanol-treated in HepG2 cells. (A) Protein level of HO-1 was assessed by cell lysates pre-treated with flavonols against ethanol-induced oxidative stress. (B) Nuclear or cytosolic fractions were prepared to determine Nrf2 protein level in nucleus. β-actin and histone H3 were used as internal control for total cell lysates and nuclear fraction, respectively. # p < 0.05 and ## p < 0.01 was considered statistically significant compared with ethanol-treated cells
Transcriptional factors activated by quercetin and its metabolites
For examination of the molecular mechanism for the up-regulation of HO-1 by quercetin and its metabolites, molecular mediators for these responses were speculated. AP-1 and NF-κB are transcription factors activated by quercetin and its metabolites because previous study reported that AP-1 and NF-κB are critical factors inducing HO-1 expression by binding to its promoter [18, 20, 35]. In luciferase assay experiment, ethanol treatment itself significantly reduced AP-1 and NF-κB activities (second bars in Fig. 6A, B). Pre-treatment of quercetin or its metabolites increased AP-1 activity in the presence of ethanol, but not NF-κB, which is correlated with HO-1 and Nrf2 expression (Fig. 6A, B). These results indicate that AP-1, but not NF-κB, is a transcription factor up-regulating the expression of HO-1 in ethanol-treated hepatocytes by quercetin and its metabolites. In summary, it suggests that two transcription factors, Nrf2 and AP-1, are activated by quercetin or its metabolites and increase HO-1 expression, which plays an important role for the activation of antioxidant gene in hepatocytes [16, 18]. Overall, this study demonstrates that quercetin and its major metabolites can be used as therapeutic agents for ameliorating alcohol-induced liver disease.
Fig. 6.
Activity of transcription factors (AP-1 or NF-κB) on quercetin and its metabolites against ethanol-treated in HepG2 cells. HepG2 cells were transiently transfected with luciferase vector reporting the activation of AP-1 or NF-κB and β-galactosidase. The activation of (A) AP-1 or (B) NF-κB transcription factor was assessed using luciferase assay system. Colorimetric β-galactosidase assay was performed to correct for the transfection efficiency. Luciferase activity was expressed as relative light unit (RLU). **p < 0.01 was considered statistically significant compared with control cells. ## p < 0.01 and ### p < 0.001 were considered statistically significant compared with ethanol-treated cells
Acknowledgements
This study was supported by Dongguk University Research Fund (Grants No. S-2016-G0001-00020).
References
- 1.Lieber CS. Biochemical factors in alcoholic liver disease. Seminars in Liver Disease. 1993;13:136–153. doi: 10.1055/s-2007-1007345. [DOI] [PubMed] [Google Scholar]
- 2.Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007;81:177–187. doi: 10.1016/j.lfs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Cameron RG, Neuman MG, Shear NH, Katz G, Bellentani S, Tiribelli C. Modulation of liver-specific cellular response to ethanol in vitro in hep G2 cells. Toxicology in Vitro: an International Journal Published in Association with BIBRA. 1998;12:111–122. doi: 10.1016/S0887-2333(97)00095-7. [DOI] [PubMed] [Google Scholar]
- 4.Ko HJ, Chen JH, Ng LT. Hepatoprotection of Gentiana scabra extract and polyphenols in liver of carbon tetrachloride-intoxicated mice. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer. 2011;30:179–187. doi: 10.1615/JEnvironPatholToxicolOncol.v30.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Tian L, Shi X, Yu L, Zhu J, Ma R, Yang X. Chemical composition and hepatoprotective effects of polyphenol-rich extract from Houttuynia cordata tea. Journal of Agricultural and Food Chemistry. 2012;60:4641–4648. doi: 10.1021/jf3008376. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radical Biology & Medicine. 2004;36:1505–1516. doi: 10.1016/j.freeradbiomed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Nussler AK, Hao L, Knobeloch D, Yao P, Nussler NC, Wang Z, Liu L, Ehnert S. Protective role of HO-1 for alcohol-dependent liver damage. Digestive Diseases. 2010;28:792–798. doi: 10.1159/000324287. [DOI] [PubMed] [Google Scholar]
- 8.Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends in Immunology. 2003;24:449–455. doi: 10.1016/S1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 9.Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radical Research. 2004;38:877–884. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- 10.Justino GC, Santos MR, Canario S, Borges C, Florencio MH, Mira L. Plasma quercetin metabolites: structure-antioxidant activity relationships. Archives of Biochemistry and Biophysics. 2004;432:109–121. doi: 10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Granado-Serrano AB, Martin MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chemico-Biological Interactions. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Angeloni C, Spencer JP, Leoncini E, Biagi PL, Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89:73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Duenas M, Surco-Laos F, Gonzalez-Manzano S, Gonzalez-Paramas AM, Santos-Buelga C. Antioxidant properties of major metabolites of quercetin. Eur Food Res Technol. 2011;232:103–111. doi: 10.1007/s00217-010-1363-y. [DOI] [Google Scholar]
- 14.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 15.Surh YJ. Transcription factors in the cellular signaling network as prime targets of chemopreventive phytochemicals. Cancer Research and Treatment: Official Journal of Korean Cancer Association. 2004;36:275–286. doi: 10.4143/crt.2004.36.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar KJ, Chu FH, Hsieh HW, Liao JW, Li WH, Lin JC, Shaw JF, Wang SY. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136:168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Hou W, Yao P, Zhang B, Sun S, Nussler AK, Liu L. Quercetin protects against ethanol-induced oxidative damage in rat primary hepatocytes. Toxicol In Vitro. 2010;24:516–522. doi: 10.1016/j.tiv.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 19.Chia AJ, Goldring CE, Kitteringham NR, Wong SQ, Morgan P, Park BK. Differential effect of covalent protein modification and glutathione depletion on the transcriptional response of Nrf2 and NF-kappaB. Biochemical pharmacology. 2010;80:410–421. doi: 10.1016/j.bcp.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada H, Sugimoto R, Watanabe A, Taketani S, Okada K, Warabi E, Siow R, Itoh K, Yamamoto M, Ishii T. Differential roles for Nrf2 and AP-1 in upregulation of HO-1 expression by arsenite in murine embryonic fibroblasts. Free Radic Res. 2008;42:297–304. doi: 10.1080/10715760801975735. [DOI] [PubMed] [Google Scholar]
- 21.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biology. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramyaa P, Krishnaswamy R, Padma VV. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells - up regulation of Nrf2 expression and down regulation of NF-kappaB and COX-2. Biochimica et Biophysica Acta. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Hou W, Yao P, Li N, Zhang B, Hao L, Nussler AK, Liu L. Heme oxygenase-1 mediates the protective role of quercetin against ethanol-induced rat hepatocytes oxidative damage. Toxicol In Vitro. 2012;26:74–80. doi: 10.1016/j.tiv.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacognosy Magazine. 2010;6:135–141. doi: 10.4103/0973-1296.62900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicology Letters. 2006;160:171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods in Molecular Biology. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 28.Switzer CH, Cheng RY, Ridnour LA, Murray MC, Tazzari V, Sparatore A, Del Soldato P, Hines HB, Glynn SA, Ambs S, Wink DA. Dithiolethiones inhibit NF-kappaB activity via covalent modification in human estrogen receptor-negative breast cancer. Cancer Research. 2012;72:2394–2404. doi: 10.1158/0008-5472.CAN-11-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badr CE, Niers JM, Tjon-Kon-Fat LA, Noske DP, Wurdinger T, Tannous BA. Real-time monitoring of nuclear factor kappaB activity in cultured cells and in animal models. Molecular Imaging. 2009;8:278–290. doi: 10.2310/7290.2009.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy BS, Reddy RK, Reddy BP, Ramakrishna S, Diwan PV. Potential in vitro antioxidant and protective effects of Soymida febrifuga on ethanol induced oxidative damage in HepG2 cells. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association. 2008;46:3429–3442. doi: 10.1016/j.fct.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Spencer JP, Kuhnle GG, Williams RJ, Rice-Evans C. Intracellular metabolism and bioactivity of quercetin and its in vivo metabolites. The Biochemical Journal. 2003;372:173–181. doi: 10.1042/bj20021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JK, Jang HD. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int J Mol Sci. 2014;15:12149–12165. doi: 10.3390/ijms150712149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death and Differentiation. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 34.Chen YL, Peng HC, Tan SW, Tsai CY, Huang YH, Wu HY, Yang SC. Amelioration of ethanol-induced liver injury in rats by nanogold flakes. Alcohol. 2013;47:467–472. doi: 10.1016/j.alcohol.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Lavrovsky Y, Song CS, Chatterjee B, Roy AK. Age-dependent increase of heme oxygenase-1 gene expression in the liver mediated by NFkappaB. Mech Ageing Dev. 2000;114:49–60. doi: 10.1016/S0047-6374(00)00087-7. [DOI] [PubMed] [Google Scholar]