Abstract
Chloropropanols such as 3-monochloropropane-1,2-diol (3-MCPD) and 1,3-dichloropropan-2-ol (1,3-DCP) are produced by heat treatment in the presence of fat and hydrochloric acid during the manufacture of food stuffs such as hydrolyzed vegetable protein and soy sauce. 3-MCPD and 1,3-DCP have been detected in several foods. An efficient, highly selective GC–MS method was developed to determine the concentration of 3-MCPD and 1,3-DCP in food. Calibration curves for 3-MCPD and 1,3-DCP were constructed, and a correlation of determination (r2) ≥ 0.9990 was obtained. The limits of detection and quantitation for 3-MCPD in food were 0.6 and 2.0 µg/kg, respectively, and those for 1,3-DCP were 0.2 and 0.6 µg/kg, respectively. To the best of our knowledge, this GC–MS-based method is a newly improved analytical procedure for the simultaneous separation and determination of 3-MCPD and 1,3-DCP, at once and at low levels (µg/kg).
Keywords: Chloropropanol; 1,3-Dichloropropan-2-ol; 3-Monochloropropane-1,2-diol; Gas chromatography–mass spectrometry; Hydrolyzed vegetable protein (HVP)
Introduction
3-Monochloropropane-1,2-diol (3-MCPD), the most common member of the group of chemical contaminants known as chloropropanols, and 1,3-dichloropropan-2-ol (1,3-DCP) are carcinogenic substances produced by the oxidation of hydrolyzed vegetable protein (HVP) [1]. During the hydrochloric acid hydrolysis of proteins containing low amounts of oil (e.g., beans, wheat, and rice), the proteins break down into amino acids while fat residues degrade into fatty acid esters (glycerol). The resulting glycerol reacts with hydrochloric acid, eliminating the fatty acid and producing chlorinated contaminants [2, 3]. Recent studies have reported that soy sauce contains the highest concentrations of MCPD and 1,3-DCP. These are also found in other foods such as bread, cookies, soups, and sauces [4]. In addition, 3-MCPD is an environmental hormone that is suspected to disrupt the endocrine system and possibly induce genotoxicity, reproductive toxicity, neurotoxicity, renal toxicity, and cancer [5, 6]. 1,3-DCP is highly volatile and flammable and can cause subcutaneous hemolysis on inhalation, sterility and other reproductive problems, acute liver damage, blood coagulation, cancer, and damage to the eyes and skin on contact [7–9]. Several countries have set reference values for the dietary intake of 3-MCPD and 1,3-DCP and strictly evaluate their content in food. In HVP, the maximum concentration of 3-MCPD has been set at 0.02 mg/kg. In soy sauces, 3-MCPD concentrations have been limited to 0.02 mg/kg for products with 40% solids and 0.05 mg/kg for 100% dried products, and these set limits are proportional to the concentration of solid foods for a solid accounting for 40–100% [10–12]. On May 22, 2002, the South Korean government provisionally set the maximum concentrations of 3-MCPD at 0.3 mg/kg for unfermented and mixed soy sauces and 1.0 mg/kg for HVP [13–15]. Therefore, it is important to develop a method for detecting and quantifying 3-MCPD and 1,3-DCP to manage the regulation of food constituents. 3-MCPD and 1,3-DCP are poorly volatile and have a high polarity, resulting in very low sensitivity because of their poor recovery and tendency to adsorb on the GC inlet or column [16]. Consequently, they must be analyzed following derivatization by methods including N,O-bis(trifluoroacetamide acid) (BSTFA), phenylboric acid (PBA), heptafluorobutyrylimidazole (HFBI), and heptafluorobutyric acid (HFBA). In this study, HFBA was used to derivatize 3-MCPD and 1,3-DCP in accordance with the Korea Food Code. In addition, there is no set limit for the concentration of 1,3-DCP in foods. Hence, this study aims to develop an effective method to simultaneously analyze 3-MCPD and 1,3-DCP, which are abundant in soups and soy sauces available across South Korea, Japan, Southeast Asia, and China. The development of such an effective analytical method will provide standard analytical techniques to prevent and regulate the amount of harmful 3-MCPD and 1,3-DCP.
Materials and methods
Analyzed samples
Food products with a high probability of detecting 3-MCPD and 1,3-DCP were primarily selected. Soybean paste, cookies, and dietary oils were purchased from three supermarkets in Sejong, Deajeon, and Cheongju, and the contents of 3-MCPD and 1,3-DCP were simultaneously analyzed. The purchased samples were stored under conditions identical to those in the supermarket until analysis.
Chemicals and reagents
3-MCPD and 1,3-DCP were purchased from Sigma-Aldrich (St Louis, USA), and 3-MCPD-d5 and 1,3-DCP-d5 internal standards were purchased from CDN Isotopes (Pointe-Claire, Quebec, Canada). The extractant dichloromethane was purchased from Wako (Osaka, Japan), and sodium chloride and sodium sulfate were purchased from Dae Jung (Gyeonggi-do, Korea). The Extrelut-NT3 cartridge, ethyl acetate, and isooctane were purchased from Merck (Darmstadt, Germany), and heptafluorobutyric anhydride (HFBA) used for derivatization was purchased from Sigma-Aldrich (St. Louis, USA). Triple-distilled water used in these experiments was obtained using a Thermo Scientific Barnstead Nanopure system (Marietta, USA).
Preparation of standard solutions and reagents
To prepare 1000 µg/mL solutions, each stock and internal standard solutions was dissolved in ethyl acetate. The working standard solution, which was used to generate the calibration curve, was prepared by diluting the stock standard solution in ethyl acetate to 0.03, 0.1, 0.5, 1, and 2 µg/mL solutions. The internal standard solution was diluted with ethyl acetate to give a 10 µg/mL solution. To perform derivatization, 50 µL of each standard solution at 5 different concentrations, 50 µL of the internal standard solution, 900 µL of isooctane, and 150 µL of HFBA were mixed in vitro in a test tube with a lid, and the results were analyzed. In this study, the samples were classified based on their fat contents (< 10, 10–50, and 70–100%), and only the main samples were selected and analyzed. The stock standard and internal standard solutions were stored in brown bottles at 4 °C until analysis. In addition, a 5 M sodium chloride solution was prepared by dissolving 292.2 g of NaCl in 1 L of triple-distilled water.
Sample preparation
First, 5 g of a homogenized sample was accurately measured, and a 5 M NaCl solution was added up to the 30-mL mark. Second, the mixture was subjected to shaking for 10 min, followed by centrifugation (4500 rpm, 4 °C, 5 min). Third, 1 g of anhydrous sodium sulfate was added on the top of the Extrelut-NT3 column, and 2 mL of the supernatant from the centrifuged sample, as well as 50 µL of the internal standard solution, was added. The resulting mixture was allowed to rest for 10 min to enable adsorption, followed by elution with 60 mL of dichloromethane at a rate of 2 mL/min. The eluted fractions were collected in an evaporating flask, followed by evaporation in a water bath at 35 °C and concentration to 1 mL under high pressure. The concentrated solution was transferred into a graduated test tube using a Pasteur pipette. The residues remaining in the flask were washed with dichloromethane and added to the test tube. The test tube was then subjected to evaporation in a water bath at 35 °C under nitrogen and concentrated to 100 µL. Next, 900 µL of isooctane and 150 µL of HFBA were added to the concentrated sample in the test tube, and the mixture was allowed to react for 30 min in a water bath at 60 °C after sealing the test tube with a lid. Next, the sample was allowed to rest for 10 min at room temperature, 5 mL of triple-distilled water was added, and the mixture was shaken to cease the reaction and separate the HFBA residues. The top organic layer was transferred to a test tube, a small amount of anhydrous sodium sulfate was added, and the layer was subjected to shaking. The mixture was allowed to rest, and the separated layer was extracted into a vial and analyzed by GC–MS.
GC–MS analysis
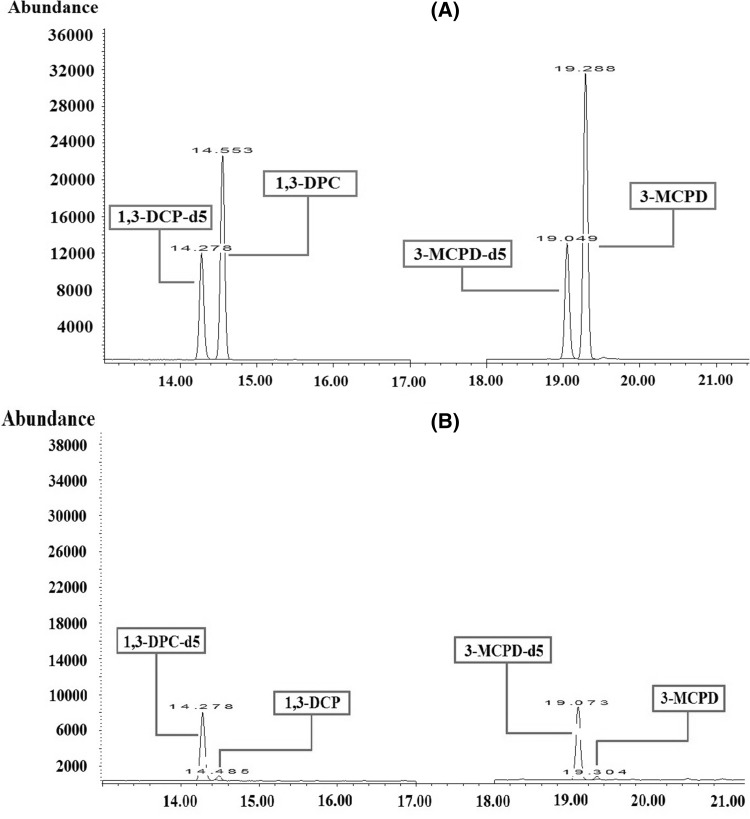
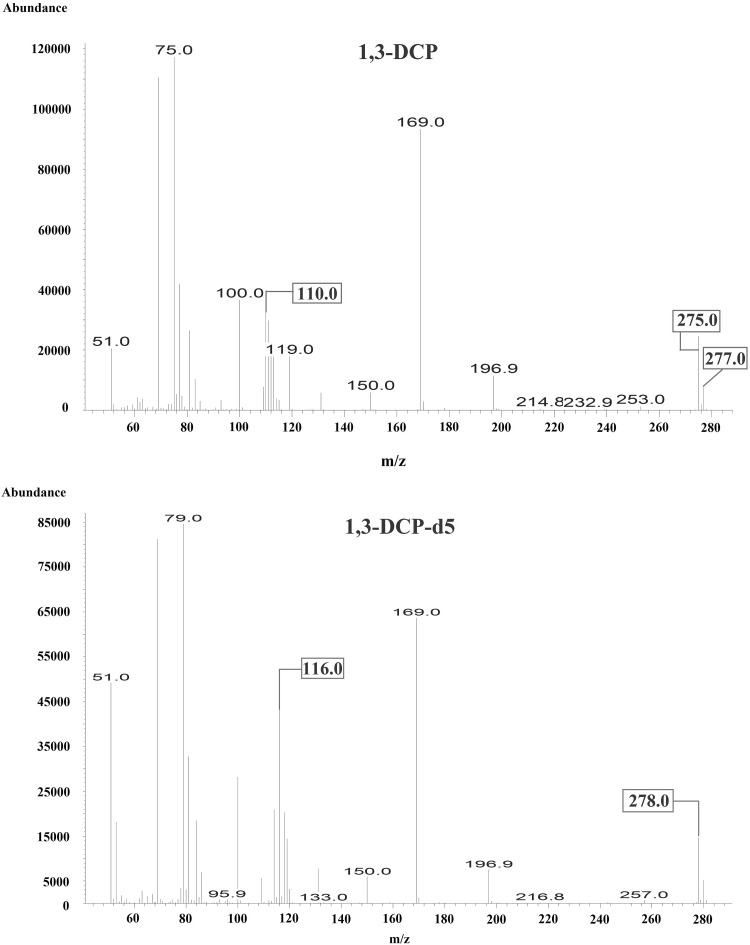
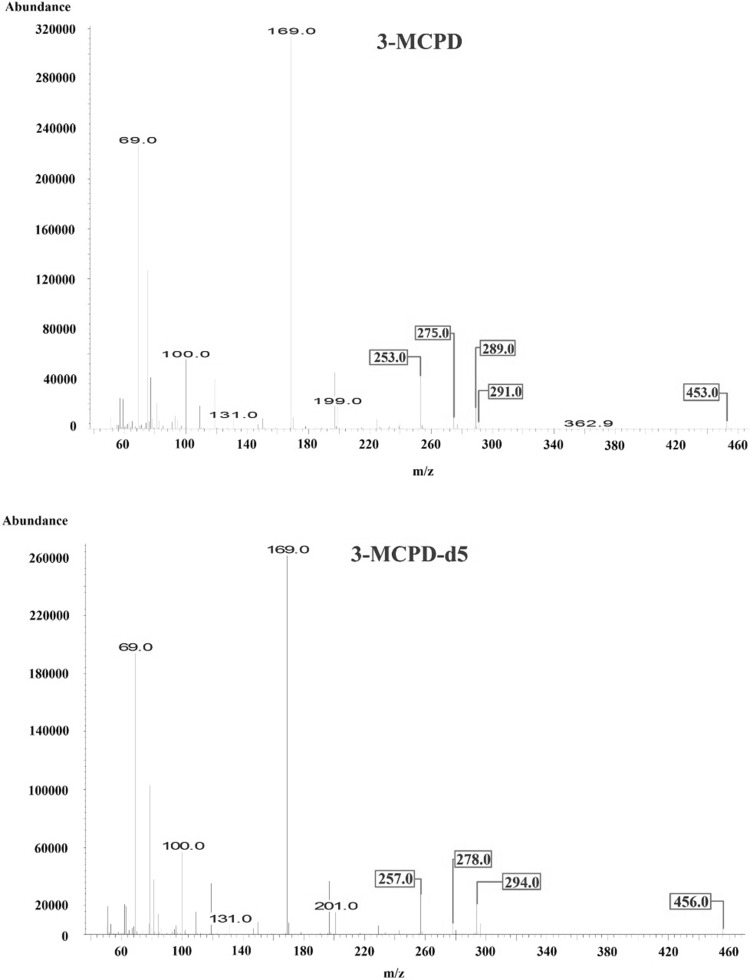
GC and MSD instruments with model numbers 7890B and 5977A (Agilent, USA), respectively, and an Agilent DB-5MS column (30 m × 0.25 mm, 0.25 µm) were used for analysis. The injector temperature was 250 °C and 1 µL of the sample was injected into the GC–MS column with He at a flow rate of 0.8 mL/min. The analysis was conducted for 40 min in the split mode (10:1). The oven temperature was set to 50 °C for the initial 5 min, increased at a rate of 2 °C/min until the temperature reached 90 °C, maintained at 90 °C for 5 min, and increased at a rate of 30 °C/min until the temperature reached 280 °C, which was maintained for the final 3 min. The ion source temperature was 230 °C and the analysis was conducted in the single-ion monitoring (SIM) mode with an ionization energy of 70 eV obtained in the electron ionization (EI) mode. In the derivatized samples, ions were observed at m/z 253, 275, 289, 291, and 453, corresponding to 3-MCPD, whereas those observed at m/z 257, 278, 294, and 456 corresponded to 3-MCPD-d5 (internal standard solution). Peaks at m/z 253 and 257 corresponded to the quantitative ions of 3-MCPD and the internal standard solution, respectively. Peaks observed at m/z 275, 110, and 277 corresponded to 1,3-DCP, whereas those at m/z 278 and 116 corresponded to 1,3-DCP-d5 (internal standard solution). Peaks at m/z 275 and 278 corresponded to the quantitative ions for 1,3-DCP and the internal standard solution, respectively. For qualitative analysis, the reference range of ion proportion was set to ± 20% (Figs. 1, 2, 3).
Fig. 1.
Chromatograms of (A) 1,3-DCP, 1,3-DCP-d5, 3-MCPD, and 3-MCPD-d5 in standard mixtures and (B) HFBA derivatized chloropropanols in soy sauce
Fig. 2.
EI mass spectra of 1,3-DCP and 1,3-DCP-d5
Fig. 3.
EI mass spectra of 3-MCPD and 3-MCPD-d5
Verification of the effectiveness of the test method
In this study, the selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, and accuracy were investigated. Standard solutions of 3-MCPD and 1,3-DCP were added to samples (soy sauce (< 10%), snack (10–50%), and rapeseed oil (> 50%)) with different fat contents to verify the selectivity of the method. Linearity was verified using the coefficient of determination (r2) and regression, which was obtained from the calibration curve plotted by the analysis of standard solutions with different concentrations. The LOD and LOQ were determined by measuring a matrix blank of fortified samples seven times with standard solutions. The background response and standard deviation were computed and defined as 3 times (3σ) and 10 times (10σ) the standard deviation (σ), respectively. Accuracy was determined by the recovery rate measured by mixing 4.5, 7.5, and 15 µg/mL of a standard solution with the sample, which was measured seven times in 1 day. Precision was verified by the percentage relative standard deviation (%RSD) obtained by conducting seven intraday assays in 1 day. Accuracy and precision were verified to be suitable on the basis of AOAC guidelines [17].
Statistical analysis
The data analysis function of EXCEL (Microsoft Office 2010) was used to calculate the mean and standard deviation. An F-test was conducted with a significance level of 99% (p = 0.01).
Results and discussion
Verification of the effectiveness of the test method
Generally, hydrolysis is carried out at 100–130 °C using 4–6 M hydrochloric acid for 4–24 h and finally neutralized with NaOH. In this process, 3-MCPD and 1,3-DCP are produced by replacing one or two of the three fatty acids of the triglyceride of glycerol with the chlorine group of hydrochloric acid [18]. 3-MCPD and 1,3-DCP reacted selectively with the isotopically labeled internal standard and chloropropanol to provide stable derivatives of HFBA because of their low volatility and high polarity. These species decompose into characteristic ions and allow quantification of chloropropanol [19]. Although studies have reported the GC–MS [20] or LC–MS [16] analysis of 3-MCPD with 1,3-DCP using chromatography glass columns [19], to the best of our knowledge, studies have not reported the simultaneous analysis of 3-MCPD and 1,3-DCP by GC–MS using Extrelut-NT3 columns in the past decade. Hence, this study verified the effectiveness of a simple, rapid GC–MS method for the simultaneous analysis of 3-MCPD and 1,3-DCP (Table 1).
Table 1.
Characteristic ions in the EI spectra of HFBA derivatives
| Compound | MW | [M-CH2Cl]+ | [M-C3F7CO2]+ | [M-C3F7CO2CH2]+ | [M-C3F7CO2-HCl]+ |
|---|---|---|---|---|---|
| 3-MCPD | 502 | 453 | 289/291 | 275 | 253 |
| 1,3-DCP | 325 | 275/277 | 110 | – | – |
| 3-MCPD-d5 | 507 | 456 | 294 | 278 | 257 |
| 1,3-DCP-d5 | 330 | 278 | 116 | – | – |
Standard solutions were added to the samples prior to GC–MS analysis to verify the selectivity of 1,3-DCP and 3-MCPD. Sample analysis indicated a peak retention time (Rt) and a spectrum identical to that observed for the standard solution and a specific peak with no noise. Standard solutions of 3-MCPD and 1,3-DCP were diluted to 0.3–2 µg/mL, followed by derivatization and GC–MS analysis. The analysis revealed that the regression equations for 3-MCPD and 1,3-DCP were y = 1.767827x + 0.001,670 and y = 1.264366x + 0.016974, respectively, while r2 was greater than 0.9990 for 3-MCPD and greater than 0.9999 for 1,3-DCP, indicating good linearity. The LOD and LOQ for 3-MCPD were 0.6 and 2.0 µg/kg, respectively. The corresponding LOD and LOQ values for 1,3-DCP were 0.2 and 0.6 µg/kg (Table 2). In other countries, 3-MCPD had LOD and LOQ values of 0.1–10 and 1.2–50 μg/kg, respectively, and the LOD and LOQ of 1,3-DCP ranged between 0.41–3.15 and 2–150 μg/kg, respectively. Results similar to those in this experiment have been obtained previously [16, 19–27]. The recovery of this study was 88–90% for 3-MCPD and 75–80% for 1,3-DCP. Previous studies have shown that the recovery of soy is lower than that of 3-MCPD (61–131%) and 1,3-DCP (71–120%) [16, 19–27]. However, this study is suitable for 75–120% of the AOAC guidelines [17]. The lower recovery of 1,3-DCP than that of 3-MCPD is probably related to how the samples were concentrated [28]; 1,3-DCP should not be completely dried in the concentration process. Experiments were carried out at three concentrations: 4.5 ppm (final concentration 30 ppb), 7.5 ppm (final concentration 50 ppb), and 15 ppm (final concentration 100 ppb). Only the lowest possible concentration of 4.5 ppm is shown (Table 2). Seven intraday assays were conducted in 1 day to verify their precision, and a standard deviation of 5% was observed, satisfying the less than 8% requirement specified by the AOAC guidelines [17]. When the significance of the intraday precision was verified with ANOVA, no significant differences were observed (p < 0.01).
Table 2.
Limits of detection (LOD), limits of quantification (LOQ), accuracy, and precision for 3-MCPD and 1,3-DCP in foods
| Sample | Compound | Spiked concentration (µg/mL) | LOD (ng/g) | LOQ (ng/g) | Accuracy (% recovery) (n = 5, a = 0.01) |
Precision (%RSD) |
|---|---|---|---|---|---|---|
| Intra-day | ||||||
| Soy sauce | 3-MCPD | 4.5 | 0.6 | 2.0 | 90 ± 0.002a | 0.5 |
| 1.3-DCP | 0.2 | 0.6 | 80 ± 0.003 | 1.6 | ||
| Snack | 3-MCPD | 4.5 | 0.05 | 0.2 | 85 ± 0.002 | 0.8 |
| 1.3-DCP | 0.03 | 0.1 | 79 ± 0.002 | 2.0 | ||
| Rapeseed oil | 3-MCPD | 4.5 | 0.2 | 0.6 | 88 ± 0.009 | 3.7 |
| 1.3-DCP | 0.2 | 0.5 | 75 ± 0.001 | 0.8 |
aValues are mean ± confidence interval
Application of the developed GC–MS method to food containing different fat contents
Food products on sale in South Korea were classified based on their fat contents (< 10, 10–50, and 70–100%), and the main samples were selected. Soy sauce was not selected as a monitoring sample because many different sample types of soy sauce, which is widely consumed in Korea, were already used as monitoring samples. Forty-five samples of food products were analyzed (i.e., soybean paste, cookies, and dietary fats). The analysis revealed the absence of 3-MCPD and 1,3-DCP in all samples. The recovery rates for 3-MCPD and 1,3-DCP for soybean paste were 91 and 76%, respectively. The corresponding recovery rates were 91 and 80% for cookies and 76 and 88% for dietary fats. High recovery rates indicated that the GC–MS method for the simultaneous determination of 3-MCPD and 1,3-DCP is reliable (Table 3).
Table 3.
Detection of 3-MCPD and 1,3-DCP in foods
| Food categories | Compound | Sample (N) | Detected (N) | Mean (µg/kg) | Recovery (%) |
|---|---|---|---|---|---|
| Doenjang (fat content < 10%) | 3-MCPD | 15 | 0 | ND* | 91 |
| 1,3-DCP | ND | 76 | |||
| Snack (fat content 10–50%) | 3-MCPD | 15 | 0 | ND | 91 |
| 1,3-DCP | ND | 80 | |||
| Rapeseed oil (fat content > 50%) | 3-MCPD | 15 | 0 | ND | 76 |
| 1,3-DCP | ND | 88 |
*ND: Below LOD (not detected)
In the present study, 3-MCPD and 1,3-DCP were not detected in food, but the results of previous studies showed that 3-MCPD was detected in 8 of 44 soy sauces (0.02–0.28 mg/kg), 20 of 28 snacks (0.09–1.43 mg/kg), and 8 of 10 oils (0.04–1.22 mg/kg) [3]. In addition, studies thus far have reported that 3-MCPD and 1,3-DCP are produced by mixing and cooking with oil, NaCl, and water. 3-MCPD and 1,3-DCP have been reportedly found in sauces, processed meat products [29], preheated grain products, e.g., cereal, bread, donuts) [30], and compounds used as a seasoning in food products (soups) [31]. Hence, it is necessary to reduce the production of harmful substances. In addition, continuous monitoring and evaluation of different food products are crucial to ensure public health and food safety.
In conclusion, a rapid GC–MS method to simultaneously determine 3-MCPD and 1,3-DCP, typically found in food products such as soup, soy sauce, and HVP, is developed. 3-MCPD and 1,3-DCP are consumed in large quantities not only in South Korea but also in several other Asian countries, which is a social problem with global ramifications. Methods for effectively analyzing both 3-MCPD and 1,3-DCP are being developed in South Korea. The concentrations of 3-MCPD and 1,3-DCP were simultaneously analyzed to determine the content of these harmful compounds in different purchased food products in South Korea. The effectiveness of the developed GC–MS test method is verified by the calculation of selectivity, linearity, the limit of detection (LOD), the limit of quantification (LOQ), precision, and accuracy. Application of the developed method to representative food samples indicated that 3-MCPD and 1,3-DCP were absent in all 45 samples. Hence, the developed GC–MS method is accurate and effective. The results obtained herein can serve as a reference for food safety regulations.
Acknowledgements
This study was supported by the Korean Ministry of Food and Drug Safety in 2016.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
References
- 1.Velisek J, Davidek J, Hajslova J, Kubelka V, Janicek G, Mankova B. Chlorohydrins in protein hydrolysates. Z. Lebensmit.-Untersuch. Forsch. 1978;167:241–244. doi: 10.1007/BF01135595. [DOI] [PubMed] [Google Scholar]
- 2.Collier PD, Cromie DDO, Davies AP. Mechanism of formation of chloropropanols present in protein hydrolysates. J. Am. Oil Chem. Soc. 1991;68:785–790. doi: 10.1007/BF02662173. [DOI] [Google Scholar]
- 3.Kang Y-W, Park S-K, Seo J-H, Kim D-S. Survey of contaminants of bound 3-MCPD in food. J. Food Hyg. Saf. 2010;25:289–293. [Google Scholar]
- 4.Crew C, Brereton P, Davies A. The effects of domestic cooking on the levels of 3-monochloropropanediol in foods. Food Addit. Contam. 2001;18:271–280. doi: 10.1080/02652030120064. [DOI] [PubMed] [Google Scholar]
- 5.Van Duuren BL, Glodschmidt BM, Katz C, Seidman CK, Paul JS. Carcinogenic activity of alkylating agents. J. Natl. Cancer Inst. 1974;53:695–700. doi: 10.1093/jnci/53.3.695. [DOI] [PubMed] [Google Scholar]
- 6.Weisburger EK, Ulland BM, Nam J-M, Gart JJ, Weisburger JH. Carcinogenicity tests of certain environmental and industrial chemicals. J. Natl. Cancer Inst. 1981;67:75–88. [PubMed] [Google Scholar]
- 7.Haratake J, Furuta A, Iwasa T, Wakasugi C, Imazu K. Submassive hepatic necrosis induced by dichloropropanol. Liver. 1993;13:123–129. doi: 10.1111/j.1600-0676.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 8.Piasecki A, Ruge A, Marquardt H. Malignant transformation of mouse M2-fibroblasts by glycerol chlorohydrines contained in protein hydrolysate and commercial food. Arzneim. Forsch. 1990;40:1054–1055. [PubMed] [Google Scholar]
- 9.Kim M-G, Kim Y-S, Lee M-J, Kim J-K, Kim K-A, Park E-M, Ko H-U, Son J-S. Survey of contaminants of 3-MCPD and 1,3-DCP in soy sauce using GC/MS. J. Food Hyg. Saf. 2006;21:153–158. [Google Scholar]
- 10.Official Journal of the European Communities. L77/12 16. 3 (2001)
- 11.European Commission: Commission Regulation (EC) No 466. Setting maximum levels for certain contaminants in foodstuffs (2001)
- 12.Katoh T, Fueta Y, Kikuchi M, Kohshi K, Munaka M, Yamamura K, Yoshikawa M, Arashidani K. Disseminated intravascular coagulation after acute hepatic injury in rats induced by 1,3-dichloro-2-propanol. Sangyo Eiseigaku Zasshi, November. 1999;41:202–203. doi: 10.1539/sangyoeisei.KJ00002990459. [DOI] [PubMed] [Google Scholar]
- 13.Song H-S, Lee B-M. Analysis of 3-monochloro-1,2-propanediol (3-MCPD) in soy sauce products in Korea. J. Toxicol. Pub. Health. 2002;18:191–194. [Google Scholar]
- 14.Oh C-H, Yoo S-S. Comparison of the analytical method for 3-monochloropropane-1,2-diol in food. Korean J. Food Sci. Technol. 2007;39:360–365. [Google Scholar]
- 15.Position Paper on Chloropropanols for the Thirty-third Session of the Code Committee on Food Additives and Contaminants, Joint FAO/WHO Food Standard program (2001)
- 16.Park G-B, Kim Y-H, Kim J-S, Jeong J-Y, Kim C-Y, Lee S-G. Quantitative analysis of 3-MCPD in water using LC-MS. Anal. Sci. Technol. 2007;20:198–203. [Google Scholar]
- 17.Association of Official Analytical Chemists (AOAC). Guidelines for standard method performance requirements. Available from: http://www.aoac.org. Accessed May 20 (2016)
- 18.Korea Food & Drug Administration. 3-MCPD Risk Profile (2010)
- 19.Chung W-C, Hui K-Y, Cheng S-C. Sensitive method for the determination of 1,3-dichloropropan-2-ol and 3-chloropropane-1,2-diol in soy sauce by capillary gas chromatography with mass spectrometric detection. J. Chromatogr. A. 2002;952:185–192. doi: 10.1016/S0021-9673(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee H-D, Oh C-H. Establishment of analytical method and survey of contaminants of 3-MCPD (monochloropropane-1,2-diol) in food. The Annual Report of KNTP. 1, 265–312 (2002)
- 21.Meierhans DC, Bruehlmann S, Meili J, Taeschler C. Sensitive method for the determination of 3-chloropropane-1,2-diol and 2-chloropropane-1,3-diol by capillary gas chromatography with mass spectrometric detection. J. Chromatogr. A. 1998;802:325–333. doi: 10.1016/S0021-9673(97)01188-6. [DOI] [Google Scholar]
- 22.Kim W-S, Jeong Y-A, On J-W, Choi A, Lee J-Y, Lee J-G, Lee K-G, Pyo H-S. Analysis of 3-MCPD and 1,3-DCP in various foodstuffs using GC-MS. Toxicol. Res. 2015;31:313–319. doi: 10.5487/TR.2015.31.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crew C, LeBrun G, Brereton P. Determination of 1,3-dichloropropanol in soy sauces by automated headspace gas chromatography-mass spectrometry. Food Addit. Contam. 2002;19:343–349. doi: 10.1080/02652030110098580. [DOI] [PubMed] [Google Scholar]
- 24.Sim U-S. Determination of 3-MCPD in Ramyun soup by GC-MSD. Seoul: Chung ang university; 2002. [Google Scholar]
- 25.Valentina CB, Julieta AT, Terry MV. 3-Monochloro-1,2-propandiol(3-MCPD) in soy sauce from the Bulgarian market. Food Addit. Contam. 2013;6:163–167. doi: 10.1080/19393210.2013.777800. [DOI] [PubMed] [Google Scholar]
- 26.Fabian MD, Milady RN. Development of an analytical method for 3-monochloropropane-1,2-diol in soy sauce using 4-heptanone as derivatizing agent. Food Addit. Contam. 2004;21:204–209. doi: 10.1080/02652030310001656352. [DOI] [PubMed] [Google Scholar]
- 27.1,3-Dichloro-2-propanol. Chem. Abstr. Serv. Reg. NO:96-23-1 (2010)
- 28.Abu-EI-Haj S, Bogusz MJ, Ibrahim Z, Hassan H, Al Tufail M. Rapid and simple determination of chloropropanols (3-MCPD and 1,3-DCP) in food products using isotope dilution GC-MS. Food Control. 2007;18:81–90. doi: 10.1016/j.foodcont.2005.08.014. [DOI] [Google Scholar]
- 29.Woo SM, Oh JH, Jang YM, Kim MH. Analysis method development for bound-MCPD. J. Food Hyg. Saf. 2010;25:294–302. [Google Scholar]
- 30.3-MCPD in soy sauce and related products-your questions answered. Food Standard Agency (2001)
- 31.Kim HJ, Chun HS, Ha JH. Dietary exposure to 3-monochloropropane-1,2-diol from sauces and instant fried noodle (Ramyun) seasoning. J. Food Hyg. Saf. 2007;22:306–310. [Google Scholar]