Abstract
The impact of heat type, sample type, temperature and time on the heat-induced conversion of gingerols to shogaols in ginger were studied by an UHPLC–ESI–MS/MS. Heat treatments greatly induced the conversion of gingerols to shogaols in ginger. As the temperature increased, the faster conversion of gingerols into shogaols were observed. However, the efficiency of the heat-induced conversion differed greatly with the heat types. Moist heat treatment induced significantly higher quantity of shogaols than dry heat treatment. The moist heat treatment at 120 °C for 360 min induced the highest conversion, reaching to 2991 mg 6-shogaol per kg ginger. In addition, dry-heat induced conversion was affected by the sample type. The dry-heat treatment on dried powder induced significantly higher quantity of shogaols than that on sliced fresh ginger. This represents the first systematic comparative study on the heat and sample types on the heat-induced conversion of gingerols into shogaols in ginger.
Keywords: Gingerol and shogaol, UHPLC–ESI–MS/MS, Dry heat, Moist heat, Ginger powder
Introduction
Ginger (Zingiber officinale Roscoe) root is commonly used in various foods, beverages, and pharmaceutical supplements due to its high contents of pungent compounds (gingerols and shogaols) [1, 2]. The bioactive compounds in ginger have been known to have various health promoting functions such as antitumor, anti-platelet activator, antioxidative and anti-inflammatory activity, and preventive effectiveness on pregnancy-related nausea and vomiting [3–8].
6-, 8, and 10-Gingerols are the most abundant pungent compounds in fresh ginger. Shogaols, the corresponding dehydration products of gingerols, are present only in trace quantities in fresh gingers, but in high quantity in thermally treated ginger roots due to the heat-induced conversion of gingerols to shogaols [9, 10]. Shogaols have been reported to exhibit more potent bioactive properties such as free radical scavenging capacity, anti-inflammatory, and anti-cancer abilities than gingerols [6, 10–14]. Shogaols have been reported to exhibit much stronger anti-proliferative activity than gingerols on H-1299 human lung cancer cells and HCT-116 human colon cancer cells [13]. Accumulated data of previous studies also suggest that 6-shogaol play an important role as a memory-enhancing and anti-oxidant agent against neurological diseases. Supplementation of 6-shogaol has been reported to greatly reduce the microgliosis and astrogliosis in intrahippocampal AbO-injected mice, and AbO and scopolamine-induced memory impairment in animal study [14]. Several studies on the heat-induced conversion (dehydration) of gingerols into shogaols in ginger have been previously reported. The heat-induced dehydration reaction of gingerols to shogaols in ginger might be affected by the heat type (dry heat or moist heat) and sample type (sliced fresh ginger or freeze-dried ginger powder). However, systematic comparative study on the effects of heat type, sample type on the conversion of gingerols to shogaols in ginger at the various temperature and duration have not been conducted.
Thus, the objective of this research was to study the comparative impacts of heat type (dry and moist heat), temperature, and heating time on the heat-induced conversion efficiency of gingerols to shogaols in ginger.
Materials and methods
Materials and chemicals
Gingerol and shogaol standards (6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). HPLC-grade acetonitrile, water, and ethyl acetate were purchased from Fisher Scientific (Fair Lawn. NJ, USA). Formic acid and ammonium formate were obtained from Merck (Darmstadt, Germany) and Fluka (St. Louis, MO, USA), respectively. Fresh gingers were purchased from a single local ginger farm in Wanju, Jeonbuk, Republic of Korea. Gingers were thinly sliced with the thickness of 5.0 mm by the slicer (SJM slicer, Samjin Machine Co., Kimpo, Kyonggido, Republic of Korea). The samples of fresh sliced gingers were divided and individually packed in flexible PE zipper bags (60 g per bag). The samples in the zipper bags were placed in a refrigerator until used.
Dry heat and moist heat treatments
We conducted two independent dry heat treatment experiments; one with the sliced fresh ginger and the other one with the freeze-dried ginger powder. For the dry heat treatment, the thinly sliced fresh gingers (30 g each) were placed in a single layer in aluminum foil for the even heat penetration. The samples in aluminum foils were placed in duplicate in a convection oven (VS1202D3HT, Vision Scientific, Seoul, Republic of Korea) set at the various different temperatures (100, 110, 120 and 130 °C). Parts of the sliced gingers were freeze-dried and ground to make ginger powder. The dry heat treatment on the freeze-dried ginger powders (5 g each) were transferred in duplicate into 100 mL capacity glass beaker and placed in the convection oven set at the various different temperatures (100, 110, 120 and 130 °C). The samples at the predetermined time (10, 30, 120, 240, and 360 min) were collected from the oven. Moist heat treatment with the sliced fresh gingers was conducted in an autoclave (AC12, Biogenics, Daejeon, Republic of Korea) set at the various different temperatures (100, 110, 120 and 130) for different heating time (10, 30, 120, 240, and 360 min). Preliminary heat treatment experiments were conducted with different sample amounts (5 and 10 g of powdered samples, and 30 and 60 g sliced fresh ginger samples) on the conversion of gingerols to shogaols in the sample to monitor the impact of sample amount on the conversion efficiency. It was found that the heat induced conversion rate was not considerably affected by the sample amount.
Sample extraction
The gingerols and shogaols were extracted from gingers with ethyl acetate according to the previous reports [15–17]. Fresh gingers and heat treated gingers were first freeze-dried and then were ground using a food grinder (GREEN-MIX, Daesung Artlon, Republic of Korea). The powdered sample (100 mg) was extracted with ethyl acetate (3 mL) according to the previous report [17]. The extracted sample solutions were diluted to 10–30 × with acetonitrile to obtain an appropriate concentration for the UHPLC–ESI–MS/MS analysis.
Quantification of gingerols and shogaols by UHPLC–MS/MS analysis
A previously validated analytical method (UHPLC–MS/MS) was used for the determination of gingerols and shogaols [17]. The quantification of gingerols and shogaols was performed with a UHPLC (Nexera ×2 system consisting of LC-30AD, SIL-30AC, STO-20AC, Shimadzu, Tokyo, Japan) coupled to a triple quadrupole mass spectrometer (TQ8040, Shimadzu). The analytical conditions including the column, mobile phase, quantitative and qualitative MRM transitions were adopted from the previous report [17]. Briefly, the column used was a short core shell column (Kinetex, 2.1 mm × 50 mm, 2.6 μm particle, Phenomenex, Torrance, CA, USA). The mobile phase system was composed of 0.05 mM ammonium formate and 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The mobile phase gradient program was as following: gradient program of 0–0.01 min, 60% B; 0.01–0.90 min, 60–90% B; 0.90–2.00 min, 90% B; 2.00–2.10 min, 90–60% B; 2.10–2.50 min, 60% B. The flow rate of mobile phase was 1.0 mL min−1. The injection volume was 1 μL and the column temperature was maintained at 40 °C. The quantitation of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol was conducted with the external calibration curves of their authentic compounds, respectively. The contents of 8-shogaol and 10-shogaol, which were not commercially available, were calculated with calibration curves of 6-shogaol. The quantity of gingerols and shogaols in ginger was expressed mg/kg ginger on the base of dry weight.
Statistical analysis
Analysis of variance (ANOVA) and Duncan’s multiple-range test (a post-hoc test) were conducted to monitor the statistical significance of the contents of gingerols and shogaols in the samples at α = 0.05 by using a SPSS statistical analysis program (SPSS 14.0 K, SPSS, Chicago, IL, USA).
Results and discussion
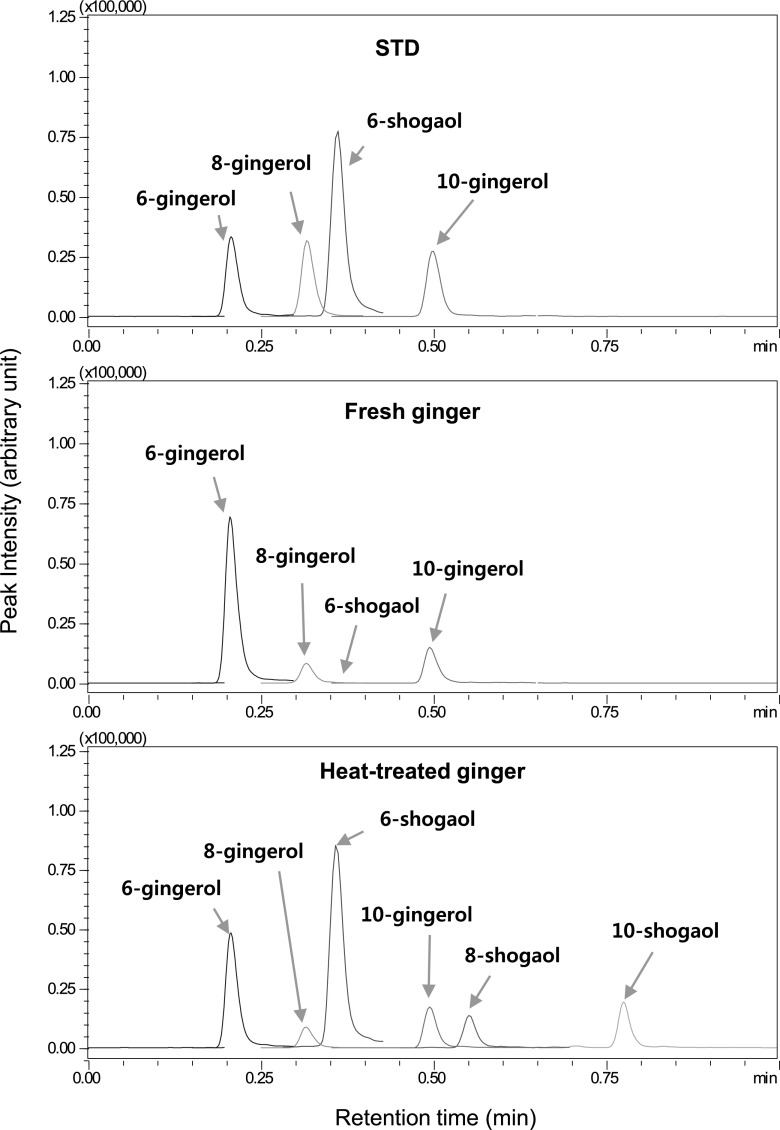
UHPLC–MS chromatograms for the standards and ginger extracts
The gingerols and shogaols in the samples were determined by a previously validated UHPLC-tandem mass spectrometry method. The representative chromatograms of authentic gingerols and shogaols and extracts of fresh and heat treated gingers obtained by the UHPLC–ESI–MS/MS are shown in Fig. 1. With this method, a full separation of the major gingerols and shogaols was obtained within 1 min. In fresh ginger, 6-gingerol was the major component, which was consistent with previous reports [9, 15, 17]. The contents of 6-, 8-, 10-gingerol in fresh ginger were 6200, 914, and 1366 mg/kg ginger (on the dry-weight basis). Only a tiny quantity of shogaols was found in the fresh ginger. The contents of 6-, 8-, 10-shogaol in fresh ginger before heat treatment were 29, 4, and 5 mg/kg ginger (on dry-weight basis), respectively.
Fig. 1.
The representative chromatograms of authentic standards (6-, 8-, and 10-gingerols and 6-shogaol, 0.5 mg/mL each) and extracts of fresh and heat treated gingers obtained by a previously validated UHPLC–ESI–MS/MS method
Dry heat induced conversion of gingerols to shogaols
The dry heat treatments on two different type ginger samples (sliced fresh ginger and freeze-dried ginger powder) were performed in a convection oven at different temperatures (100, 110, 120 and 130 °C) for the different heating times (10–360 min) [Figs. 1, 2(A, B), respectively]. Heat treatment greatly decreased the gingerols and increased the shogaols, supporting the previous observation that shogaols are artifacts converted from gingerols through dehydration reaction [18]. Dry heat treatment on the sliced fresh ginger at 130 °C for 360 min decreased the 6-gingerol from 6243 to 2877 mg/kg ginger (on dry weight basis), but induced the formation of 6-shogaol, reaching to 1350 mg 6-shogaol/kg ginger (on dry weight basis). However, dry heat treatment on the dried ginger powder at 130 °C for 360 min decreased the 6-gingerol from 6288 to 2265 mg/kg ginger (on dry weight basis), but induced the formation of 6-shogaol, reaching to 1611 mg 6-shogaol/kg ginger (on dry weight basis). It is noteworthy that the dry heat treatment on the powder type ginger was significantly more efficient for the conversion of gingerols to shogaols than that on sliced fresh ginger (p < 0.05). This result clearly showed that the conversion of gingerols to shogaols were greatly affected by the sample type (sliced fresh ginger or dried powder). The low conversion efficiency in sliced fresh ginger may be due to the water vaporization energy required during the initial stage of dry-heat treatment. The vaporization energy exerted on the sliced ginger products resulted in less heat energy on the sliced fresh gingers as compared with dried ginger powder. The maximum content of 6-shogaol was achieved by the dry heat treatment on dried ginger powder at 130 °C for 240 min. After then, the content of 6-shogaol slightly decreased. The same phenomena has been also previously observed by the Ho and Su [10]. Ho and Su [10] studied the effects of dry heat treatment on the conversion of gingerols to shogaols in the ginger powder at 75, 100, 125, and 150 °C in a convection oven for 20, 40, 60, 80, and 180 min. The authors reported that the dry heat treatment at 150 °C rapidly increased the 6-shogaol concentration up to 80 min heating time. After then, the 6-shogaol contents in ginger decreased significantly. However, in the previous study [10], the authors used the ginger powder, which were prepared by drying the fresh ginger in a convection oven at 50 °C for 48 h. The initial ginger powder sample already contained significant quantity of shogaols [10]. However, in our study, we prepared the ginger powder by the freeze drying technique. Thus, only negligible quantity of shogaols were present in our ginger powder samples. Furthermore, the dry heat treatment on the sliced fresh ginger was not studied in the previous study [10]. Our present data clearly showed that dry heat treatment on powdered ginger sample exerted significantly higher conversion than that on sliced fresh ginger at the same heat treatment conditions.
Fig. 2.

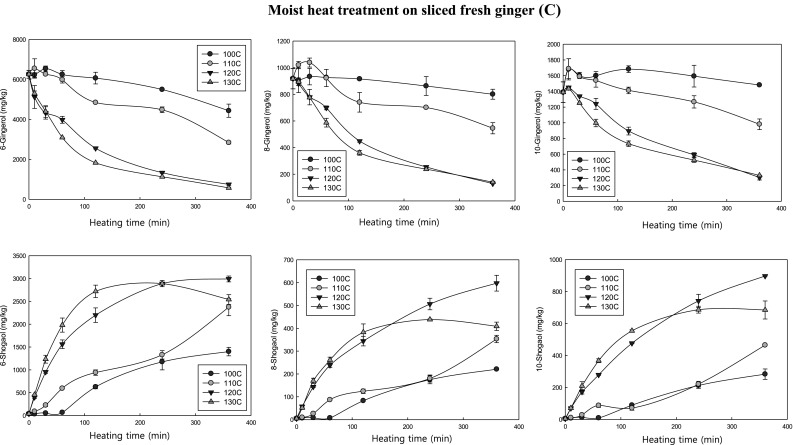
The conversions of gingerols to shogaols in sliced fresh ginger (A) and dried ginger powder (B) during the dry-heat treatments and sliced fresh ginger during wet heat treatment (C) at the different temperatures (100, 110, 120 and 130 °C) for the different time (10, 30, 60, 120, 240 and 360 min)
Moist heat induced conversion of gingerols to shogaols
Figure 2(C) shows the impacts of moist heat on contents of gingerols and shogaols in sliced gingers. The moist heat treatment was conducted in an autoclave. Here again, the temperature and time greatly affected the contents of gingerols and shogaols in the ginger. The higher the temperature was, the faster the decrease of gingerols and the faster the increase of shogaols were observed. The 6-gingerol content in ginger after the heat treatment of 360 min at 100, 110, 120 and 130 °C decreased from 6258 mg/kg to 4436, 2840, 746, and 571 mg/kg ginger (on dry weight basis), respectively. The moist heat treatment at 130 °C rapidly increased the content of 6-shogaol up to maximum 2890 mg/kg (on dry weight basis) during the 240 min treatment. After then, further heat treatment decreased the contents of shogaols. However, at 120 °C, the contents of shogaols continuously increased until the end of the heating time (360 min), reaching to 2991 mg/kg (on dry weight basis). It is interesting to note that the moist heat induced significantly higher quantity of shogaols than dry heat. Note that the dry heat treatment on the sliced fresh ginger at 130 °C for 360 min induced the maximum 1161 mg 6-shogaol/kg ginger (on dry weight basis), whereas the moist heat treatment at 130 °C induced the formation of the 6-shogaol up to 2890 mg/kg (on dry weight basis) in ginger. Yang et al. [19] conducted the heat treatment of ginger with 9 repeated steaming and drying (each time required 30 min steaming and 12 h drying) to obtain black ginger with high quantity of shogaol. The content of 6-shogaol in the 9 repeated steaming and drying heat-treated ginger was 2099 mg/kg (on dry weight basis) (total required time was 112.5 h) [19]. Cheng et al. [11] previously conduced the moist heat treatment experiment on raw ginger at 120 °C for 0.5, 1, 2, 4, and 6 h to study the heat induced conversion of gingerols to shogaols. The authors reported that the level of 6-shogaol significantly increased during the steaming process, and achieved to the maximum quantity at 4 h [11]. When the steaming lasted for 6 h, 6-shogaol was found to slightly decrease. However, the authors did not study the experiment of the moist heat treatment at different temperature for the various different time. The only other heat treatment test was done at 100 °C for 1 h as a control. However, we conducted the experiments at various different temperatures for different heating times to check the time-course changes of the conversion of gingerols to shogaols. Our results clearly showed that the moist heat-induced conversion of gingerols to shogaols was also greatly dependent on the temperatures. The higher the temperature induced the faster conversion rate. So high temperature of moist heat treatment could efficiently reduce the required heat-treatment time for the ginger products with high quantity of shogaols.
In a brief conclusion, the heat-induced conversion of gingerols to shogaols were found to be significantly affected by the heating type (dry heat or moist heat) and sample type (sliced fresh ginger or freeze-fried ginger powder), heating temperature and time. Generally the higher the temperature, the faster and higher the conversion. Our results clearly suggested that moist heat treatment at a higher temperature (120 or 130 °C) for the preset time is advantageous to obtain the ginger products with high quantity of bioactive components of shogaols.
Acknowledgements
Authors greatly appreciate Ms. Hong Yu Cheng for her technical assistance.
Compliance with ethical standards
Conflict of interest
This research was supported by the Ministry of Agriculture, Food and Rural Affairs (MAFRA), through the 2015 Healthy Local Food Branding Project of the Rural Resources Complex Industrialization Support Program.
References
- 1.Bartley JP, Jacobs AL. Effects of drying on flavour compounds in Australian-grown ginger (Zingiber officinale) J. Sci. Food Agric. 2000;80:209–215. doi: 10.1002/(SICI)1097-0010(20000115)80:2<209::AID-JSFA516>3.0.CO;2-8. [DOI] [Google Scholar]
- 2.Pawar N, Pai S, Nimbalkar M, Dixit G. RP-HPLC analysis of phenolic antioxidant compound 6-gingerol from different ginger cultivars. Food Chem. 2011;126:1330–1336. doi: 10.1016/j.foodchem.2010.11.090. [DOI] [Google Scholar]
- 3.Katiyar SK, Agrwal R, Mukhtar H. Inhibition of tumor promotion in SENCAR mouse skin by ethanol extract of Zingiber officinale Rhizome. Cancer Res. 1996;56:1023–1030. [PubMed] [Google Scholar]
- 4.Kim SO, Kim KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 5.Manju V. Nalini. N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin. Chim. Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Hsu YL, Chen CY, Lin IP, Tsai EM, Kuo PL, Hou MF. 4-Shogaol, an active constituent of dietary ginger, Inhibits metastasis of MDA-MB-231 human breast adenocarcinoma cells by decreasing the repression of NF-κB/Snail on RKIP. J. Agric. Food Chem. 2012;60:852–861. doi: 10.1021/jf2052515. [DOI] [PubMed] [Google Scholar]
- 7.Young HY, Luo YL, Cheng HY, Hsieh WC, Liao JC, Peng WH. Analgesic and anti-inflammatory activities of [6]-gingerol. J. Ethnopharm. 2005;96:207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Shanmugam KR, Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2010;124:1436–1442. doi: 10.1016/j.foodchem.2010.07.104. [DOI] [Google Scholar]
- 9.Park JS, Jung MY. Development of high-performance liquid chromatography-time-of- flight mass spectrometry for the simultaneous characterization and quantitative analysis of gingerol-related compounds in ginger products. J. Agric. Food Chem. 2012;60:10015–10026. doi: 10.1021/jf302944p. [DOI] [PubMed] [Google Scholar]
- 10.Ho S, Su M. Optimized heat treatment enhances the anti-inflammatory capacity of ginger. Int. J. Food Prop. 2016;19:1884–1898. doi: 10.1080/10942912.2015.1084633. [DOI] [Google Scholar]
- 11.Cheng X, Liu Q, Peng Y, Qi L, Li P. Steamed ginger (Zingiber officinale): Changed chemical profile and increased anticancer potential. Food Chem. 2011;129:1785–1792. doi: 10.1016/j.foodchem.2011.06.026. [DOI] [Google Scholar]
- 12.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-Inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol, and [6]-shogaol. J. Ethnopharm. 2010;127:515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Sang S, Hong J, Wu H, Liu J, Yang CS, Pan M, Badmaev V, Ho C. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009;57:10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon M, Kim HG, Choi JG, Oh H, Lee PKJ, Ha SK, Kim SY, Park Y, Huh Y, Oh MS. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Sep. Purif. Technol. 2015;146:219–226. doi: 10.1016/j.seppur.2015.03.049. [DOI] [Google Scholar]
- 15.Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages. J Chromatogr. B. 2007;856:41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Yudthavorasit S, Wongravee K, Leepipatpiboon N. Characteristic fingerprint based on ginger derivative analysis for discrimination of ginger (Zinger officiale) according to geographical origin using HPLC-DAD combined with chemometrics. Food Chem. 2014;158:101–111. doi: 10.1016/j.foodchem.2014.02.086. [DOI] [PubMed] [Google Scholar]
- 17.Park SY, Jung MY. UHPLC-ESI-MS/MS for the quantification of eight major gingerols and shogaols in ginger products: Effects of ionization polarity and mobile phase modifier on the sensitivity. J. Food Sci. 2016;81:C2457–C2465. doi: 10.1111/1750-3841.13429. [DOI] [PubMed] [Google Scholar]
- 18.Shao X, Lv L, Parks T, Wu H, Ho CT, Sang S. Quantitative analysis of ginger components in commercial products using liquid chromatography with electrochemical array detection. J. Agric. Food Chem. 2010;58:12608–12614. doi: 10.1021/jf1029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang BW, Park HS, Park JW, Baik MY, Kim BY, Kim HK, Hahm YT. Physicochemical Properties of repetitive heat-treated ginger and its quantitative conversion of gingerol to shogaol. Food Eng. Prog. 2017;21:22–28. doi: 10.13050/foodengprog.2017.21.1.22. [DOI] [Google Scholar]