Abstract
Pectins were extracted from banana peels of five different varieties using citric acid solution. The chemical characteristics of banana peel pectins were investigated and compared with citrus peel and apple pomace pectins which were extracted under the same extraction conditions to assess the potential of banana peels as an alternative source of commercial pectin. The yield of banana peel pectins ranged from 15.89 to 24.08%. The extracted banana peel pectins were categorized as high methoxyl pectin with the degree of esterification between 63.15 and 72.03% comparable to those of conventional pectin sources from citrus peel (62.83%) and apple pomace (58.44%). The anhydrouronic acid (AUA) content of banana peel pectins varied from 34.56 to 66.67%. Among various banana varieties being studied, pectin from Kluai Nam Wa variety had the highest AUA content (66.67%) which met the criteria for food additive pectin indicating its commercial significance as an alternative pectin source.
Keywords: Banana peels, Pectin, Citric acid extraction, FT-IR
Introduction
Large quantities of by-products are generated from the fruit processing industry, especially as peel and stones which can be used for animal feed [1]. These by-products are a cheap source of raw material for animal feed stuff, and the peel also contains high amounts of pectin. Citrus peel and apple pomace have long been utilized for commercial scale pectin production [2]. Pectin is a structural hetero-polysaccharide found in the primary cell walls of most plants. It provides mechanical strength and flexibility due to interaction with other cell wall components [3]. Pectin is a high value functional food ingredient because of its excellent emulsifying properties and stability which can be used as a gelling agent and stabilizer [2].
Commercially, pectin is extracted from raw materials such as apple pomace or citrus peel by acid at a high temperature. The pH, temperature and extraction method affect pectin yield and quality [4]. Extraction and characterization of pectin have been studied for many plant materials. Many researchers focused on the extraction of pectin from pumpkin, soy hull, peach pomace, sugar beet, Krueo Ma Noy (Cissampelos pareira; an herb traditionally used in warm regions of Asia, East Africa and South America), apple pomace, mango, Ambarella, banana peel, cocoa husks, citrus peel, jackfruit waste and Saba banana waste [4–15]. Interest is now evident in alternative sources of pectin such as banana peel.
Banana (Musa spp.) is an important fruit crop in tropical and subtropical regions of the world. Banana fruit is a good source of energy and minerals which is normally consumed fresh or processed to make many products such as chips, powder, jam and wine. Banana peels accounts for 40% of the total weight of the fresh fruit [16]. The peel is not used and it is discarded as solid waste at large expense. With the development of the banana processing industry and increased production of processed fruit products, large quantities of banana peel are wasted or cheaply consumed as animal feed. This is both uneconomical and non-environmentally friendly [11]. Therefore, the banana-processing industry has been searching for applications of banana peel as a source of pectin [17].
In this study, pectin was extracted from banana peels of five different banana varieties using a conventional hot acid extraction method. These banana varieties were selected based on their high consumption in Thailand. The yield and properties of the obtained pectin were investigated in order to determine whether there is significant variation in the pectin yield and characteristics among different varieties. In addition, citrus peel and apple pomace, which are the conventional source for pectin production, were also included in this study. The pectins extracted from these two conventional sources using the same extraction conditions and method were then characterized for their properties and the results were compared with the banana peel pectins.
Materials and methods
Raw materials
Banana (green or unripe) peels from five different varieties, including Kluai Khai and Kluai Leb Mu Nang from Musa (AA); Kluai Hom Thong from Musa (AAA); Kluai Nam Wa and Kluai Hin from Musa (ABB), citrus peel (Citrus reticulate Blanco.), and apple pomace were used as pectin sources in this study. The fruits were purchased from the local market. The peels were removed from the banana and citrus fruits, washed with water and chopped into 1 cm2 pieces using a stainless steel knife. Apple was first washed and minced in an electric grinder. The crushed pulp was then pressed to remove juice. Apple pomace and all fruit peels were then dried at 60 °C for 24 h, ground to pass through a 60-mesh sieve and stored in a desiccator at room temperature prior to analysis.
Alcohol insoluble solids (AIS) preparation
Dried powder of the samples were homogenized in boiling ethanol (solid–liquid ratio of 1:10, w/v) with a final ethanol concentration of 80% to inactivate possible endogenous enzymes and remove alcohol-soluble solids. After boiling for 20 min, the residue was filtered through a nylon cloth and washed with 70% ethanol. It was then washed successively with ethanol (95%, 3 times), acetone (3 times) and vacuum-dried at 40 °C overnight to remove the moisture and weighed.
Pectin extraction
The extraction procedure was modified from Happi Emaga et al. [18] and Rascón-Chu et al. [19]. Pectin was extracted from banana peels of five different banana varieties, citrus peel and apple pomace using 6% citric acid solution and the results were compared to determine the best source for good quality pectin recovery [18]. Ten grams of AIS was heated with 200 mL (solid–liquid ratio of 1:20, w/v) of extraction solutions. After extraction, the mixture was cooled to room temperature and then centrifuged at 8000 rpm for 10 min. The supernatant was filtered through a Whatman No. 1 filter paper to remove the impurities. The filtrate was dispersed in an equal volume of 95% ethanol containing 0.05 M HCl, stirred for 5 min for proper mixing and allowed to stand for 24 h. The precipitates were collected and washed 3–4 times with 95% ethanol. The product was then dried at 40 °C in an air oven to constant weight. The dried pectin was ground to pass 100-mesh sieve and stored at room temperature. Pectin yield was calculated as follows:
Determination of ash content, moisture content and water activity
Ash content was determined by incinerating 1 g of sample in a furnace at 550 °C for 4 h. The subsequent ash was cooled and stored in a desiccator with blue silica gel until weighing. Moisture content was determined by drying pectin samples in an air-circulated oven at 105 °C, for 24 h. All values were calculated on a dry-weight basis [20].
Water activity was measured three times per treatment using an AquaLab water activity meter (CX-2, Decagon Devices, Inc., Washington, DC, USA). A water activity for the pectin below 0.60 would indicate microbial stability for the product.
Determination of equivalent weight
Equivalent weight was determined by Ranganna’s method [21]. Pectin sample of 0.5 g was placed in a 250 mL flask, moistened with 2 mL of ethanol and dissolved in 100 mL of carbon dioxide-free water. Sodium chloride (1 g) was added to sharpen the end point and 6 drops of phenol red indicator were added. The mixture was then stirred rapidly to dissolve all pectin substance. Titration was done slowly with 0.1 N sodium hydroxide until the color of the indicator changed to pink (pH 7.5). The neutralized solution was used for the methoxyl determination. Equivalent weight was calculated using the equation:
Determination of methoxyl content (MeO)
Determination of MeO was done by using the Ranganna’s method [21]. This was done by adding 25 mL of 0.25 N sodium hydroxide to the neutralized solution obtained from the determination of equivalent weight. The mixture was stirred thoroughly and allowed to stand for 30 min at room temperature in a stoppered flask. Twenty-five milliliters of 0.25 N hydrochloric acid was then added and titrated to the same end point as before. The following equation was used to calculate the methoxyl content:
where 31 is the molecular weight of the methoxyl group.
Determination of anhydrouronic acid content (AUA)
Total AUA of pectin was obtained following a formula reported in the previous publication [22]. By using the titration volumes obtained from the determination of equivalent weight and methoxyl content, the AUA was calculated as follows:
where molecular unit of AUA (1 unit) = 176 g, z = mL (titre) of sodium hydroxide from equivalent weight determination, y = mL (titre) of sodium hydroxide from methoxyl content determination, w = weight of sample.
Determination of degree of esterification (DE)
The DE of pectin was calculated according to the formula reported previously [15, 23].
Color measurement
The color parameters of the pectin samples were determined using a Hunter Lab colorimeter by measuring L*, a* and b* values in the CIE system [24].
FT-IR spectroscopic method
All samples were dried and desiccated in a vacuum jar containing blue silica gel prior to FT-IR analysis. FT-IR spectra of samples were obtained using a Golden Gate Diamond single reflectance ATR on an FTS 7000 FT-IR spectrophotometer with a DTGS detector (DIGILAB, Randolph, MA). The spectra were recorded at the absorbance mode from 4000 to 400 cm−1 (mid-infrared region) at a resolution of 4 cm−1 and 128 interferograms were collected to obtain a high signal to noise ratio [3].
Statistical analysis
All experiments were carried out at least in triplicate. Data were interpreted by one-way analysis of variance (ANOVA) using SPSS 21 software. Statistical significance was evaluated at p < 0.05 level. Duncan’s multiple range test was applied for mean comparison.
Results and discussion
In this study, the peels from five banana varieties were used for pectin extraction. These banana varieties were selected based on their high consumption in Thailand. The five varieties were from 3 different genomic groups. Kluai Khai and Kluai Leb Mu Nang belonged to Musa (AA), Kluai Hom Thong belonged to Musa (AAA) and Kluai Nam Wa and Kluai Hin belonged to Musa (ABB). These banana varieties are also commonly cultivated in other parts of the world; however, they may be called by other local names. The readers interested in the local names of these varieties in specific countries can find details in the previous reviews by Valmayor et al. [25] and Ploetz et al. [26]. The genomic group, subgroup and geographic distribution of each variety [25–27] are provided in Table 1.
Table 1.
Genomic group, subgroup and geographic distribution of banana varieties used in the study
| Variety (local name) | Genomic groupa,b | Subgroupb | Geographic distributionb,c |
|---|---|---|---|
| Kluai Khai | AA | Sucrier | All continents |
| Kluai Leb Mu Nang | AA | – | – |
| Kluai Hom Thong | AAA | Gros Michel | All continents |
| Kluai Nam Wa | ABB | Pisang Awak | Southeast Asia, East Africa, Australia |
| Kluai Hin | ABB | Saba | Philippines, Indonesia, Malaysia, Thailand |
Yield, ash content, moisture content and water activity of pectin
The yield, ash content, moisture content and water activity of pectin extracted from banana peels, citrus peel and apple pomace are presented in Table 2. The yields of pectin extracted from different sources were significantly different (p < 0.05) and varied from 10.91 to 24.08%. The highest pectin yield was obtained from banana peel namely Kluai Leb Mu Nang (24.08%) whereas the lowest yield was obtained from apple pomace (10.91%). Previous studies have shown that pectin yield decreases with an increase in fruit maturation [15, 23]. Castillo-Israel et al. [15] reported that the yield of pectin extracted from the peel of unripe Saba banana was 16.54% which was higher than that obtained from the peel of ripe Saba banana (11.87%). In our study, we used the peels from unripe bananas. The pectin yields (15.89–24.08%) in this study were consistent with the value from unripe Saba banana peel reported earlier [15]. Pectin yields from banana peels were comparable to the values obtained from the conventional sources of pectin (i.e. citrus peel and apple pomace), indicating the potential of banana peel as an alternative source for commercial pectin production.
Table 2.
Extraction yield (% dried peel powder), ash content, moisture content and water activity of pectin extracted from banana peels, citrus peel and apple pomace
| Pectin sources | Pectin yield (%) | Ash content (%) | Moisture content (%) | Water activity |
|---|---|---|---|---|
| Banana peels | ||||
| Kluai Khai | 16.24 ± 0.13e | 2.76 ± 0.01c | 6.30 ± 0.11b | 0.26 ± 0.00d |
| Kluai Leb Mu Nang | 24.08 ± 0.05a | 1.38 ± 0.01g | 6.39 ± 0.07b | 0.28 ± 0.00b |
| Kluai Hom Thong | 17.31 ± 0.07d | 2.87 ± 0.01b | 6.24 ± 0.08b | 0.31 ± 0.01a |
| Kluai Nam Wa | 15.89 ± 0.02f | 2.49 ± 0.13d | 4.55 ± 0.13c | 0.22 ± 0.00f |
| Kluai Hin | 19.48 ± 0.08b | 1.43 ± 0.01f | 6.51 ± 0.49b | 0.27 ± 0.01c |
| Citrus peel | 19.25 ± 0.04c | 3.46 ± 0.01a | 7.92 ± 0.43a | 0.22 ± 0.00f |
| Apple pomace | 10.91 ± 0.02g | 1.96 ± 0.01e | 4.54 ± 0.02c | 0.25 ± 0.00e |
Mean followed by different letters within the same column are significantly different (p < 0.05)
Moisture content of all pectin samples ranged from 4.54 to 7.92%. This observation was comparable to the values of soy hull pectin (6–7%) reported by Kalapathy and Proctor [6]. Water activity (aw) indicates the amount of water in the food which can be used by microorganisms. Low aw material has longer shelf life. The aw of all pectins obtained in this study was quite low (0.22–0.31). Low moisture content and water activity are necessary for safe storage because they inhibit the growth of microorganisms and pectinase enzymes that adversely affect pectin quality [28].
The ash content of pectins extracted from various banana peels was between 1.38 and 2.87% which was in similar range to that obtained from the conventional pectin sources, citrus peel (3.46%) and apple pomace (1.96%). The ash content indicates the purity of the pectin. Lower ash content means higher purity.
Characterization of pectin
The results for chemical characterization of pectins extracted from various varieties of banana peels as compared to citrus peel and apple pomace are shown in Table 3. The equivalent weight of pectins from banana peels ranged from 943 to 1456 which was higher than those obtained from citrus peel (577) and apple pomace (551). Pectin extracted from Kluai Nam Wa peel had methoxyl content (8.46%) comparable to those from citrus peel and apple pomace (9.06 and 7.92%, respectively) while banana peels of other varieties had lower methoxyl contents (3.86–5.97%). Depending on the degree of esterification (DE), pectin is divided into two groups: pectin with DE higher than 50% is known as high methoxyl pectin while low methoxyl pectin has a DE lower than 50% [24]. The DE of extracted pectins from various banana varieties ranged between 63.15 and 72.03%, indicating that all banana peel pectins were categorized as high methoxyl pectin similar to those from citrus peel (62.83%) and apple pomace (58.44%).
Table 3.
Chemical characterization of pectin obtained from banana peels compared with citrus peel and apple pomace
| Pectin sources | Equivalent weight (g/mol) | MeO (%) | AUA (%) | DE (%) |
|---|---|---|---|---|
| Banana peels | ||||
| Kluai Khai | 1140.30 ± 0.30d | 5.97 ± 0.01d | 49.43 ± 0.03d | 68.74 ± 0.04b |
| Kluai Leb Mu Nang | 1456.93 ± 0.04a | 4.31 ± 0.02e | 36.46 ± 0.02f | 67.07 ± 0.02c |
| Kluai Hom Thong | 1312.20 ± 0.15c | 4.25 ± 0.02f | 37.49 ± 0.01e | 64.25 ± 0.03d |
| Kluai Nam Wa | 943.14 ± 0.07e | 8.46 ± 0.01b | 66.67 ± 0.02c | 72.03 ± 0.04a |
| Kluai Hin | 1380.06 ± 0.03b | 3.86 ± 0.02g | 34.56 ± 0.01g | 63.15 ± 0.03e |
| Citrus peel | 577.72 ± 0.09f | 9.06 ± 0.03a | 82.05 ± 0.03a | 62.83 ± 0.02f |
| Apple pomace | 551.29 ± 0.10g | 7.92 ± 0.02c | 76.80 ± 0.01b | 58.44 ± 0.03g |
Mean followed by different letters within the same column are significantly different (p < 0.05)
The content of anhydrouronic acid (AUA) indicates the purity of the extracted pectin with a recommended value of not less than 65% for pectin used as food additives or for pharmaceutical purpose [2, 29]. This requirement has limited the potential sources of food and pharmaceutical pectins. The search for alternative pectin sources for commercial production using hot diluted acid extraction technique which is the most convenient approach for industrial extraction of pectin has been the topic of many studies [6, 15, 18, 23, 30]. Results in Table 3 show that the pectins from citrus peel and apple pomace had AUA content at 82.05 and 76.80%, respectively, indicating their high purity. The AUA contents of banana peel pectins ranged from 34.56% for Kluai Hin to 66.67% for Kluai Nam Wa. These results were relatively similar to those previously reported for Saba banana peel pectins [15] and Grande Naine banana peel pectins [17] which were from 39.68 to 57.32% and from 40.0 to 69.1%, respectively. Based on the AUA content, only banana peel pectin from Kluai Nam Wa variety had AUA content higher than 65% and met the criteria for commercial pectin; thus, banana peel from Kluai Nam Wa variety can be an alternative source of high methoxyl pectin.
Color of pectin
Color parameters of pectin isolated from banana peels were determined using a Hunter Lab colorimeter and the value shown in Table 4. The lightness (L*) of extracted pectins ranged from 73.53 to 85.30 with significant difference (p < 0.05). In general, pectins extracted from banana peels showed higher lightness than citrus peel and apple pomace pectins. The redness (a*) and yellowness (b*) of the apple pomace and citrus peel pectins were greater than banana peel pectins. This would be reflected from different color component contained in the raw materials.
Table 4.
Color parameters of pectin from banana peels compared with citrus peel and apple pomace
| Pectin sources | Color parameter | ||
|---|---|---|---|
| L* | a* | b* | |
| Banana peels | |||
| Kluai Khai | 77.33 ± 0.12f | 3.88 ± 0.07b | 7.55 ± 0.07d |
| Kluai Leb Mu Nang | 82.30 ± 0.12c | 2.09 ± 0.03e | 5.37 ± 0.03g |
| Kluai Hom Thong | 82.82 ± 0.06b | 2.81 ± 0.03c | 6.02 ± 0.07f |
| Kluai Nam Wa | 80.67 ± 0.09d | 3.85 ± 0.03b | 8.59 ± 0.07c |
| Kluai Hin | 85.30 ± 0.38a | 2.67 ± 0.03d | 7.18 ± 0.07e |
| Citrus peel | 78.83 ± 0.12e | 2.20 ± 0.06e | 11.44 ± 0.06b |
| Apple pomace | 73.53 ± 0.18g | 9.09 ± 0.09a | 12.93 ± 0.06a |
Mean followed by the different letters within the same column are significantly different (p < 0.05)
FT-IR spectroscopy
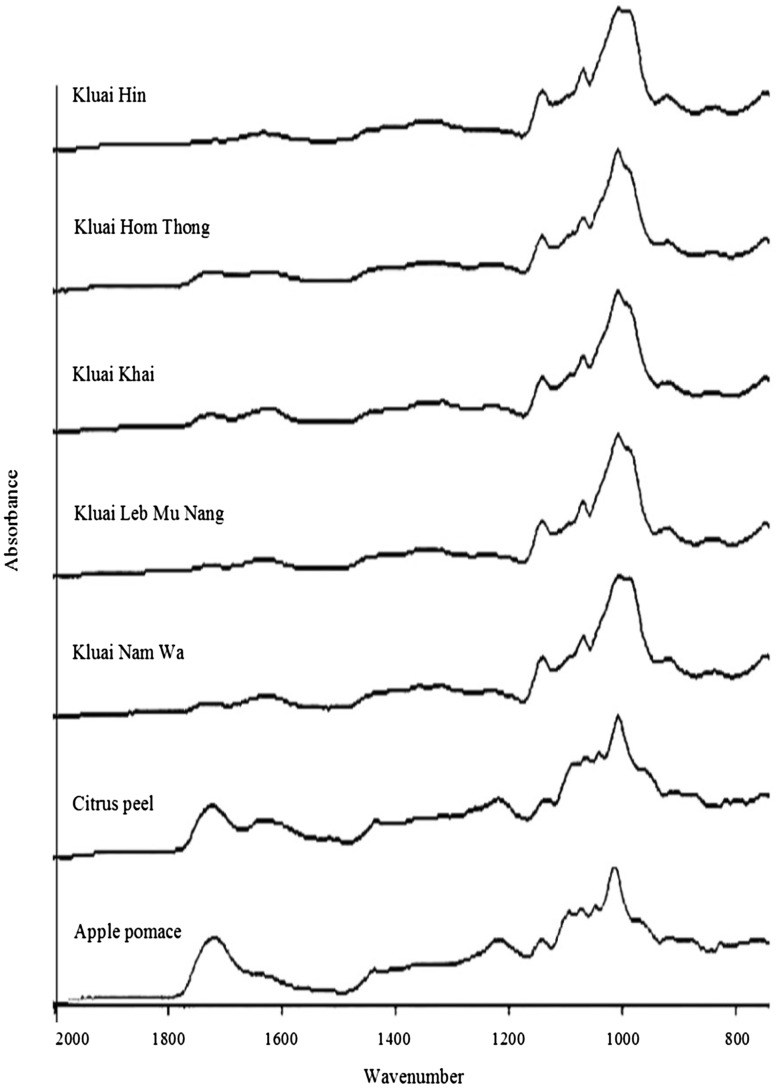
The chemical structure of pectins extracted from various banana peels, citrus peel and apple pomace was characterized by FTIR and their spectra are presented in Fig. 1. FT-IR spectra in the region between 800 and 1300 cm−1 are considered as the ‘finger print’ region for carbohydrates which allow an identification of major chemical groups specific for particular polysaccharides [31, 32]. It can be observed that the samples extracted from banana peels have the spectra in the ‘finger print’ region similar to those of citrus peel and apple pomace and also similar to the reported spectra of pectin in previous studies [3, 30–32] suggesting that the extracted polysaccharides obtained in this study were pectin.
Fig. 1.
Fourier transform infrared spectra of pectins extracted from banana peels, citrus peel and apple pomace
Absorption bands observed at 1730–1760 cm−1 and 1600–1630 cm−1 were attributed to stretching vibration of ester carbonyl groups (C=O) and carboxyl groups (COO−), respectively [9, 32]. A stronger absorption of ester carbonyl groups with a weaker absorption of the carboxyl stretching band indicated that citrus peel and apple pomace pectins were high methoxyl pectins. The intensity of these two absorption bands of extracts from banana peels was much weaker probably due to the lower content of anhydrouronic acid in these samples.
In conclusion, banana peels from all five different varieties gave pectin yield comparable to the conventional sources (citrus peel and apple pomace). The extracted banana peel pectins were classified as high methoxyl type similar to citrus peel and apple pomace pectins. Based on the value of AUA content, pectin from the peel of Kluai Nam Wa variety had the highest purity which met the criteria for use as food additive, signifying its potential use as an alternative source of commercial pectin production.
Acknowledgements
The authors are thankful to faculty of biotechnology, Rangsit University, Thailand, for providing financial support as well as the laboratory facilities to carry out this work.
References
- 1.Min B, Lim J, Koa S, Lee KG, Lee SH, Lee S. Environmentally friendly preparation of pectins from agricultural byproducts and their structural/rheological characterization. Bioresource Technol. 2011;102(4):3855–3860. doi: 10.1016/j.biortech.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 2.May C. Industrial pectins: Sources, production and applications. Carbohyd. Polym. 1990;12:79–99. doi: 10.1016/0144-8617(90)90105-2. [DOI] [Google Scholar]
- 3.Singthong J, Cui SW, Ningsanond S, Goff HD. Structural characterization degree of estertification and some gelling properties of Krueo Ma Noy pectin (Cissampelos pareira) pectin. Cabohyd. Polym. 2004;58:391–400. doi: 10.1016/j.carbpol.2004.07.018. [DOI] [Google Scholar]
- 4.Wang S, Chen F, Wu J, Wang Z, Liao X, Hu X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J. Food Eng. 2007;78:693–700. doi: 10.1016/j.jfoodeng.2005.11.008. [DOI] [Google Scholar]
- 5.Shkodina OG, Zeltser OA, Selivanov NY, Ignatov VV. Enzymic extraction of pectin preparations from pumpkin. Food Hydrocolloids. 1998;12:313–316. doi: 10.1016/S0268-005X(98)00020-4. [DOI] [Google Scholar]
- 6.Kalapathy U, Proctor A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy hull pectin. Food Chem. 2001;73:393–396. doi: 10.1016/S0308-8146(00)00307-1. [DOI] [Google Scholar]
- 7.Pagàn J, Ibarz A, Llorca M, Pagàn A, Barbosa-Cànovas GV. Extraction and characterization of pectin from stored peach pomace. Food Res. Int. 2001;34:605–612. doi: 10.1016/S0963-9969(01)00078-3. [DOI] [Google Scholar]
- 8.Levigne S, Ralet MC, Thibault JF. Characterisation of pectins extracted from fresh sugar beet under different conditions using an experimental design. Carbohyd. Polym. 2002;49:145–153. doi: 10.1016/S0144-8617(01)00314-9. [DOI] [Google Scholar]
- 9.Singthong J, Ningsanond S, Cui SW, Goff HD. Extraction and physicochemical characterization of Krueo Ma Noy pectin. Food Hydrocolloids. 2005;19:719–801. doi: 10.1016/j.foodhyd.2004.09.007. [DOI] [Google Scholar]
- 10.Koubala BB, Kansci G, Garnier C, Mbome LI, Durand S, Thibault JF, Ralet MC. Rheological and high gelling properties of mango (Mangifera indica) and Ambarella (Spondias cytherea) peel pectins. Int. J. Food Sci. Tech. 2009;44(9):1809–1817. doi: 10.1111/j.1365-2621.2009.02003.x. [DOI] [Google Scholar]
- 11.Li Ping Q, Guang Lei Z, Hui W, Lu J, Xiao feng L, Jun Juan L. Investigation of combined effects of independent variables on extraction of pectin from banana peel using response surface methodology. Carbohyd. Polym. 80:326–331 (2010)
- 12.Chan SY, Choo WS. Effect of extraction conditions on the yield and chemical properties of pectin from cocoa husks. Food Chem. 2013;141:3752–3758. doi: 10.1016/j.foodchem.2013.06.097. [DOI] [PubMed] [Google Scholar]
- 13.Kanmani P, Dhivya E, Aravind J, Kumaresan K. Extraction and analysis of pectin from citrus peels: augmenting the yield from Citrus limon using statistical experimental design. Iranica J. Energy. Environ. 2014;5:303–309. doi: 10.5829/idosi.ijee.2014.05.03.10. [DOI] [Google Scholar]
- 14.Begum R, Aziz GM, Uddin BM, Yusof AY. Characterization of jackfruit (Artocarpus heterophyllus) waste pectin as influenced by various extraction conditions. Agric. Sci. Procedia. 2014;2:244–251. doi: 10.1016/j.aaspro.2014.11.035. [DOI] [Google Scholar]
- 15.Castillo-Israel KAT, Diasanta SF, Lizardo MDB, Dizon RCMEI, Mejico MIF. Extraction and characterization of pectin from Saba banana [Musa ‘saba’ (Musa acuminata x Musa balbisiana)] peel wastes: A preliminary study. Int. Food Res. J. 22(1):202–207 (2015)
- 16.Naggarajaiah SB, Prakash J. Chemical composition and antioxidant potential of peels from three varieties of banana. Asian J. Food Ag-Ind. 2011;4:31–46. [Google Scholar]
- 17.Happi Emaga T, Robert C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresource Technol. 2008;99:4346–4354. doi: 10.1016/j.biortech.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Happi Emaga T, Ronkart S, Robert C, Wathelet B, Paquot M. Characterisation of pectins extracted from banana peels (Musa AAA) under different conditions using an experimental design. Food Chem. 2008;108:463–471. doi: 10.1016/j.foodchem.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 19.composition and gelling capability Rascón-Chu A, Martínez-López AL, Carvajal-Millán E, Ponce de León N, Renova J, Márquez-Escalante, Romo-Chacón A. Pectin from low quality ‘golden delicious’ apples. Food Chem. 2009;116:101–103. doi: 10.1016/j.foodchem.2009.02.016. [DOI] [Google Scholar]
- 20.AOAC. Official methods of analysis. (17th ed.). Association of official analytical chemists. Arlington, Virginia, USA (2000)
- 21.Ranganna S. Hand book of analysis and quality control for fruits and vegetable products. 2. New Delhi, India: McGraw Hill publishing Co., Ltd; 1995. [Google Scholar]
- 22.Mohamed S, Hasan Z. Extraction and characterization of pectin from various tropical agro wastes. ASEAN Food J. 1995;2:43–50. [Google Scholar]
- 23.Azad AKM, Ali MA, Akter MS, Rahman MJ, Ahmed M. Isolation and characterization of pectin extracted from lemon pomace during ripening. J. Food Nutr. Sci. 2014;2:30–35. [Google Scholar]
- 24.Mesbahi G, Jamaliana J, Farahnaky A. A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocolloids. 2005;19:731–738. doi: 10.1016/j.foodhyd.2004.08.002. [DOI] [Google Scholar]
- 25.Valmayor RV, Jamaluddin SH, Silayoi B, Kusumo S, Danh LD, Pascua OC, Espino RRC. Banana cultivar names and synonyms in Southeast Asia. In: International Network for the Improvement of Banana and Plantain-Asia and the Pacific Office, Laguna, Philippines (2000)
- 26.Ploetz RC, Kepler AK, Daniells J, Nelson SC. Banana and plantain: an overview with emphasis on Pacific Island cultivars. In: Elevitch CR, editor. Species Profiles for Pacific Island Agroforestry. Holualoa, Hawaii: Permanent Agriculture Resources (PAR); 2007. [Google Scholar]
- 27.Pereira A, Maraschin M. Banana (Musa spp.) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J. Ethnopharmacol. 2015;160:149–163. doi: 10.1016/j.jep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Muhmadzadeh J, Sadeghi-Mahoonak AR, Yaghbani M, Aalami M. Extraction of pectin from sunflower head residues of selected Iranian cultivars. World Appl. Sci. J. 2010;8:21–24. [Google Scholar]
- 29.Food Chemical Codex . IV monographs. Washington DC, USA: National Academy Press; 1996. p. 283. [Google Scholar]
- 30.Oliveira TIS, Rosa MF, Cavalcante FL, Pereira PHF, Moates GK, Wellner N. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016;198:113–118. doi: 10.1016/j.foodchem.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 31.Muhammad K, Zahari NIM, Gannasin SP, Adzahan NM, Bakar J. High methoxyl pectin from dragon fruit (Hylocereus polyrhizus) peel. Food Hydrocolloids. 2014;42:289–297. doi: 10.1016/j.foodhyd.2014.03.021. [DOI] [Google Scholar]
- 32.Kamnev AA, Colina M, Rodriguez J, Ptitchkina NM, Ignatov VV. Comparative spectroscopic characterization of different pectins and their sources. Food Hydrocolloids. 1998;12:263–271. doi: 10.1016/S0268-005X(98)00014-9. [DOI] [Google Scholar]