Abstract
A novel bacteriocin-producing strain, Lactobacillus plantarum JY22 isolated from golden carp intestine, was screened and identified by its physiobiochemical characteristics and 16S rRNA gene sequence analysis. This bacteriocin, named plantaricin JY22, was purified using ethyl acetate extraction and gel filtration. Its molecular weight was approximately 4.1 kDa by SDS-PAGE analysis. The partial amino acid sequence of plantaricin JY22 was DFGFDIPDEV. It was highly heat-stable and remained active at pH range from 2.5 to 5.5, but was sensitive to protease. Plantaricin JY22 had a bactericidal mode. Scanning electron microscope analysis indicated that plantaricin JY22 damaged the morphology of cells and spores for Bacillus cereus. Moreover, the plantaricin JY22 destroyed cell membrane integrity as confirmed by the leakage of electrolytes, the losses of Na+K+-ATP, AKP, nucleic acids (OD260nm) and proteins. SDS-PAGE of B. cereus proteins further demonstrated that plantaricin JY22 had a remarkable effect on bacterial proteins.
Keywords: Lactobacillus plantarum, Bacteriocin, Purification and characterization, Action mechanism, Bacillus cereus
Introduction
Bacillus cereus, an aerobic endospore-forming, is food-borne pathogen that may cause two types of gastrointestinal disorders: the emetic, caused by ingestion of a preformed toxin, and diarrheal syndrome, caused by several enterotoxins [1–4]. In 2014, statistical data from the Health Ministry showed that 19 cases of food-borne disease, accounting 5.4% of the total bacterial illness, were caused by B. cereus in China. Due to its widespread distribution in the environment, it frequently contaminates cereal products and farinaceous foods and causes food poisoning. Levels of B. cereus higher than 103 CFU/g have been found in both cooked or uncooked rice and cereal products all over the world [5]. Moreover, B. cereus spores are able to survive in pasteurization processes as well as cooking procedures because of their thermal stability [6]. Therefore, under specific conditions, some treatments might activate rather than inactivate dormant spores, thereby increasing the risk of food-borne illness.
To solve this problem, various strategies such as physical treatments, chemical preservatives and bio-preservatives have been applied to foods to inhibit the growth of B. cereus. Among these strategies, bio-preservative based on living microorganisms and/or their antimicrobial products has become increasingly popular. Bacteriocins produced by lactic acid bacteria (LAB) have attracted much attention because of their potential use as non-toxic and safe bio-preservative for improving the safety of foods [7]. Although fermented foods have been known as a mainly source for bacteriocin-producing LAB, isolates from the intestinal tract of animals and humans have become an increasingly important source for bio-preservation [8]. LAB are naturally present in fish intestinal, and the bio-preservation potential of some strains and/or their bacteriocins have been highlighted in the last years, such as Lb. casei AP8 and Lb. plantarum H5 from beluga and Persian sturgeon, Lactococcus lactis ssp. lactis USC-39, Enterococcus faecium USC-46 and E. mundtii USC-51 from turbot [9]. However, in comparison with fermented foods, only few bacteriocin-producing LAB strains have been obtained from fish products. Therefore, screening of bacteriocin-producing LAB from fish has great significance to increase source of bio-preservative.
Before a bacteriocin is considered for application in foods, its information on antimicrobial spectrum, biochemical and genetic characteristics, especially the action mechanism should be known [10]. Currently, antibacterial mechanism of nisin has been widely known, it typically dissipates proton motive force of the target cells by forming transmembrane channels permeable to various ions [11]. However, it was generally found that bacteriocins from different sources can produce different antibacterial mechanism in a strain-dependent manner [12]. Therefore, the action mechanism of bacteriocins is prerequisite to increasing its efficacy on inhibitory action of different biochemical conditions naturally occurring in foods [13].
The objectives of this study were to screen bacteriocin-producing LAB from freshwater fish intestine, then to purify and characterize of the bacteriocin, and to preliminarily elucidate the action mechanism of the bacteriocin using physiological and morphological indices, in order to develop a natural and high efficient bio-preservative to prevent contamination of Bacillus spp. and to extend the shelf-life of foods.
Materials and methods
Samples, bacterial strains and growth conditions
A total of six freshwater fish samples, including golden carp, silver carp, grass carp, bighead carp, mirror carp and Chinese carp, were obtained from Jinzhou aquatic products market (Jinzhou, Liaoning province, China). The intestinal mucosa and contents of freshwater fish were homogenized in sterile saline buffer, serially diluted and plated onto de Mann Rogosa Sharpe (MRS, Aoboxing, Beijing, China) agar plates containing calcium carbonate and incubated at 37 °C for 48 h. Then these strains were further purified on MRS agar plates by scribing method. Cell free supernatant (CFS) produced by LAB was obtained by centrifugation of overnight cultures (8000 g at 4 °C for 10 min) and filtered through 0.45 μm filters.
Target bacteria Bacillus cereus CMCC 63301 was purchased from China Microbiological Culture Collection Center. B. cereus was cultured in Luria–Bertani (LB, Aoboxing, Beijing, China) broth at 37 °C for 24 h. Spores of B. cereus was performed according to the method as described by Wang et al. [14].
Screening for LAB strains with bacteriocin activity
Bacteriocin-producing LAB was screened agar well diffusion method with slight modification [15]. The CFS was adjusted to pH 5.0 with NaOH (4 mol/l) and catalase was added, thus the effects of low pH and hydrogen peroxide was eliminated. The diameter of inhibitory zones was measured by vernier caliper.
Identification of bacteriocin-producing strain
The identification of bacteriocin-producing strain JY22 from was preliminarily identified based on morphologicaland biochemical characteristics as previous study [16]. The molecular level of strains identification was carried out by 16S rRNA gene sequence analysis using universal 27F and 1492R primers. The sequence was submitted to NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for blast search and phylogenetic analysis was carried out by MEGA 5.0.
Purification of bacteriocin
One liter of MRS broth was inoculated with 2% (v/v) overnight seed culture of strain JY22 and statically cultivated at 37 °C for 24 h. After removing the cells by centrifugation (8000 g at 4 °C for 10 min), the CFS was extracted with equal volume of ethyl acetate using a separating funnel. The extracted liquid was vacuum concentrated to eliminate the ethyl acetate and the crude extracts obtained were further purified.
A Sephadex G50 column (1.6 cm × 60 cm) was equilibrated with phosphate buffer (pH 6.0). After the equilibration, 2 ml of extracted sample filtered through a 0.45 µm filter membrane was eluted at a flow rate of 0.5 ml/min with phosphate buffer (pH 6.0). Each fraction (4 ml) was collected and the antibacterial activity against B. cereus was evaluated by the agar diffusion method. The active fraction with antibacterial activity was collected and concentrated by vacuum freeze-drying.
Molecular weight and amino acid sequence of bacteriocin
The molecular weight of bacteriocin was measured by SDS-PAGE with 5% stacking gel and 15% separating gel. Half of the gel was stained by Coomassie blue R250 for molecular mass determination, meanwhile another half for antimicrobial activity assay was equilibrated in water and then overlaid by LB agar containing B. cereus and incubated at 37 °C for 12 h.
The partial N-terminal amino acid sequence of the bacteriocin was determined based on Edman degradation using a PPSQ-33A automated protein sequencer (Shimadzu, Japan). The amino acid sequence that we obtained was compared with the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast) to determine homology with sequences that were reported previously.
Effects of temperature, pH and enzymes on antibacterial activity of bacteriocin
Purified bacteriocin was incubated in water bath at 40, 60, 80, and 100 °C for 30 min and in an autoclave at 121 °C for 20 min. Meanwhile two other samples were stored at 4 °C and − 20 °C for 30 days. The effect of pH was tested by adjusting the pH in a range from 2.5 to 7.5. The bacteriocin was co-incubated with the following enzymes at 37 °C for 4 h, pepsin, nutrase, papain and α-amylase. For all the experiments here, controls were maintained without any treatment.
Minimum inhibitory concentration (MIC) of bacteriocin
Firstly, purified bacteriocin was dissolved into LB broth to achieve a concentration of 24 mg/ml. Then a serial two fold diluted to obtain final concentrations ranging from 12 to 0.375 mg/ml. Equal volumes (100 µl) of diluted sample and B. cereus suspensions (106 CFU/ml) were mixed in 96 well plates and incubated at 37 °C for 24 h. Then OD600 was determined on a microplate reader (Imark, BIO-RAD, US). MIC was defined as the lowest concentration without any indicators had grown.
Mode of action of bacteriocin
The bacteriocin was added to logarithmic phase of B. cereus suspensions (6 h) to obtain final concentrations of 1 and 3 MIC, and then incubated at 37 °C. The control group was conducted without bacteriocin. Viable cells counts (CFU/ml) were counted by plating on LB agar plates and changes in cell growth were recorded at 600 nm using a UV–visible spectrophotometer (UV2550, Shimadzu, Japan).
Effect of bacteriocin on permeability and integrity of cell membrane
Electrical conductivity
The purified bacteriocin was added to B. cereus suspensions (106 CFU/ml) to obtain final concentrations of 1 and 3 MIC, and then incubated at 37 °C. Bacterial suspension without bacteriocin was used as control. Samples were removed from flask at 2 h intervals for 12 h, and then centrifuged at 8000 g for 10 min. Supernatant conductivity was determined using a conductivity meter (DDS-307, Precision & Scientific Instrument Co. Ltd., Shanghai, China).
Na+K+-ATP and AKP
The purified bacteriocin was added to B. cereus suspensions (106 CFU/ml) to obtain final concentrations of 0, 1 and 3 MIC. Bacterial cells were collected at 8000 g for 10 min at 4 °C after 12 h incubation. Intracellular Na+K+-ATP and AKP activity was determined using ATP kit and AKP kit (Nanjing Jiancheng Technology Co. Ltd., Nanjing, China) according to the manufacturer’s instructions.
UV-absorbing materials and leakage of proteins
The purified bacteriocin was added to B. cereus suspensions (106 CFU/ml) to obtain final concentrations of 0, 1 and 3 MIC. The cultures were incubated at 37 °C for 12 h, and then centrifuged at 8000 g for 10 min. The supernatant was immediately determined by using micro protein assay [17]. In addition, absorbance of the supernatants was recorded at 260 nm using a UV–visible spectrophotometer (UV2550, Shimadzu, Japan).
Effects of bacteriocin on the cell and spore morphology of B. cereus
The purified bacteriocin was added to the B. cereus suspensions and spores suspension to obtain final concentrations 0, 1 and 3 MIC. Bacterial cells were collected at 8000 g for 10 min at 4 °C after 12 h incubation. And then cells were rinsed with sterile water, and fixed with 2.5% glutaraldehyde for 24 h at 4 °C. Subsequently, cells were gradually dehydrated by a graded series of ethanol (50, 70, 80, 90 and 100%) for 20 min. The dehydrated cells were placed on a silicon wafer, covered with gold–palladium, and imaged using S-4800 SEM (Hitachi, Hitachi City, Japan).
SDS-PAGE analysis of bacterial proteins
The purified bacteriocin was added to the B. cereus suspension (106 CFU/ml) to obtain final concentrations 0, 1 and 3 MIC. Bacterial cells were collected at 8000 g for 10 min at 4 °C after 12 h incubation. The total soluble proteins were obtained by protein extraction kit (GenMed Scientifics, USA), and then protein concentration was determined by Coomassie blue staining. The loading buffer was added into samples with a protein concentration of 0.6 mg/ml. The protein was boiled for 5 min and cooled on ice. Subsequently, ten microlitres of protein was loaded on a 4% stacking gel and 15% resolving gel. After electrophoresis, the gel was stained with Coomassie brilliant blue R-250 and then decolorized to obtain the separated protein bands.
Statistical analysis
All experiments were performed as three independent replicates and expressed as mean ± standard deviation. Statistical analyses were performed using the Origin Pro 8.0 (Origin Lab Corporation, USA) and SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA).
Results and discussion
Screening and identification of bacteriocin-producing LAB
A total of 137 strains LAB were isolated from freshwater fish intestine samples. Among them, the CFS of 21 strains showed antibacterial activity against B. cereus. After eliminating the effects of low pH and hydrogen peroxide, the CFS of strain JY22 isolated from golden carp intestine, still exhibited strong antimicrobial activity towards B.cereus (data not shown). Hence, strain JY22 was selected for further research.
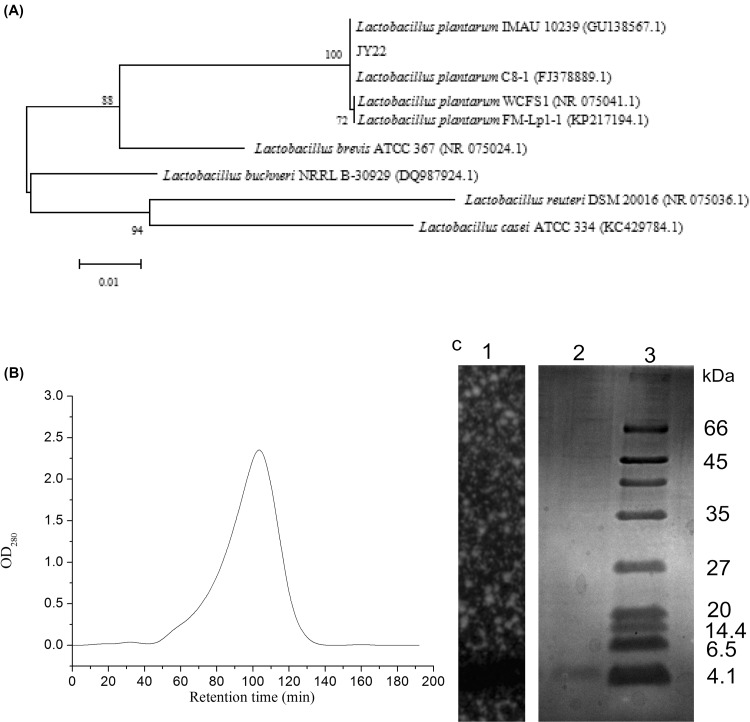
Strain JY22 was a Gram-positive, catalase negative and rod-shaped bacillus. To further characterize this strain, sugar fermentation assay tests indicated that it could metabolize glucose, cellobiose, aesculin, d-fructose, lactose, d-mannose, mannitol, melezitose, melibiose, raffinose, sucrose, xylose and maltose, but not L-arabinose, sorbitol, rhamnose. Regarding 16S rRNA gene sequence analysis, the nucleotide sequence of a 1225 bp fragment was amplified from strain JY22 genomic DNA. A neighbor-joining phylogenetic tree was draw from sequence alignment and comparison [Fig. 1(A)], which showed 100% similarity to Lb. plantarum. Therefore, on the basis of biochemical and morphological characteristics, strain JY22 was identified as Lb. plantarum and its GenBank access number was KX538909.
Fig. 1.
(A) The phylogenetic tree of Lb. plantarum JY22 based on 16S rRNA; (B) purification of bacteriocin by Sephadex G-50 chromatogram; (C) determination of molecular weight of the purified bacteriocin by SDS-PAGE, lane 1: gel overlaid with LB soft agar containing B. cereus; lane 2: plantaricin JY22; lane 3: protein Marker
LAB is found on seafood products, fresh fish, or in the intestinal contents of fish [18–21]. Some strains of Carnobacterium, Lactobacillus spp., Enterococcus spp. and Pediococcus spp. have received attention as bacteriocin-producing cultures. In comparison with fermented foods such as dairy and meat, few bacteriocin-producing strains and their bacteriocins recovered from aquatic environments, fish products and even less from non-fermented seafood, have been identified and characterized [20]. Strain Lb. plantarum JY22 isolated from golden carp intestine was capable of secreting high concentrations of bacteriocin. Therefore, it has potential for application in the aquatic products preservation field.
Purification and identification of plantaricin JY22
The bacteriocin produced by Lb. plantarum JY22 was purified from CFS by ethyl acetate and gel filtration chromatography. As shown in Fig. 1(B), the sample was separated into one fraction by Sephadex G50 gel filtration chromatography. The eluent of the peak was collected and then concentrated by vacuum freeze-drying. After testing, the fraction of the peak showed distinct antibacterial activity. So the eluent of the peak was collected and used for SDS-PAGE. As can be seen in Fig. 1(C), the molecular weight of the bacteriocin was approximately 4.1 kDa. The bacteriocin was named as plantaricin JY22.
In the past few years, several new bacteriocins from fish products have been successfully purified and characterized. The molecular weight of plantaricin JY22 was close to that reported for small bacteriocins (< 10 kDa) produced by Enterococcus, Lactobacillus and Pediococcus. Some bacteriocins produced by LAB form fish products are as follows: plantaricin H5 (3.0 kDa) from Lb. plantarum H5 [9], bacteriocin AP8 (5.0 kDa) from Lb. casei AP8 [9], bacteriocin TW34 (4.5 kDa) from Lac. lactis TW34 [22], bacteriocin bacALP57 (< 6.5 kDa) from P. pentosaceus ALP57 [20] and bacteriocin GM1 (4.5 kDa) from E. feacium GM1 [23].
The partial amino acids sequence of plantaricin JY22 was DFGFDIPDEV. The sequence of plantaricin JY22 showed no homology with other known bacteriocins using protein BLAST against the NCBI database. Thus, plantaricin JY22 may be identified as a novel bacteriocin. To obtain more information about amino acid sequences of plantaricin JY22, more mass spectrometry techniques need to be performed in the future.
Effect of temperature, pH and enzymes on bacteriocin
As shown in Table 1, plantarincin JY22 was heat-stable even after autoclaved at 121 °C for 20 min. Due to the high heat resistance of B. cereus spores and the increasing of heating processing procedures in foods. The heat-stable characteristics of plantaricin JY22 further showed its potential use as a food preservative for the control of B. cereus.
Table 1.
Effect of temperature, pH and enzymes on bacteriocin activity
| Treatment | Residual bacteriocin activity (%)a |
|---|---|
| pH | |
| 2.5 | 100 ± 0.26 a |
| 3.5 | 100 ± 0.17 a |
| 4.5 | 96.4 ± 0.43 d |
| 5.5 | 74.2 ± 0.31 f |
| 6.5 | 0.0 ± 0.0 g |
| 7.5 | 0.0 ± 0.0 g |
| Enzymes | |
| Nutrase | 0.0 ± 0.0 g |
| Pepsin | 0.0 ± 0.0 g |
| Papain | 0.0 ± 0.0 g |
| a-amylase | 100 ± 0.14 a |
| Temperature (°C) | |
| 40 (30 min) | 100 ± 0.31 a |
| 60 (30 min) | 99.5 ± 0.42 ab |
| 80 (30 min) | 98.1 ± 0.36 c |
| 100 (30 min) | 98.1 ± 0.28 c |
| 121 (20 min) | 95.0 ± 0.33 e |
| 4 (30 days) | 99.1 ± 0.31 b |
| − 20 (30 days) | 99.5 ± 0.27 ab |
aValues were mean of three independent experiments carried out in duplicate
Bacteriocins produced by LAB are generally highly stable under acidic conditions, but many of them are easily inactivated under neutral and alkaline conditions [24]. Its bacteriocin activity remained stable at pH values between 2.5 and 5.5. However, the antibacterial activity disappeared entirely at pH values between 6.5 and 7.5, which indicated that the bacteriocin had no antibacterial effect under alkaline conditions. This result was similar to bacteriocin-like ACU-1 [25] bacteriocin Si3 [26] and plantaricin LC74 [27].
The inhibitory activity of bacteriocin was completely disappeared after treatment with nutrase, papain and pepsin, whereas it was not affected by a-amylase. Protease sensitivity demonstrated that the bacteriocin was a peptide. Moreover, resistance of the antibacterial substances to treatment with a-amylase suggested that the bacteriocin were not glycosylated.
Mode of action of bacteriocin
The MIC of plantaricin JY22 against B. cereus cells was 6 mg/ml. Addition of 1 and 3 MIC of bacteriocin to 6 h-old culture B. cereus resulted in nearly entire inhibition of cell growth for at least 18 h [Fig. 2(A)]. Meanwhile, the number of viable cells decreased 1.74 log CFU/ml and 2.04 log CFU/ml after 18 h of treatment with 1 and 3 MIC bacteriocin, respectively [Fig. 2(A)]. This result indicated that the mode of action of plantaricin JY22 against B. cereus was considered as bactericidal. Similar results were reported for bacteriocin GM3 [28], plantaricin ZJ008 [29] and sakacin C2 [30].
Fig. 2.
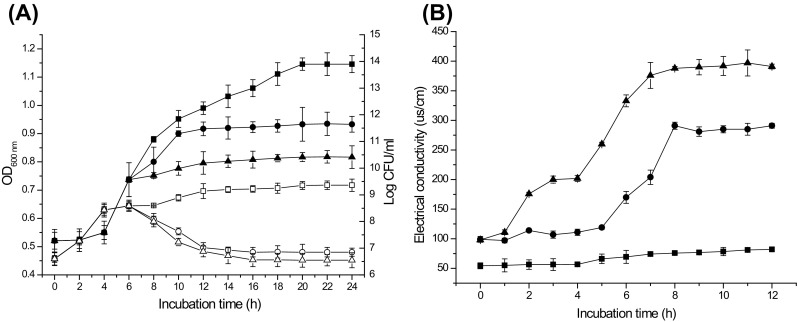
Effect of plantaricin JY22 on cell growth of B. cereus (A). OD600 was observed with 1 MIC (filled circle), 3 MIC (filled triangle) and without (filled square) plantaricin JY22, viable cell counts (log CFU/ml) were observed with 1 MIC (circle), 3 MIC (triangle) and without (square) plantaricin JY22. Effect of plantaricin JY22 on permeability of cell membrane of B. cereus (B). Electrical conductivity was observed with 1 MIC (filled circle), 3 MIC (filled triangle) and without (filled square) plantaricin JY22
Effect of bacteriocin on cell membrane permeability
As shown in Fig. 2(B), the electric conductivity of B. cereus treated and untreated was basically stable at 50 and 100 μs/cm within 5 h, respectively. This reason might be that plantaricin JY22 did not affect B. cereus in the initial period. After 12 h incubation, the electric conductivity of B. cereus was essentially constant in control. However, when B. cereus treated with plantaricin JY22 at 1 and 3 MIC, the conductivity values of cells significantly increased throughout 12 h. The increase in electric conductivity of cells with increasing concentrations of plantaricin JY22 suggested that the cell membranes permeability was disrupted, which caused intracellular components leakage.
The bacterial cell membrane provides a permeability barrier to the passage of small ions such as K+, Na+ which are necessary electrolytes, facilitate cell membrane functions and maintain proper enzyme activity [31]. Increase in the release of electrolytes will indicate a disruption of this permeability barrier. Our results showed that the level of electric conductivity increased rapidly with the increasing concentration of plantaricin JY22, which meant that the cell membrane permeability was disrupted, This result was similar to Zhang et al. [32] who reported that bacteriocin PlnEF caused the increased in electric conductivity of Lb. plantarum pl2.
Effect of bacteriocin on cell membrane integrity
The integrity of cell membrane was determined by the measurement of the leakage of intracellular ingredient including Na+K+-ATP, AKP, nucleic acids (OD260nm) and proteins in B. cereus. Table 2 showed the effect of different concentrations of plantaricin JY22 on cell membrane integrity during 12 h. Compared to the control, the level of intracellular Na+K+-ATP and AKP in B. cereus treated with 1 MIC plantaricin JY22 decreased 78.46 and 35.93%, respectively, while they decreased 98.69 and 79.70% respectively when treatment at 3 MIC. The absorbance of nucleic acids (OD260nm) and concentration of proteins in B. cereus suspension treated with 1 MIC bacteriocin increased 81.39% and 96.12%, respectively, when treatment at 3 MIC, they increased by 93.39 and 97.73% respectively. This result indicated that the leakage of intracellular ingredient increased significantly with the increased concentration of plantaricin JY22. It was confirmed that plantaricin JY22 had detrimental effect on damaging cell membrane integrity, resulting in the leakage of intracellular ingredient from the treated cells.
Table 2.
Effects of plantaricin JY22 produced by Lb. plantarum JY22 on the change of intracellular ingredients in Bacillus cereus
| Concentrations | Intracellular ingredients | |||
|---|---|---|---|---|
| Na+K+-ATP (U/mgprot) | AKP(U/L) | Nucleic acids (OD260nm) | Protein(ug/ml) | |
| Control | 7.66 ± 0.21 a | 56.83 ± 0.53 a | 0.08 ± 0.02 c | 8.16 ± 2.16 c |
| 1 × MIC | 1.65 ± 0.17 b | 36.41 ± 0.31 b | 0.43 ± 0.04 b | 210.15 ± 53 b |
| 3 × MIC | 0.10 ± 0.01 c | 11.57 ± 0.17 a | 1.21 ± 0.10 a | 359.22 ± 39 a |
Values represent means of three independent replicates ± SD. Different letters within a column indicate statistically significant differences between the means (p < 0.05)
The Na+K+-ATP, AKP, nucleic acids (OD260nm) and proteins which reside throughout the interior of the cell, in the cytoplasm, are the key intracellular components. Measurement of specific intracellular components leakage markers is an indicator of membrane integrity to specific antimicrobial agent in relationship to untreated cells [33]. Our experimental results showed that the plantaricin JY22 significantly caused massive losses of Na+K+-ATP, AKP, nucleic acids (OD260nm) and proteins from the treated B. cereus cells. Similar results have been observed with the other baceriocins such as bifidocin A against E. coli [7] and pentocin 31-1 against Listeria monocytogenes [13].
Effect of bacteriocin on the cell and spore morphology of B. cereus
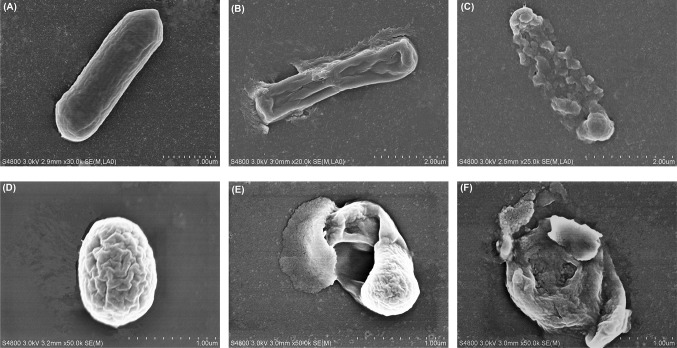
As observed in Fig. 3(A), untreated B. cereus cell exhibited distinctive features characterized by typical rod-shaped, pump and smooth surface. B. cereus cell treated with 1 MIC bacteriocin became deformed, shriveled, wrinkled and cracked with intracellular component leakage [Fig. 3(B)]. And the damages were more evident with the increase of concentration of plantaricin JY22. When treated with plantaricin JY22 at 3 MIC, the integrity of cell was damaged and cell gradually dissolved [Fig. 3(C)]. This supported the results of the permeability and integrity of cell membrane assays, and indicated that the plantaricin JY22 had severe effects on the cell membrane. The present findings were in agreement with the finding of Liu et al. [7] who reported that untreated E. coli displayed striated cell wall and smooth cell membrane, whereas, those treated with bifidocin A exhibited wrinkled surfaces by SEM analysis.
Fig. 3.
Scanning electron micrograph of B. cereus cells without plantaricin JY22 (A) and treated with 1 MIC (B) and 3 MIC (C), spores of B. cereus without plantaricin JY22 (D) and treated with 1 MIC (E) and 3 MIC (F)
Moreover, the morphology change of B. cereus spores was also evaluated by SEM analysis. For control, B. cereus spores displayed intact circular structure. However, B. cereus spores treated with plantaricin JY22 at 1 MIC were obviously disrupted, with exosporium peeled out and out layer depressed [Fig. 3(E)]. With the increased of concentration of plantaricin JY22, the damaging effect to spore was stronger. When treated with plantaricin JY22 at 3 MIC, B. cereus spores were hollow with leaking spore contents [Fig. 3(F)]. This indicated that bacteriocin had also severe effects on the integrity of spore.
SDS-PAGE profiles
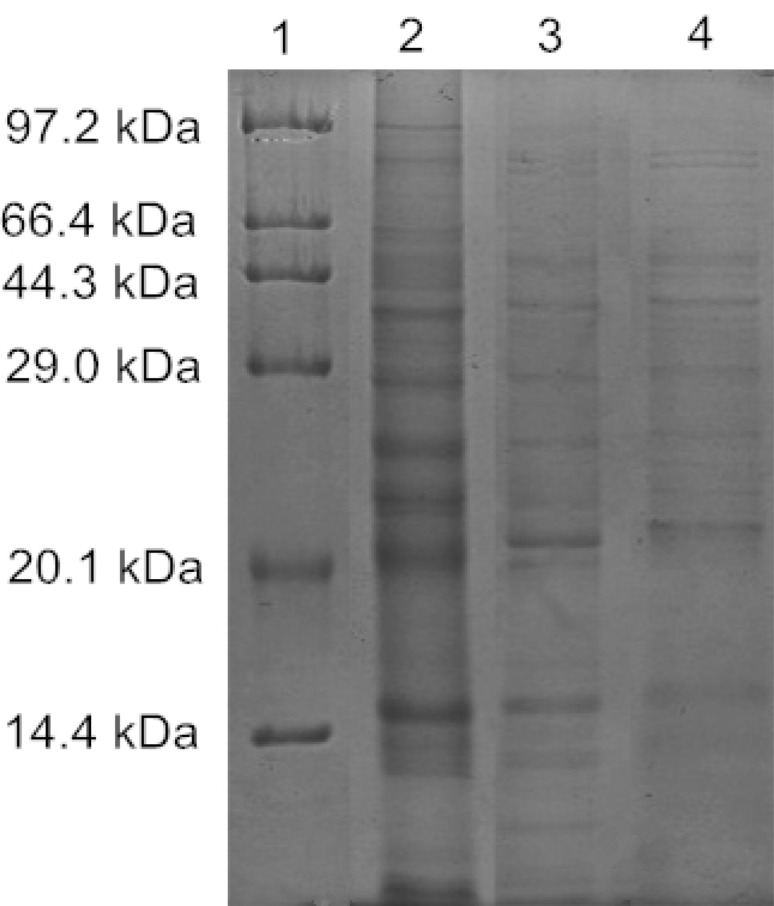
Soluble protein electrophoresis bands of B. cereus treated with plantaricin JY22 were significantly different from its control group in Fig. 4. The bands of cells all-molecular-weight proteins appeared strong and clear in control group but obviously shallow in the treated groups. The bands of proteins reduced more obviously with the increasing concentration of plantaricin JY22 and even disappeared. These results suggested that plantaricin JY22 decreased the content of cellular soluble proteins by damaging bacterial cell membrane integrity, with releasing intracellular proteins subsequently. This result confirmed that plantaricin JY22 disrupt the integrity of cell membrane, resulting in the leakage of proteins. Similar results have been reported with glycinin, some protein bands of L. monocytogenes cells disappeaed after treatment with glycinin [34].
Fig. 4.
SDS-PAGE profiles of B. cereus total soluble proteins treated with plantaricin JY22. Lane 1: Marker bands; lane 2: untreated B. cereus; 3: B. cereus treated with 1 MIC plantaricin JY22; 4: B. cereus treated with 3 MIC plantaricin JY22
Acknowledgements
This study was funded by the Research Project from Science and Technology Department of Liaoning Province of China (No. 2015103020) and the Research and Demonstration of Quality Traceability Technology and Supervision System of Famous Aquatic Products (No. 2015BAD17B05).
References
- 1.Bauer R, Chikindas ML, Dicks LM. Purification, partial amino acid sequence and mode of action of pediocin PD-1, a bacteriocin produced by Pediococcus damnosus NCFB 1832. Int. J. Food Microbiol. 2005;101:17–27. doi: 10.1016/j.ijfoodmicro.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Chang H-J, Lee J-H, Han B-R, Kwak T-K, Kim J. Prevalence of the levels of Bacillus cereus in fried rice dishes and its exposure assessment from Chinese-style restaurants. Food Sci. Biotechnol. 2011;20:1351–1359. doi: 10.1007/s10068-011-0186-3. [DOI] [Google Scholar]
- 3.Soria MC, Audisio MC. Inhibition of Bacillus cereus Strains by antimicrobial metabolites from Lactobacillus johnsonii CRL1647 and Enterococcus faecium SM21. Probiotics Antimicrob. 2014;6:208–216. doi: 10.1007/s12602-014-9169-z. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Tao W-Y, Liu Y-J, Zhu F. Inhibition of Bacillus cereus by lactic acid bacteria starter cultures in rice fermentation. Food Control. 2008;19:159–161. doi: 10.1016/j.foodcont.2007.03.002. [DOI] [Google Scholar]
- 5.Grande MJ, Lucas R, Abriouel H, Valdivia E, Omar NB, Maqueda M, Martinez-Bueno M, Martinez-Canamero M, Galvez A. Inhibition of toxicogenic Bacillus cereus in rice-based foods by enterocin AS-48. Int. J. Food Microbiol. 2006;106:185–194. doi: 10.1016/j.ijfoodmicro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Røssland E. Inhibition of Bacillus cereus by strains of Lactobacillus and Lactococcus in milk. Int. J. Food Microbiol. 2003;89:205–212. doi: 10.1016/S0168-1605(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Song Z, Yang X, Gao Y, Wang C, Sun B. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control. 2016;62:309–316. doi: 10.1016/j.foodcont.2015.10.033. [DOI] [Google Scholar]
- 8.Ghanbari M, Jami M, Domig KJ, Kneifel W. Seafood biopreservation by lactic acid bacteria—A review. LWT Food Sci. Technol. 2013;54:315–324. doi: 10.1016/j.lwt.2013.05.039. [DOI] [Google Scholar]
- 9.Ghanbari M, Jami M, Kneifel W, Domig KJ. Antimicrobial activity and partial characterization of bacteriocins produced by Lactobacilli isolated from Sturgeon fish. Food Control. 2013;32:379–385. doi: 10.1016/j.foodcont.2012.12.024. [DOI] [Google Scholar]
- 10.Gao Y, Li D, Sheng Y, Liu X. Mode of action of sakacin C2 against Escherichia coli. Food Control. 2011;22:657–661. doi: 10.1016/j.foodcont.2010.07.010. [DOI] [Google Scholar]
- 11.Todorov SD, Holzapfel W, Nero LA. Characterization of a novel bacteriocin produced by Lactobacillus plantarum ST8SH and some aspects of its mode of action. Ann Microbiolo. 2015;66:949–962. doi: 10.1007/s13213-015-1180-4. [DOI] [Google Scholar]
- 12.Yi L, Dang J, Zhang L, Wu Y, Liu B, Lü X. Purification, characterization and bactericidal mechanism of a broad spectrum bacteriocin with antimicrobial activity against multidrug-resistant strains produced by Lactobacillus coryniformis XN8. Food Control. 2016;67:53–62. doi: 10.1016/j.foodcont.2016.02.008. [DOI] [Google Scholar]
- 13.Zhou K, Zhou W, Li P, Liu G, Zhang J, Dai Y. Mode of action of pentocin 31-1: An antilisteria bacteriocin produced by Lactobacillus pentosus from Chinese traditional ham. Food Control. 2008;19:817–822. doi: 10.1016/j.foodcont.2007.08.008. [DOI] [Google Scholar]
- 14.Wang G, Manns DC, Guron GK, Churey JJ, Worobo RW. Large-scale purification, characterization, and spore outgrowth inhibitory effect of thurincin H, a bacteriocin produced by Bacillus thuringiensis SF361. Probiotics. Antimicro. 2014;6:105–113. doi: 10.1007/s12602-014-9159-1. [DOI] [PubMed] [Google Scholar]
- 15.Chahad OB, El Bour M, Calo-Mata P, Boudabous A, Barros-Velazquez J. Discovery of novel biopreservation agents with inhibitory effects on growth of food-borne pathogens and their application to seafood products. Res. Microbiol. 2012;163:44–54. doi: 10.1016/j.resmic.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Hu Y, Liu X, Shan C, Xia X, Wang Y, Dong M, Zhou J. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: Purification, identification and antimicrobial characteristics. Food Control. 2017;77:290–297. doi: 10.1016/j.foodcont.2017.02.007. [DOI] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Campos CA, Rodríguez Ó, Calo-Mata P, Prado M, Barros-Velázquez J. Preliminary characterization of bacteriocins from Lactococcus lactis, Enterococcus faecium and Enterococcus mundtii strains isolated from turbot (Psetta maxima) Food Res. Int. 2006;39:356–364. doi: 10.1016/j.foodres.2005.08.008. [DOI] [Google Scholar]
- 19.Gómez-Sala B, Herranz C, Díaz-Freitas B, Hernández PE, Sala A, Cintas LM. Strategies to increase the hygienic and economic value of fresh fish: Biopreservation using lactic acid bacteria of marine origin. Int. J. Food Microbiol. 2016;223:41–49. doi: 10.1016/j.ijfoodmicro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Pinto A, Fernandes M, Pinto C, Albano H, Castilho F, Teixeira P, Gibbs P. Characterization of anti-Listeria bacteriocins isolated from shellfish: Potential antimicrobials to control non-fermented seafood. Int. J. Food Microbiol. 2009;129:50–58. doi: 10.1016/j.ijfoodmicro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki K, Suzuki M, Kawai Y, Inoue N, Montville TJ. Inhibition of Listeria monocytogenes in cold-smoked salmon by Carnobacterium piscicola CS526 isolated from frozen surimi. J. Food Protec. 2003;66:1420–1425. doi: 10.4315/0362-028X-66.8.1420. [DOI] [PubMed] [Google Scholar]
- 22.Sequeiros C, Garces ME, Vallejo M, Marguet ER, Olivera NL. Potential aquaculture probiont Lactococcus lactis TW34 produces nisin Z and inhibits the fish pathogen Lactococcus garvieae. Arch. Microbiol. 2015;197:449–458. doi: 10.1007/s00203-014-1076-x. [DOI] [PubMed] [Google Scholar]
- 23.Sarra M, Taoufik G, Le CP, Benjamin B, Yannick F, Khaled H. Isolation and characterization of Enterococci bacteriocinic strains from Tunisian fish viscera. Food. Nutr. Sci. 2013;4:701–708. [Google Scholar]
- 24.Iranmanesh M, Ezzatpanah H, Mojgani N. Antibacterial activity and cholesterol assimilation of lactic acid bacteria isolated from traditional Iranian dairy products. LWT Food Sci. Technol. 2014;58:355–359. doi: 10.1016/j.lwt.2013.10.005. [DOI] [Google Scholar]
- 25.Castro MP, Palavecino NZ, Herman C, Garro OA, Campos CA. Lactic acid bacteria isolated from artisanal dry sausages: characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci. 2011;87:321–329. doi: 10.1016/j.meatsci.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson J, Schnurer J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ Microbiol. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atrih A, Rekhif N, Moir AJG, Lebrihi A, Lefebvre G. Mode of action, purification and amino acid sequence of plantaricin C19, an anti- Listeria bacteriocin produced by Lactobacillus plantarum C19. Int. J. Food Microbiol. 2001;68:93–104. doi: 10.1016/S0168-1605(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 28.Devi Avaiyarasi N, David Ravindran A, Venkatesh P, Arul V. In vitro selection, characterization and cytotoxic effect of bacteriocin of Lactobacillus sakei GM3 isolated from goat milk. Food Control. 2016;69:124–133. doi: 10.1016/j.foodcont.2016.04.036. [DOI] [Google Scholar]
- 29.Zhu X, Zhao Y, Sun Y, Gu Q. Purification and characterisation of plantaricin ZJ008, a novel bacteriocin against Staphylococcus spp. from Lactobacillus plantarum ZJ008. Food Chem. 2014;165:216–223. doi: 10.1016/j.foodchem.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Jia S, Gao Q, Tan Z. A novel bacteriocin with a broad inhibitory spectrum produced by Lactobacillus sake C2, isolated from traditional Chinese fermented cabbage. Food Control. 2010;21:76–81. doi: 10.1016/j.foodcont.2009.04.003. [DOI] [Google Scholar]
- 31.Diao W-R, Hu Q-P, Zhang H, Xu J-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 32.Zhang X, Wang Y, Liu L, Wei Y, Shang N, Zhang X, Li P. Two-peptide bacteriocin PlnEF causes cell membrane damage to Lactobacillus plantarum. BBA-Biomembranes. 2016;1858:274–280. doi: 10.1016/j.bbamem.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Bajpai VK, Sharma A, Baek K-H. Antibacterial mode of action of Cudrania tricuspidata fruit essential oil, affecting membrane permeability and surface characteristics of food-borne pathogens. Food Control. 2013;32:582–590. doi: 10.1016/j.foodcont.2013.01.032. [DOI] [Google Scholar]
- 34.Sitohy MZ, Mahgoub SA, Osman AO. In vitro and in situ antimicrobial action and mechanism of glycinin and its basic subunit. Int. J. Food Microbiol. 2012;154:19–29. doi: 10.1016/j.ijfoodmicro.2011.12.004. [DOI] [PubMed] [Google Scholar]