Abstract
Lactobacillus gasseri BNR17 is a strain isolated from human breast milk. The objective of this randomized, double-blind, placebo-controlled, and preliminary dose-finding trial was to find the effective dose and evaluate the effect of Lb. gasseri BNR17 on irritable bowel syndrome (IBS) symptoms. A total of 55 volunteers aged over 20 years with body mass index over 23 kg/m2 were randomized to intake a placebo, low-dose (BNR-L, 2 × 108 CFU/day), intermediate-dose (BNR-M, 2 × 109 CFU/day), or high-dose BNR (BNR-H, 2 × 5 × 109 CFU/day) for four weeks. Questionnaire for IBS symptoms scores and Lb. gasseri BNR17 in feces were assessed at the beginning and end of the trial. Among IBS symptoms scores, abdominal pain score was significantly reduced in BNR-H group. Lb. gasseri BNR17 was detected in all intake groups except placebo. In the preliminary study, Lb. gasseri BNR17 was confirmed to have probiotic properties.
Keywords: Lactobacillus gasseri BNR17, Irritable bowel syndrome, Probiotics
Introduction
Probiotics are live and nonpathogenic microorganisms with beneficial effects on host’s health [1]. Health benefits of probiotics are mostly focused on gut health or treatment efficacy. Among functional gastrointestinal disorders, irritable bowel syndrome (IBS) is a common digestive disorder characterized by abdominal pain and discomfort including diarrhea, constipation, or both without consistently demonstrable structural or biochemical causes [2]. In westernized countries including Korea, IBS is one main reason that lowers quality of life. In a nationwide analysis, 6% of Korean population seeks medication for IBS. Such high prevalence leads to increase medical cost [3]. Due to obscure etiology of IBS, main treatment strategies involve the use of anti-spasmodics or anti-depressants at low dose to relieve abdominal pain [4, 5]. Some probiotics have been investigated to be effective in the management of IBS. However, their benefits are likely to be strain-specific [6]. Moreover, few randomized controlled trials have tested the efficacy of probiotics for IBS with controversial results [6, 7].
Lactobacillus gasseri BNR17 is a strain isolated from human breast milk. Its probiotic properties have been evaluated [8]. Because this strain was isolated in the attempt to find anti-diabetic probiotic strains, previous studies were focused on lowering blood glucose and reducing body fat levels [9–11]. However, as a probiotic strain, its effect in improving IBS symptoms should be confirmed and its optimal dose should be identified. Therefore, this preliminary study was performed to find the effective dose and evaluate the effect of Lb. gasseri BNR17 on IBS symptoms in subjects with IBS.
Materials and methods
Test materials
Lb. gasseri BNR17 was kindly provided by Bioneer Ltd (Daejeon, Korea). Placebo contained only dextrin. Final products had identical shape, texture, and appearance. All test products were labeled and randomized by the study coordinator who was not a participant of this study.
Participants
A total of 78 subjects aged between 20 and 54 years were recruited for this study through advertisement posted on several websites. To be eligible for this study, subjects were required to have a body mass index (BMI) over 23 kg/m2 and subjective symptoms of IBS. Those who were taking medication and/or dietary supplements within one month prior to screening visit or having inflammatory disease or any other diseases that could affect results of this study were excluded. All subjects were generally healthy based on their medical history and laboratory tests.
Study design
This study was a double-blind, randomized, placebo-controlled, parallel study. They were instructed to avoid foods, medicines, and other dietary supplements that might affect the efficacy of the test material. After 7 days of run-in period, subjects were randomly assigned into four groups (placebo; BNR-L, 2 × 108 CFU/day; BNR-M, 2 × 109 CFU/day; BNR-H, 2 × 5 × 109 CFU/day). They were asked to consume test materials or placebo for four weeks. Venous blood was collected to check safety markers. A questionnaire was used to investigate symptom severity of IBS. Fecal samples were collected at baseline and the final visit. They were kept frozen at − 54 °C for PCR analysis. Study protocol was approved by Institutional Review Board (IRB) of Ewha Womans University (No. 2011-12-11). It was carried out in accordance with the Helsinki declaration and registered at WHO International Clinical Trial Registry Platform (www.who.int/ictrp) with the following identification number: KCT0000368.
Questionnaires
At baseline and the final visit, subjects were asked to answer questions about the frequency of their abdominal pain, bloating, and feeling of incomplete evacuation. The severity of symptoms was assessed on a five-point Likert visual analogue scale (1 = not at all; 5 = extremely severe that affected daily life). Symptom scale was adapted from the previously validated 5-point scale [12–14].
Fecal preparation and extraction of DNA
In order to separate fecal Lb. species, 1 g of thawed fecal sample was diluted 100 times with PBS. Then 100 µL of diluted sample was seeded onto LBS agar plate (Difco; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and incubated at 37 °C with 5% CO2 for 24–48 h. After incubation, cells were counted to evaluate total Lb. species and colonies were incubated in MRS broth (Difco) at 37 °C with 5% CO2 environment for 24 h. Incubated MRS broth was centrifuged (4 °C, 3000 rpm for 15 min) and the cell pellet was suspended in 6.7% sucrose solution pre-warmed at 37 °C for 15 min. DNA was extracted from cell pellet using Accuprep Genomic DNA Extraction Kit (Bioneer Ltd).
Polymerase chain reaction (PCR)
PCR was performed using Accupower Hot Start PCR premix (Bioneer Ltd) and Lb. gasseri specific primers [15] Lgas_F (5′-AGCGACCGAGAAGAGAGAGA-3′) and Lgas_R (5′-TGCTATCGCTTCAAGTGCTT-3′) using MY GenieTM 96 Gradient Thermal Block (Bioneer Ltd). PCR amplification was performed under the following conditions: denaturation at 95 °C for 10 min, 35 cycles of denaturation at 95 °C for 30 s, annealing at 65 °C for 2 min, and extension at 74 °C for 2 min, followed by a final extension step at 74 °C for 5 min.
Gel electrophoresis and gene sequencing
To confirm Lb. gasseri BNR17 species, PCR products were subjected to agarose gel electrophoresis. Briefly, 2 g of agarose, 100 mL TBE buffer, and 5 µL EtBr were used to pour the gel. After 30 min, 2 µL DNA size marker (100 bp DNA Ladder) and PCR products mixed with 2 µL 6× Agarose Gel Loading Buffer were loaded to the gel. Gel electrophoresis was performed for 30–40 min. Samples that showed bands at the same position of Lb. gasseri BNR 17 (positive control) were purified using Accuprep PCR Purification Kit (Bioneer Corporation, Daejeon, Korea) and subjected to DNA sequencing to confirm homology (≥ 98%) with Lb. gasseri BNR17.
Statistical analysis
A Shapiro–Wilk W test was used to assess the normality of each variable. Differences among groups were tested with Fisher’s exact test for categorical variables and ANOVA for continuous variables. Difference within group was tested with paired t test. P < 0.05 was considered statistically significant. SAS program package version 9.3 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results and discussion
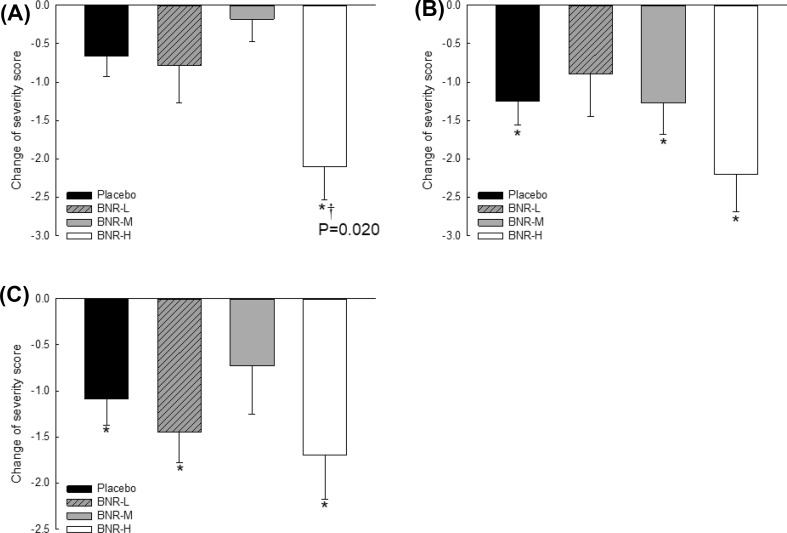
A total of 78 subjects were screened for eligibility and 55 subjects were enrolled. Thirteen subjects were lost during the study period. Finally, 42 subjects were analyzed. There were no significant differences in baseline characteristics among these participants (Table 1). Changes in IBS symptoms scores are shown in Fig. 1. Because IBS symptoms scores are subjective measuring markers, changes in placebo group have been reported in numerous papers [16, 17]. In the present study, severity scores for bloating and feeling incomplete evacuation were significantly changed in BNR-L and BNR-M groups as well as the placebo group compared to baseline values. In BNR-H group, all severity scores were significantly changed (P < 0.05). For abdominal pain scores, changes in values from baseline to 4-week in BNR-H group were significantly (P = 0.020) lower when compared to those in the placebo group. There was no placebo effect in abdominal pain scores. Probiotic properties should be determined at strain level [18]. Although numerous papers have published beneficial effects of Lb. gasseri on health [19–21], those strains are different from our stain isolated from breast milk. Therefore, probiotic properties and optimal intake level of this strain should be confirmed using human subjects. In the present study, Lb. gasseri BNR17 was confirmed to have probiotic properties by reducing IBS symptom severity scores, especially it had effect in relieving abdominal pain. Its optimal dose was found to be 2 × 5 × 109 CFU/day.
Table 1.
Baseline characteristics of subjects
| Placebo (n = 12) | BNR-L (n = 9) | BNR-M (n = 11) | BNR-H (n = 10) | P valuea | |
|---|---|---|---|---|---|
| Age (years) | 26.7 ± 2.3 | 35.6 ± 3.3 | 24.9 ± 1.7 | 28.6 ± 2.4 | 0.103 |
| Female/male (n) | 6/6 | 6/3 | 2/9 | 3/7 | 0.132 |
| Weight (kg) | 75.43 ± 2.73 | 71.11 ± 2.18 | 75.35 ± 3.51 | 75.7 ± 3.32 | 0.679 |
| Body mass index (kg/m2) | 25.5 ± 0.6 | 25.0 ± 0.7 | 24.3 ± 0.8 | 24.9 ± 0.5 | 0.059 |
| Systolic blood pressure (mmHg) | 129.0 ± 4.5 | 120.7 ± 3.3 | 125.2 ± 3.3 | 125.2 ± 4.7 | 0.674 |
| Diastolic blood pressure (mmHg) | 84.2 ± 2.0 | 81.1 ± 2.3 | 84.5 ± 2.7 | 82.0 ± 2.3 | 0.659 |
| Fasting blood glucose (mg/dL) | 101.29 ± 2.36 | 100.92 ± 4.42 | 101.40 ± 2.01 | 97.15 ± 2.64 | 0.706 |
| Total cholesterol (mg/dL) | 183.64 ± 6.63 | 204.46 ± 8.61 | 182.00 ± 8.23 | 173.38 ± 9.38 | 0.080 |
| HDL- Cholesterol (mg/dL) | 55.71 ± 2.40 | 52.39 ± 2.58 | 58.13 ± 4.30 | 50.31 ± 3.05 | 0.503 |
| LDL- Cholesterol (mg/dL) | 105.21 ± 6.32 | 124.00 ± 7.51 | 103.87 ± 7.06 | 98.15 ± 6.64 | 0.070 |
| Triacylglyceride (mg/dL) | 101.79 ± 14.07 | 142.23 ± 27.54 | 103.00 ± 11.74 | 140.15 ± 23.50 | 0.284 |
Mean ± SE (all variables)
aANOVA test was used for continuous variables. Fisher’s exact test was used for categorical variables
Fig. 1.
Changes in severity score for symptoms. (A) Abdominal pain; (B) Bloating; (C) Feeling of incomplete evacuation. Data are presented as mean ± standard error (SE). *P < 0.05 versus baseline values by paired t test. † P < 0.05 versus placebo group by ANOVA with Dunnet’s multiple comparison test as post hoc analysis
In order to confirm gut colonization of Lb. gasseri BNR17, fecal Lb. species were quantified through in vitro cultivation and Lb. gasseri BNR17 was detected by PCR followed by gel electrophoresis and gene sequencing (Table 2). When changes were compared among treated groups, BNR-H group showed the highest increase in fecal Lb. species (P = 0.015). After intervention for four weeks, Lb. gasseri BNR17 was found in all subjects who consumed Lb. gasseri BNR17. Their difference was statistically significant (P < 0.0001). For probiotics, colonization through digestive tract is the foremost important characteristic [22]. Lb. acidophilus NCFM has been tested for colonization. It is found in feces of 65% of participants after supplementation, suggesting satisfactory compliance [23]. In BNR-L, BNR-M, and BMR-H subjects who participated in this trial, 62, 90, and 75% showed positive responses to Lb. gasseri BNR17, respectively. Adhesion capacity of our strain was found to be superior to Lb. acidophilus NCFM. This shows that our strain has high potential to be developed as a probiotic strain.
Table 2.
Number of fecal Lactobacillus species and detection of Lactobacillus gasseri BNR17 in fecal samples by PCR
| Placebo (n = 12) | BNR-L (n = 9) | BNR-M (n = 11) | BNR-H (n = 10) | P value3 | ||
|---|---|---|---|---|---|---|
| Lactobacillus species | ||||||
| Cell count1 (Log10 CFU/g) | Week 0 | 4.07 ± 0.59 | 3.97 ± 0.66 | 4.14 ± 0.32 | 2.45 ± 0.69 | 0.141 |
| Week 4 | 4.42 ± 0.38 | 5.25 ± 0.36 | 4.73 ± 0.23 | 5.13 ± 0.39 | 0.310 | |
| Changes | 0.35 ± 0.62ab | 1.28 ± 0.41bc | 0.58 ± 0.29ab | 2.68 ± 0.66c | 0.015 | |
| P value2 | 0.585 | 0.014 | 0.073 | 0.003 | ||
| Positive/tested (n) | Week 0 | 10/12 | 8/9 | 11/11 | 8/10 | 0.093 |
| Week 4 | 12/12 | 9/9 | 11/11 | 10/10 | – | |
| Lactobacillus gasseri BNR17 | ||||||
| Positive/tested (n) | Week 0 | 0/12 | 2/9 | 0/11 | 1/10 | 0.083 |
| Week 4 | 0/12 | 7/9 | 10/11 | 9/10 | <0.0001 |
1Mean ± SE (all variables)
2Paired t test was used for comparisons within each group
3ANOVA was used for comparisons of continuous variables among groups. Fisher’s exact test was used for comparisons of categorical variable among groups
In summary, our results confirmed probiotic properties of Lb. gasseri BNR17 in terms of relieving IBS symptoms and colonization. In addition, its optimal intake level was found to be 2 × 5 × 109 CFU/day. However, this was a preliminary trial without detecting biochemical or metabolomics changes in feces. In addition, only IBS symptoms were measured. In order to provide pronounce evidence on its beneficial effects, more objective measures should be determined such as transit time, gut microbial changes and metabolites in feces. In the next main trial, biochemical or metabolomic markers related to IBS should be studied using the present strain. Additionally, changes in population of gut microbiomes should be identified.
Acknowledgements
This study was supported by a Bio-Synergy Research Project (2012M3A9C4048761) of the National Research Foundation funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Wang JR, Xing ZQ, Tang W, Zheng YN, Wang YP. Isolation, identification, and potential probiotic characterization of one Lactococcus from Kefir grain. Food Sci Biotechnol. 2015;24:1775–1780. doi: 10.1007/s10068-015-0231-8. [DOI] [Google Scholar]
- 2.Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, Guo X, Sun G, Shang J, Wang WF, Feng J, Huang Q. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2012;27:760–765. doi: 10.1111/j.1440-1746.2011.06930.x. [DOI] [PubMed] [Google Scholar]
- 3.Jung HK, Kim YH, Park JY, Jang BH, Park SY, Nam MH, Choi MG. Estimating the burden of irritable bowel syndrome: analysis of a nationwide korean database. J Neurogastroenterol Motil. 2014;20:242–252. doi: 10.5056/jnm.2014.20.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Gastroenterology Task Force on Irritable, Bowel S, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 5.Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, Quigley E, Schoenfeld P, Schuster M, Talley N. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7–S26. doi: 10.1016/s0002-9270(02)05657-5. [DOI] [PubMed] [Google Scholar]
- 6.Whelan K. Probiotics and prebiotics in the management of irritable bowel syndrome: a review of recent clinical trials and systematic reviews. Curr Opin Clin Nutr Metab Care. 2011;14:581–587. doi: 10.1097/MCO.0b013e32834b8082. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol 104: 1033–49; quiz 1050 (2009) [DOI] [PubMed]
- 8.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107:1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 9.Jung SP, Lee KM, Kang JH, Yun SI, Park HO, Moon Y, Kim JY. Effect of Lactobacillus gasseri BNR17 on Overweight and Obese Adults: A Randomized, Double-Blind Clinical Trial. Korean J Fam Med. 2013;34:80–89. doi: 10.4082/kjfm.2013.34.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 11.Kang JH, Yun SI, Park MH, Park JH, Jeong SY, Park HO. Anti-obesity effect of Lactobacillus gasseri BNR17 in high-sucrose diet-induced obese mice. PLoS One. 2013;8:e54617. doi: 10.1371/journal.pone.0054617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan C, Talley NJ, Cross S, Jones M, Hammer J, Giles N, Horowitz M. Development and validation of the Diabetes Bowel Symptom Questionnaire. Aliment Pharmacol Ther. 2003;17:1179–1187. doi: 10.1046/j.1365-2036.2003.01553.x. [DOI] [PubMed] [Google Scholar]
- 13.Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–394. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman J. Extraintestinal symptoms in irritable bowel syndrome and inflammatory bowel diseases: nature, severity, and relationship to gastrointestinal symptoms. Dig Dis Sci. 2003;48:743–749. doi: 10.1023/A:1022840910283. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi H, Fujita T, Suzuki Y, Benno Y. Monitoring and survival of Lactobacillus gasseri SBT2055 in the human intestinal tract. Microbiol Immunol. 2006;50:867–870. doi: 10.1111/j.1348-0421.2006.tb03862.x. [DOI] [PubMed] [Google Scholar]
- 16.Ducrotte P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroentero. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr. 2010;140:690S–697S. doi: 10.3945/jn.109.113746. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic I, Sybesma W, Phothirath P, Ananta E, Mercenier A. Application of probiotics in food products-challenges and new approaches. Curr Opin Biotech. 2010;21:175–181. doi: 10.1016/j.copbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara S, Seto Y, Kimura A, Hashiba H. Establishment of orally-administered Lactobacillus gasseri SBT2055SR in the gastrointestinal tract of humans and its influence on intestinal microflora and metabolism. J Appl Microbiol. 2001;90:343–352. doi: 10.1046/j.1365-2672.2001.01251.x. [DOI] [PubMed] [Google Scholar]
- 20.Olivares M, Diaz-Ropero MA, Gomez N, Lara-Villoslada F, Sierra S, Maldonado JA, Martin R, Lopez-Huertas E, Rodriguez JM, Xaus J. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol. 2006;107:104–111. doi: 10.1016/j.ijfoodmicro.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Takagi A, Yanagi H, Ozawa H, Uemura N, Nakajima S, Inoue K, Kawai T, Ohtsu T, Koga Y. Effects of Lactobacillus gasseri OLL2716 on Helicobacter pylori-Associated Dyspepsia: a Multicenter Randomized Double-Blind Controlled Trial. Gastroenterol Res Pract. 2016;2016:7490452. doi: 10.1155/2016/7490452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alok A, Singh ID, Singh S, Kishore M, Jha PC, Iqubal MA. Probiotics: a New Era of Biotherapy. Adv Biomed Res. 2017;6:31. doi: 10.4103/2277-9175.192625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreasen AS, Larsen N, Pedersen-Skovsgaard T, Berg RM, Moller K, Svendsen KD, Jakobsen M, Pedersen BK. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr. 2010;104:1831–1838. doi: 10.1017/S0007114510002874. [DOI] [PubMed] [Google Scholar]