Abstract
A total of fourteen roasted coffee samples were collected from different local markets in Nayarit, Mexico. Twenty-two fungi isolates were related to the genera Aspergillus (54.54%) and Penicillium (4.5%). The strains R16 (0.33 μg/kg), 6N (1.16 μg/kg) and 11 (0.36 μg/kg) tested positive for OTA (ochratoxin A) production in PDA, the other fungi samples were not toxigenic. According to the sequence analysis of their ITS1-5.8S-ITS2 rDNA region, fungi OTA producers correspond to A. niger, A. versicolor and Byssochlamys spectabilis. These three strains were able to produce OTA when inoculated in roasted coffee in concentrations ranging from 75 to 90 μg/kg, after 21 days. Different production stages of roasted coffee (crop management, postharvest practices and storage) along with environmental conditions do not ensure mycotoxigenic fungi free products. This is the first report of OTA natural occurrence in roasted coffee from Nayarit.
Keywords: Roasted coffee, Byssochlamys spectabilis, Toxigenic fungi, Ochratoxin A, Immunoaffinity column
Introduction
Coffee is one of the most widely-consumed food products, with an important economic and cultural role. However, coffee beans, like other crops, can be contaminated by microorganisms during the different stages of growing, harvesting, processing, transport and storage. Many studies have revealed that important toxigenic fungal genera (Aspergillus and Penicillium) are natural coffee contaminants [1–3]. OTA is a mycotoxin naturally found in various food products including green coffee beans, roasted coffee and instant coffee [2]. This mycotoxin produced by toxigenic fungi has been shown to exhibit hepatotoxic, nephrotoxic, teratogenic, and carcinogenic properties [4]. The International Agency for Research on Cancer (IARC) classified OTA as carcinogenic for humans (group 2B) [5, 6]. According to the European Commission Regulation [7], the maximum levels for OTA are 5 μg/kg for roasted coffee beans and ground roasted coffee, and 10 μg/kg for soluble coffee [8].
One of the main economic activities in Nayarit is coffee production. Environmental conditions such as high temperatures and humidity favor fungal development in coffee beans. De Lourdes et al. [9], reported the presence of OTA in 70% of green coffee samples with an average concentration of 30.1 μg/kg. Franco et al. [10] detected OTA from 4.90 to 37.73 μg/kg and total aflatoxins were found from 1.51 to 1.93 μg/kg. It was found that four out of the 21 samples in Panamanian exportation coffee tested positive for OTA and three tested positive for presence of total aflatoxins. These findings highlight the importance of determining the presence of potential ochratoxin and aflatoxin producing fungi in roasted coffee beans (Coffea arabica L.) from Nayarit, an important coffee producer in Mexico.
Materials and methods
Roasted coffee
Experiments were carried out using 14 ground roasted coffee samples (Coffea arabica L.) from different local markets in Nayarit, Mexico.
Identification of fungal isolates
Two methods were employed for fungal isolation. In the first method, processed coffee beans were plated directly onto filter paper moistened with sterile distilled water. The beans were collected randomly from each coffee bean sample; they were then disinfected by immersion in a 1% hypochlorite solution for 2 min (w/v). The beans were then put on the surface of potato dextrose agar (PDA) medium and Rose-Bengal (1/15,000) was added as a bacteriostatic agent. Plates were incubated at 28 °C for 7–10 days [11]. In the second method, ten grams of ground roasted coffee were transferred to a wide mouth reagent bottles containing 90 mL sterile distilled water. The bottles were shaken for 15 min. This gave an approximate dilution of 1/10. Sterile dilutions from 1/100 to 1/10,000, were made using test tubes containing 9 mL sterilized distilled water. One mL of each selected dilution was put in a sterile petri dish and 10 mL of the medium (Czapek Yeast Extract Agar, Malt Extract Agar, Czapek Dox Agar) were poured, the plate was then gently moved for homogeneous distribution. The plates were incubated at 28 °C for 7–14 days. Bacterial growth was inhibited by using streptomycin (300 ppm). Sodium bicarbonate (NaHCO3) was added to 50% of the plates in order to substantially inhibit growth of A. niger [12].
Isolates were purified on Czapek Yeast Extract Agar. The fungi were allowed to grow at 25 °C for 7 days, and were then pre-identified following the traditional morphological methods. Macroscopic (mycelium type, color and growth type) as well as microscopic optical characteristics (at 40×, mycelium type, conidiophores morphology and spore morphology) were considered for identification [13, 14].
Ochratoxin and aflatoxin production ability of the isolates
Mycotoxin production by isolated fungal strains was determined using HPLC following the methodology described by Mounjouenpou et al. [15]. After 10 days of incubation on PDA agar (25 °C), direct extraction was carried out on 3 agar discs taken from the center of the colony. Extraction was carried out in 2.5 mL of solvent (methanol/formic acid 25:1 v/v) for 15 min in an ultrasound bath.
The production of OTA was detected and quantified by reversed phase high (zorbax SB-C18 2.1 × 50 mm id: 1.8 μm) HPLC with electrospray ion (ESI) MS (Agilent Technologies), in ionization mode, positive mode capillary 3500 V, nebulizer 25 psi (nitrogen), dry gas nitrogen at 9 L/min, dry gas temperature 350 °C, fragmentor voltage 95; selected ion monitoring (SIM), m/z 404 (OTA). The mobile phase (5 mM ammonium acetate/acetonitrile, 65: 35 at 40 °C) was pumped at a rate of 0.2 mL/min. The injection volume was 20 μL and the retention time was around 4 ± 1 min.
In all cases, aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G2 (AFG2), aflatoxin G1 (AFG1) were quantified on extracts by HPLC with fluorometric detection (Shimadzu LC-10 ADVP, Japan) [1]. The operating conditions were as follows: 100 μL injection loop, C18 reverse phase HPLC column, ODS 5 μm (Supelco, Interchim, Montluçon, France) with an identical pre-column thermostatically controlled at 35 °C, an isocratic flow of 1 mL/min, an excitation wavelength of 362 nm and an emission wavelength of 425 nm were used. Aflatoxins were carried out with potassium bromide. Contents were calculated from a calibration curve established from a standard (1 μg/mL; ref PD 226 R. Biopharm Rhône Ltd, Glasgow, UK).
PCR identification
Fungi were grown in Potato Dextrose Agar at 28 °C for 5–7 days. DNA extraction was then performed according to the protocol proposed by Sambrook and Russell [16]. PCR was conducted using the primers ITS1 (ITS1-5, 8s-ITS-2) of ribosomal DNA using universal primers ITS1 (5′CAACTCCCAAACCCCTGTGA-3′) and ITS4 (5′- GCGACGATTACCAGTAACGA-3′) for molds [17]. Two amplifications were carried out in a Technethermocycler (iCycler Biorad, USA). Thermal cycling parameters for first amplification were: initial denaturation at 95 °C for 2 min, followed by 30 cycles of heat denaturation at 95 °C for 1 min, annealing at 50 °C for 30 s, extension at 72 °C for 2 min and final extension at 72 °C for 10 min. Thermal cycling parameters for second amplification were: initial denaturation at 95 °C for 5 min, followed by 30 cycles of heat denaturation at 95 °C for 1 min, annealing at 56.5 °C for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min. PCR products from these amplifications were separated by electrophoresis on 1% (w/v) agarose gel, stained in syber safe (Sigma-Aldrich), the sample was mixed with buffer TAE 1X. A molecular marker from 100 to 1000 bp was used. Electrophoresis was performed under the following conditions: 1 h and 30 min, 60 V and 400 mA. PCR products were visualized using a UV transilluminator (UVP BioDoc-IT Imaging System, USA). The PCR products were sent to GENEWIZ Inc. (USA), for sequencing and species identification. The results were analyzed using the BLAST (The Basic Local Alignment Search Tool) of NCBI (National Center for Biotechnological Information) with the support of Codon Code Aligner 2.0 editing program sequences.
OTA production by fungi isolates
Isolates of A. niger, A. versicolor and Byssochlamys spectabilis were grown on PDA, at 27 °C for 7 days. Secondly, deionized water (3 mL) was added to each coffee sample (1 g) and autoclaved at 121 °C for 15 min. The coffee was inoculated with 2 culture discs of the strain [18]. After 21 days, OTA production was detected and quantified by HPLC system 1260 series Agilent Technologies model consisting of a quaternary pump, autosampler, a column thermostat and a Quadrupole detector (6120).
OTA production was analyzed according to Mounjouenpou et al. [19] with some modifications. Samples were extracted for 50 min (60 °C) with a solution of methanol/3% sodium bicarbonate (20:80), the extracts were filtered and diluted with phosphate-buffered saline and applied to an immunoaffinity column (Ochrastar R). OTA was eluted with 6 mL HPLC grade methanol. The eluate was evaporated to dryness (3 min) under an oven at 70 °C, re-dissolved in 1 mL of HPLC mobile phase water/acetonitrile (50/50, v/v) and then quantified by LC–MS.
Chromatographic and spectrometric conditions
Chromatographic separation was performed on a zorbax SB-C18 (2.1 × 50 mm id: 1.8 μm) column (Agilent Technologies) using a mobile phase of 5 mM ammonium acetate/acetonitrile (65/35, v/v) at 40 °C with a flow rate of 0.2 mL/min for 3 min run. The detection of OTA was performed in LC–MS system model 6120 series LC quadrupole equipped with an electrospray ion (ESI) MS (Agilent Technologies), in ionization mode, positive mode (capillary 3500 V, nebulizer 25 psi (nitrogen), dry gas nitrogen at 9 L/min, dry gas temperature 350 °C, fragmentor voltage 95); selected ion monitoring (SIM), m/z 404 (OTA) [20].
Results and discussion
Isolation and identification of fungi
Macroscopic and microscopic presumptive identification revealed two fungal genera: Aspergillus and Penicillium. Aspergillus was predominant in roasted coffee samples with a recovery of 95.43% and Penicillium with 4.54%. From the 22 isolates, the predominant species determined by dichotomous characters were Aspergillus ochraceus (4.54%), Aspergillus carbonarius (4.54%), A. niger (27.27%), Aspergillus fumigatus (4.54%) and other Aspergillus spp. (54.54%). Aspergillus spp. propagules get on grain in different ways, most often with dust from soil, from the surface of plant remnants during harvesting, transportation, storage, and processing [21]. It has great metabolic versatility and ability to disperse conidia in the environment to such an extent that it can sustain growth even under adverse conditions such as low humidity and low water activity [22].
Table 1 shows the production of mycotoxins by isolates from roasted coffee beans inoculated in PDA. Three fungi isolates produced OTA. Strain 11 with a concentration of 0.36 μg/kg, R16 with 0.33 μg/kg and 6N with 1.16 μg/kg mycelium. No OTA or aflatoxin were detected in the rest of the isolates.
Table 1.
Relationship between levels of contamination of aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G2 (AFG2), aflatoxin G1 (AFG1) and ochratoxin A (OTA) and toxigenic filamentous fungi in roasted coffee beans
| Strain | AFG1 (μg/kg) | AFG2 (μg/kg) | AFB1 (μg/kg) | AFB2 (μg/kg) | OTA (μg/kg) |
|---|---|---|---|---|---|
| A | ND | ND | ND | ND | ND |
| A-B | ND | ND | ND | ND | ND |
| 2B | ND | ND | ND | ND | ND |
| R5 | ND | ND | ND | ND | ND |
| R10 | ND | ND | ND | ND | ND |
| R11 | ND | ND | ND | ND | ND |
| R14 | ND | ND | ND | ND | ND |
| R15 | ND | ND | ND | ND | ND |
| R16a | ND | ND | ND | ND | 0.33 |
| R17 | ND | ND | ND | ND | ND |
| R18 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND | ND |
| 3 | ND | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND | ND |
| 5 | ND | ND | ND | ND | ND |
| 6Nb | ND | ND | ND | ND | 1.162 |
| 7 | ND | ND | ND | ND | ND |
| 8-2 | ND | ND | ND | ND | ND |
| 9 | ND | ND | ND | ND | ND |
| 10 | ND | ND | ND | ND | ND |
| 11c | ND | ND | ND | ND | 0.36 |
| V | ND | ND | ND | ND | ND |
ND Not detectable
a A. niger
b Byssochlamys spectabilis
c A. versicolor
Therefore, as was observed, the strains vary in their capacity to produce OTA, as well as in the quantity produced. This is because fungal growth occurs under favorable environmental conditions and is associated with the production of a wide range of secondary metabolites. There are more important factors that rule the growth of fungi and the production of mycotoxins such as the amount of nutrients available, the ambient temperature, water activity and available oxygen [23]. In spite of OTA being a stable metabolite, in some cases OTA content decreased considerably as incubation time increased or just at the final incubation time. Some authors have suggested that this may be due to the fact that the strains remove and assimilate the phenylalanine moiety from the OTA molecule as well as nitrogen sources in the culture media when they become exhausted [24].
Fungal growth and mycotoxin production are influenced by numerous abiotic and biotic parameters and their complex interactions. Water availability is probably the single most important factor affecting germination, growth and establishment of fungi on nutrient rich substrates. The second most important is temperature (22–30 °C) [8, 25]. It has been shown that both factors influence interactions between different mycotoxigenic and non-mycotoxigenic fungi [26]. The next most important factors for mycotoxin production and mold growth are high moisture content (20–25%) and high relative humidity (70–90%) [8].
The strains that tested positive for OTA production were molecularly analyzed, the amplification of the ITS1-5.8S-ITS2 region of rDNA from the two relevant Aspergillus strains generated PCR products of 550 bp and were detected as A. niger and A. versicolor (Fig. 1). Another PCR product of 500 bp was observed and identified as B. spectabilis (Fig. 1). Sequences reported at BLAST v2.3.0 (Basic Local Alignment Search Tool http://www.ncbi.nlm.nih.gov/) at the National Center for Biotechnology Information, NCBI, USA, were used to compare the obtained sequences (Table 2).
Fig. 1.
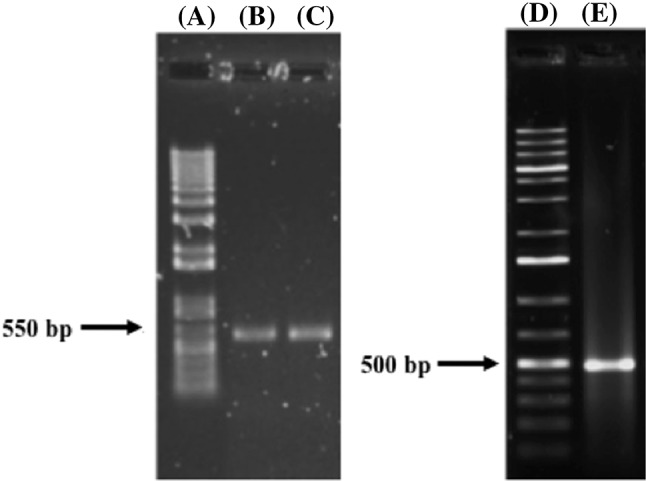
Amplification of ITS1-5.8S-ITS2 rDNA region from mycotoxigenic fungi isolated from roasted coffee from Nayarit, Mexico. (A) Molecular weight marker, 1KB plus DNA ladder (Invitrogen)., (B) A. niger., (C) A. versicolor., (D) molecular weight marker, 1KB plus DNA ladder (Invitrogen)., (E) Byssochlamys spectabilis
Table 2.
Sequence homology of toxigenic fungi isolated from coffee (Coffea arabica L.), according to N.C.B.I. (National Center for Biotechnology Information)
| Strain | Homology | Sequence | Access number |
|---|---|---|---|
| R16 | 95% A. niger | 1 tacgagcgcg aggtctttgg gccacctccc atccgtgtct attgtaccct gttgcttcgg 61 cgggcccgcc gcttgtcggc cgccgggggg gcgcctctgc cccccgggcc cgtgcccgcc 121 ggagacccca acacgaacac tgtctgaaag cgtgcagtct gagttgattg aatgcaatca 181 gttaaaactt tcaacaatgg atctcttggt tccggcatcg atgaagaacg cagcgaaatg 241 cgataactaa tgtgaattgc agaattcagt gaatcatcga gtctttgaac gcacattgcg 301 ccccctggta ttccgggggg catgcctgtc cgagcgtcat tgctgccctc aagcccggct 361 tgtgtgttgg gtcgccgtcc ccctctccgg ggggacgggc ccgaaaggca gcggcggcac 421 cgcgtccgat cctcgagcgt atggggcttt gtcacatgct ctgtaggatt ggccggcgcc 481 tgccgacgtt ttccaaccat tctttccagg ttgacctcgg |
JN226991.1 |
| 11 | 99% A. versicolor | 1 tccgtaggtg aacctgcgga aggatcatta ctgagtgcgg gctgcctccg ggcgcccaac 61 ctcccacccg tgactaccta acactgttgc ttcggcgggg agccctctcg ggggcgagcc 121 gccggggact actgaacttc atgcctgaga gtgatgcagt ctgagtctga atataaaatc 181 agtcaaaact ttcaacaatg gatctcttgg ttccggcatc gatgaagaac gcagcgaact 241 gcgataagta atgtgaattg cagaattcag tgaatcatcg agtctttgaa cgcacattgc 301 gccccctggc attccggggg gcatgcctgt ccgagcgtca ttgctgccca tcaagcccgg 361 cttgtgtgtt gggtcgtcgt cccccccggg ggacgggccc gaaaggcagc ggcggcaccg 421 tgtccggtcc tcgagcgtat ggggctttgt cacccgctcg atttagggcc ggccgggcgc 481 cagccgacgt ccaaccattt ttcttcaggt tgacctcgga tcaggtaggg atacccgctg 541 aacttaagca tatcaataag cggaggaaaa gaaaccaacc gggattgccc c |
NR_131277.1 |
| 6N | 100% Byssochlamys spectabilis | 1 ctgcggaagg atcattaccg agtgagggtc cctcggggcc caacctccca tccgtgttgt 61 cctgacacct gttgcttcgg cgggcccgcc gtggttcacg ccccggccgc cggggggttc 121 acgcccccgg gcccgcgccc gccgaagacc cctggaacgc tgcctggaag gttgccgtct 181 gagtatacaa tcaatcaatt aaaactttca acaacggatc tcttggttcc ggcatcgatg 241 aagaacgcag cgaaatgcga taagtaatgt gaattgcaga attccgtgaa tcatcgaatc 301 tttgaacgca cattgcgccc cctggcattc cggggggcat gcctgtccga gcgtcattgc 361 taaccctcca gcccggctgg tgtgttgggc cgccgtcccc ccccccgggg gacgggcccg 421 aaaggcagcg gcggcgtcgc gtccggtcct cgagcgtatg gggctttgtc acacgcttca 481 gtagaaccgg ccggcttgct ggccacacga ccttcacggt cacctatatt tctcttaggt 541 tgacctcgga tcaggtaggg atacccgctg aacttaagca tatcaataag cg |
KC157703.1 |
The strain A. niger presented a colony on CYA 65.6 ± 1.75 mm (7 days/25 °C); conidia coffee brown to black and white mycelium. Its texture is irregular, plane and velvety; reverse pale yellow; it formed radial furrows very close to each other. The conidial heads were smooth and pigmented brown, vesicle is globose and produces phallus around it, phialides are biseriate and conidia are globose and smooth. Conidiophore size is 538.03 ± 113.34 μm × 6.5 ± 0.30 μm, phialides 7.9 ± 1.00 μm × 3.36 ± 0.25 μm, vesicles 44.7 ± 10.25 μm, conidia 4.5 ± 0.05 μm (Fig. 2).
Fig. 2.
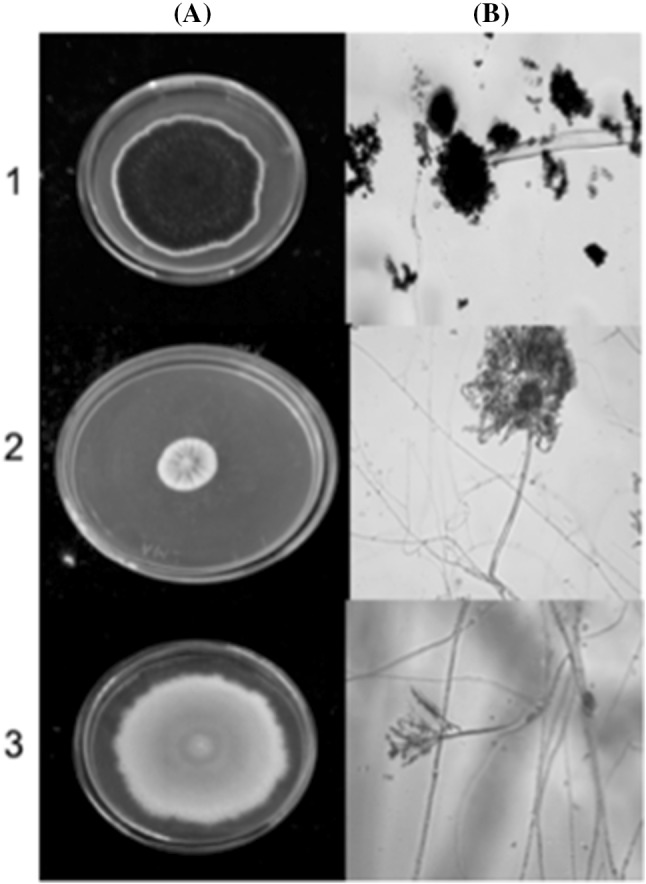
Mycotoxigenic fungi isolated from roasted coffee. (1) A. niger colonies on CYA, (2) A. versicolor on CYA, (3) Byssochlamys spectabilis on CYA. (A) macroscopic morphology, (B) optical microscopic ×40
The morphological characteristics of A. versicolor show colony diameter on CYA, 14 ± 1.80 mm (7 days/25 °C). The colonies were initially colored white, though they gradually turned grayish-green with white edges, granular shape, convex elevation, rough texture. Reverse is brownish orange or reddish brown. No exudates. Conidiophore were smooth to slightly rough walls. The conidia head is biseriate, phialides radially cover the vesicle. Conidia are spherical. Conidiophore size is 370.91 ± 220.01 × 9.82 ± 3.73 μm, vesicle 24.33 ± 0.90 μm, conidia 2.6 ± 0.2 μm. Another fungal species detected was B. spectabilis whose colony diameter on CYA was 78.43 ± 3.30 mm, plane and filamentous (7 days/25 °C). The colonies are pale yellow–brown, dusty texture with white border. Reverse is white color. No exudates. Conidiophores biverticilate and have smooth and thick wall. Conidia are ellipsoidal. Conidiophore size is 173.09 ± 110.01 × 3.1 ± 1.1 μm, phialides 21.30 ± 3.06 μm × 2.5 ± 3.0 μm, conidia 4.23 ± 0.25 μm (Fig. 2).
Gautam and Bhadauria [27] characterized at the molecular level the identification of mycoflora associated with Aspergillus species in samples of Triphala churn and ingredients (mixture of dry fruits of medicinal plants: Embilica officinalis, Terminalia bellirica, Terminalia chebula) and obtained the amplified product of 500, 700 and 1110 bp for A. versicolor.
According to the identification, only the strain of A. niger produced OTA in the analyzed sample of roasted coffee, the production of OTA in coffee by A. versicolor has not been reported before, but it has been reported in wheat, where the strain produced 0.01–0.07 μg/g [28].
In this study, B. spectabilis was first identified in roasted coffee using primers ITS1 and ITS4 amplified with 500 bp approximately. Byssochlamys species are abundant in soil and recognized as important spoilage molds in fruit and fruit products [29]. Byssochlamys spectabilis (anamorph P. variotii s.s.) commonly occurs in air, compost, various foodstuffs (including pasteurized fruit juices, rye bread) [30]. In general, this fungus can survive considerable periods of heat above 85 °C and can grow under very low oxygen conditions, produce mycotoxins such as vomitoxin and deoxynivalenol; the production of OTA by this strain had not been reported before [30, 31].
The occurrence of OTA in all inoculated roasted coffee samples demonstrates fungi isolated may produce this mycotoxin in this kind of food product. A mycotoxin may be produced by several different fungi (Table 3, Fig. 3). The mycotoxigenic potential depends on species and strain of fungus, matrix composition and environmental factors (temperature and moisture).
Table 3.
Level of OTA production (μg/kg) for A. niger, A. versicolor and Byssochlamys spectabilis
| Isolate | Coffee medium |
|---|---|
| OTA production (μg/kg) | |
| A. niger | 91.03 |
| A. versicolor | 76.74 |
| Byssochlamys spectabilis | 81.97 |
Fig. 3.
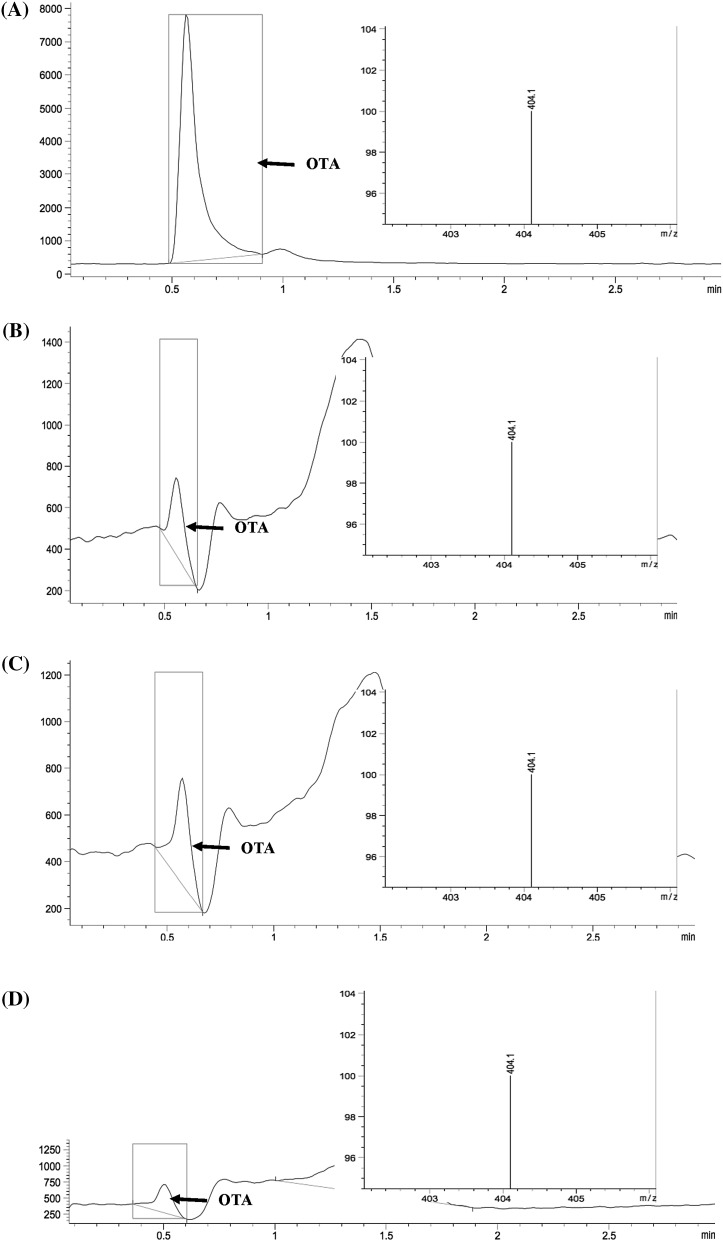
LC–MS chromatograms: (A) standard solution (OTA 0.021 μg/mL), roasted coffee sample contaminated with: (B) A. niger, (C) A. versicolor, (D) Byssochlamys spectabilis
According to these results, it can be concluded that the fungi with the potential to produce OTA in roasted coffee samples (Coffea arabica L.) are A. niger, A. versicolor and B. spectabilis. It was also observed that once a toxigenic strain was isolated from a coffee sample, they were able to produce OTA in roasted coffee samples. The presence of toxigenic strains implicates a great risk of OTA presence.
OTA is a stable compound not destroyed by common food preparation procedures. Temperatures above 250 °C for several minutes are necessary to reduce the concentration of this toxin [32]. Roasting treatment for green coffee at 200 °C for 20 min reduced levels of OTA by only 0–12% in the dried whole beans [33]. Therefore, there is a high chance that the population get contaminated by this toxin, since the roasting of coffee does not assure its total destruction. Consequently, a cup of coffee could contain high amounts of ochratoxin. Because of its high affinity with plasma proteins, their persistence in the organism (average 840 h) is ensured [34]. OTA is efficiently absorbed from the gastrointestinal tract, mainly in the small intestine and distributed via the blood, mainly to the kidneys, with lower concentrations found in liver, muscle and fat. Specific transporters may be involved in the cellular uptake of OTA into the kidney, where it accumulates. OTA could be implicated in the pathogenesis of some renal diseases including kidney tumors and chronic interstitial nephropathy [35].
Good manufacturing practices and hygiene throughout the coffee production and processing chain is highly recommended in order to reduce the risk of contamination of processed coffee. Furthermore, the OTA presence risk implies the necessity to develop effective technologies to detoxify coffee products.
In Mexico, there are no legal limits established for ochratoxin contamination and there are few studies on the occurrence of OTA in foods and beverages. More research is necessary to evaluate the real exposure of the population to this mycotoxin through the ingestion of contaminated food.
Acknowledgements
This study was carried out with the support of the “Tecnologico Nacional de Mexico” (Project No. 5851.16-P). The authors thank CONACYT (Mexico) for the scholarship Granted to Paloma Patricia CASAS-JUNCO.
References
- 1.Nakajima M, Tsubouchi H, Miyabe M, Ueno Y. Survey of aflatoxin B1 and ochratoxin A in commercial green coffee beans by high-performance liquid – chromatography linked with immunoaffinity chromatography. Food and Agricultural Immunology. 1997;9:77–83. doi: 10.1080/09540109709354938. [DOI] [Google Scholar]
- 2.Noonim P, Mahakarnchanakul W, Nielsen KF, Frisvad JC, Samson RA. Isolation, identification and toxigenic potential of ochratoxin A-producing Aspergillus species from coffee beans grown in two regions of Thailand. International Journal of Food Microbiology. 2008;128:197–202. doi: 10.1016/j.ijfoodmicro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Silva CF, Schwan RF, Dias ËS, Wheals AE. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. International Journal of Food Microbiology. 2000;60:251–260. doi: 10.1016/S0168-1605(00)00315-9. [DOI] [PubMed] [Google Scholar]
- 4.Pitt J. Toxigenic fungi: which are important? Medical mycology. 2000;38:17–22. doi: 10.1080/mmy.38.s1.17.22. [DOI] [PubMed] [Google Scholar]
- 5.Nogaim Q, Gowri P. Determination of ochratoxin A in Yemeni green coffee. Scholars Academic Journal of Biosciences. 2013;1:253–262. [Google Scholar]
- 6.IARC. Evaluation of the Carcinogenic Risk of Chemicals to Humans. pp. 56. In: Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. International Agency for Research on Cancer (1993)
- 7.European Commission. Setting maximum levels for certain contaminants in foodstuffs. Commission Regulation (EC) No 1881/2006. Off. J. Eur Union. L 364: 5–24 (2006)
- 8.Vanesa D, Ana P. Occurrence of Ochratoxin A in coffee beans, ground roasted coffee and soluble coffee and method validation. Food Control. 2013;30:675–678. doi: 10.1016/j.foodcont.2012.09.004. [DOI] [Google Scholar]
- 9.de Lourdes RM, Marín S, Ramos AJ. Contaminación natural con micotoxinas en maíz forrajero y granos de café verde en el Estado de Nayarit (México) Rev Iberoam Micol. 2001;18:141–144. [PubMed] [Google Scholar]
- 10.Franco H, Vega A, Reyes S, De León J, Bonilla A. Niveles de Ocratoxina A y Aflatoxinas totales en cafés de exportación de Panamá por un método de ELISA. Archivos Latinoamericanos de Nutrición. 2014;64:42–49. [PubMed] [Google Scholar]
- 11.Bokhari FM. Mycotoxins and toxigenic fungi in arabic coffee beans in Saudi Arabia. Advances in Biological Research. 2007;1:56–66. [Google Scholar]
- 12.Depasquale DA, El-Nabarawy A, Rosen JD, Montville TJ. Ammonium bicarbonate inhibition of mycotoxigenic fungi and spoilage yeasts. Journal of Food Protection. 1990;53:324–328. doi: 10.4315/0362-028X-53.4.324. [DOI] [PubMed] [Google Scholar]
- 13.Benítez EM. Estudio de especies micotoxígenas del género Penicillium: Penicillium verrucosum Dierckx. Ph.D. Thesis, Universitat Autònoma de Barcelona. Department de Sanitat I d´ Anatomia Animals. Facultat de Veterinária (2004)
- 14.Pitt JI, Hocking AD. Aspergillus and Related Teleomorphs. pp. 299, 300, 301, 312, 314, 318. In: Fungi and Food Spilage. Pitt JI. Hocking, AD (ed). Springer, Boston, MA (2009)
- 15.Mounjouenpou P, Gueule D, Fontana-Tachon A, Guyot B, Tondje PR, Guiraud JP. Filamentous fungi producing ochratoxin A during cocoa processing in Cameroon. International Journal of Food Microbiology. 2008;121:234–241. doi: 10.1016/j.ijfoodmicro.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Russell D. Molecular Cloning: A Laboratory Manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, New York; 2001. [Google Scholar]
- 17.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. 1990;18:315–322. [Google Scholar]
- 18.Cuadrench-Tripiana A, Agut M, Comellas L. Evaluación mediante HPLC-MS de la capacidad de producción de Aflatoxinas y Ocratoxina A por parte de 20 cepas de Aspergillus y Penicillium aisladas de compost. Afinidad: revista de quimica teórica y Aplicada. 2014;71:14–16. [Google Scholar]
- 19.Mounjouenpou P, Durand N, Guyot B, Guiraud JP. Effect of operating conditions on ochratoxin A extraction from roasted coffee. Food additives and contaminants. 2007;24:730–734. doi: 10.1080/02652030701199972. [DOI] [PubMed] [Google Scholar]
- 20.Saito K, Ikeuchi R, Kataoka H. Determination of ochratoxins in nuts and grain samples by in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. Journal of Chromatography. 2012;1220:1–6. doi: 10.1016/j.chroma.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Klich MA. Indentification of common Aspergillus species: Centraalbureau voor Schimmelcultures. Utrecht, The Netherlands (2002)
- 22.Borges VB, Maia MC, Couto MA, Vital HC, Souza MC. In: Abastract: Morphological changes of Aspergillus ochraceus irradiated on peanut grains, October 24–28, Belo Horizonte, MG, Brazil, International Nuclear Atlantic Conference-INAC (2011)
- 23.Filtenborg O, Frisvad JC, Samson R. Specific association of fungi to foods and influence of physical environmental factors. pp. 306–320. In: Introduction to food – and aiborne fungi. RA. Samson, ES. Hoekstra, JC. Frisvad O. Filtenborg (ed). Sixt Edition. Amer Society for Mycrobioloy (2000)
- 24.Astoreca A, Magnoli C, Barberis C, Chiacchiera S, Combina M, Dalcero A. Ochratoxin A production in relation to ecophysiological factors by Aspergillus section Nigri strains isolated from different substrates in Argentina. Science of the total environment. 2007;388:16–23. doi: 10.1016/j.scitotenv.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 25.Bouras N, Kim YM, Strelkov SE. Influence of water activity and temperature on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. International Journal of Food Microbiology. 2009;131:251–255. doi: 10.1016/j.ijfoodmicro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Akbar A, Magan N. The impact of water and temperature interactions on lag phase, growth and potential ochratoxin A production by two new species, Aspergillus aculeatinus and A. sclerotiicarbonarius, on a green coffee-based medium. International Journal of Food Microbiology. 2014;188:116–121. doi: 10.1016/j.ijfoodmicro.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Gautam A, Bhadauria R. Diversity of fungi and mycotoxins associated with stored Triphala churn and its ingredients. Journal of Biological Sciences. 2011;11:226–235. doi: 10.3923/jbs.2011.226.235. [DOI] [Google Scholar]
- 28.Riba A, Mokrane S, Mathieu F, Lebrihi A, Sabaou N. Mycoflora and ochratoxin A producing strains of Aspergillus in Algerian wheat. International Journal of Food Microbiology. 2008;122:85–92. doi: 10.1016/j.ijfoodmicro.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 29.Silva F. Inactivation of Byssochlamys nivea ascospores in strawberry puree by high pressure, power ultrasound and thermal processing. International Journal of Food Microbiology. 2015;214:129–136. doi: 10.1016/j.ijfoodmicro.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Houbraken J, Varga J, Rico-Munoz E, Johnson S, Samson RA. Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph Paecilomyces variotii) Applied and Environmental Microbiology. 2008;74:1613–1619. doi: 10.1128/AEM.01761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samson R, Houbraken J, Varga J, Frisvad JC. Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia-Molecular Phylogeny and Evolution of Fungi. 2009;22:14–27. doi: 10.3767/003158509X418925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudra H, Le Bars P, Le Bars J. Thermostability of Ochratoxin A in wheat under two moisture conditions. Applied and Environmental Microbiology. 1995;61:1156–1158. doi: 10.1128/aem.61.3.1156-1158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsubouchi H, Yamamoto K, Hisada K, Sakabe Y, Udagawa S. Effect of roasting on ochratoxin A level in green coffee beans inoculated with Aspergillus ochraceus. Mycopathologia. 1987;97:111–115. doi: 10.1007/BF00436848. [DOI] [PubMed] [Google Scholar]
- 34.de Cerain López. A, Jiménez AM, Ezpelea O, Bello J. Efectos tóxicos de la ocratoxina A. Revista de toxicología. 2000;17:61–69. [Google Scholar]
- 35.Malir F, Ostry V, Pfohl-Leszkowicz A, Malir J, Toman J. Ochratoxin A: 50 years of research. Toxins. 2016;8:191. doi: 10.3390/toxins8070191. [DOI] [PMC free article] [PubMed] [Google Scholar]