Abstract
The microbial and physicochemical properties of brown and white cooked rice treated by atmospheric pressure plasma (APP). APP was produced (250 W, 15 kHz, ambient air) and applied to brown and white cooked rice for 5, 10, and 20 min. The 20-min plasma treatment reduced in bacterial counts by 2.01 log CFU/g when cooked rice were inoculated with Bacillus cereus or Escherichia coli O157:H7. The pH of the brown cooked rice was decreased by the 5-min plasma. The hardness values of APP-treated brown and white cooked rice were lower than untreated samples. The reducing sugar contents of brown and white cooked rice were significantly higher than those in untreated samples. Lipid oxidation of APP-treated brown and white cooked rice were higher compared to untreated samples. These results indicate that APP improves microbial quality, although further studies should be conducted to change the physicochemical qualities of brown and white cooked rice induced by APP.
Keywords: Atmospheric pressure plasma, Cooked rice, Quality
Introduction
Cooked rice is a main food in most Asian regions and has been increasingly used in processed foods, such as rice cakes, snacks, and alcoholic drinks. There is an increased demand for vacuum-packed cooked rice products due to lifestyle changes in modern society [1]. However, levels of Bacillus cereus > 3 log CFU/g have been found in both cooked and raw rice as well as in cereal products worldwide [2, 3]. Luo et al. [1] reported that approximately 2.0 log CFU/g of Clostridium perfrigens spores were detected in 15 different types of cooked rice products. In addition, contamination of rice-based food products with B. subtilis cause foodborne diseases [4]. Major food-borne pathogens found in food also include Escherichia coli, which has increased worldwide over recent years. Kim et al. [5] showed that the contamination of E. coli were 1.0–3.4 log CFU/g in commercial available Sunsik product. Therefore, effective treatments to inactivate the pathogens are required.
Atmospheric pressure plasma (APP) is a nonthermal technology used to improve food safety by inactivating food-borne pathogens. APP is ionized gas characterized by reactive species particles that is produced by applying energy to a gas or a gas mixture [6, 7]. Operative and configuration conditions of atmospheric plasma generators and assessments of the efficiency of the ionized gas on microbial inactivation have been reviewed [8]. The effect of this technology is significant since, unlike thermal sterilization, it does not cause nutritional or quality changes; in addition, it is more cost-effective and easier to install compared with other non-thermal technologies, such as irradiation and high pressure [9, 10]. Recent studies indicate that APP significantly affects pathogen such as Bacillus spp. and E. coli inactivation efficiency of various foods, such as brown rice, kiwifruit, and meats [7, 9, 11]. However, very few researches have examined the inactivation of pathogens on cooked rice by APP.
The aim of this study was to investigate the microbial and physicochemical characteristics of brown and white cooked rice following treatment with APP.
Materials and methods
Sample preparation
Brown and white rice (Oryza sativa cv. Samkwang) were harvested at the National Institute of Crop Science, Rural Development Administration, Korea in 2015. The rice grains were washed in water. It was cooked by placing 100 g of rice and 120 mL of distilled water (1:1.2) into a multi-programmable rice cooker (CR-0671V, CUCKOO, Ltd., Yangsan, Korea). After cooling each cooked rice sample, it was vacuum-packaged and stored at refrigerator temperature (4 °C) before analysis.
Pathogen inactivation
Sterilization
Samples were treated with gamma irradiation of 30 kGy (point source AECL, IR-79; MDS Nordion, Kanata, ONT, Canada) for sterilization using a cobalt-60 irradiator (Korea Atomic Energy Research Institute, Jeongeup, Korea) for the inoculation test.
Cultivation of pathogens and inoculation
Bacillus cereus (KCTC 3624) and E. coli O157:H7 (KCCM 40406) were cultivated to the mid-log phase at 37 °C for 48 h in nutrient broth (Difco Laboratories, Detroit, MI, USA) and tryptic soy broth (Difco), respectively. The cultures were centrifuged at 3000 rpm for 10 min at 4 °C. The pellet was washed twice and re-suspended in the sterile saline solution (0.85%) to a cell density of 107–108 CFU/mL levels.
APP application
The APP apparatus was used according to Kim et al. [12]. Briefly, a plasma source (dielectric barrier discharge) was constructed using a rectangular, parallel-piped plastic container (137 × 104 × 53 mm). The actuator was made of copper electrodes, and a polytetrafluoroethylene sheet was attached to the inner walls of the container. A 15-kHz bipolar square-waveform voltage was applied to one electrode while the other electrode was grounded. Plasma was produced inside the container with input power of 250 W. The samples were treated with the APP source for 5, 10, and 20 min.
Microbial analysis
Sterile cooked rice samples (3 g) were plasma-treated and then mixed in a sterile stomacher bag containing 27 mL of sterile saline solution. Serial dilutions were prepared with the sterile saline solution. Nutrient agar (Difco) was used to record the growth of B. cereus, and tryptic soy agar was used for E. coli O157:H7 (Difco). After incubation (37 °C, 48 h) of plate, all colonies were counted and the number of microorganisms were expressed as Log CFU/g.
Physicochemical properties
APP application
APP of brown and white cooked rice was treated with the same APP apparatus conditions explained in microbial analysis section.
pH
The plasma-treated sample (1 g) was added to 9 mL of distilled water, and homogenized for 30 s. pH was then measured using a pH meter (Model 750; iSTEC, Seoul, Korea).
Pasting properties
Pasting properties were determined using a Rapid-Visco Analyzer (RVA, Model RVA-3D; Newport Scientific, Warriewood, NSW, Australia) following Kim et al. [13]. Each RVA canister contained 3 g of grinded rice samples and was made up to 25 mL using deionized water. Peak viscosity, hot paste viscosity, final viscosity, breakdown (peak viscosity–hot paste viscosity), and setback (final viscosity–peak viscosity) were recorded.
Textural profile analysis
Plasma treated and untreated cooked rice were heated for 1 min using microwave and prepared (3 cm in diameter × 2 cm thickness; approximately 20 g). The textural profile analysis of the cooked rice was performed using a texture analyzer (testXpert II; Zwick Roell, Ulm, Germany) with a 50 kg load cell using two cycle compression. Cooked rice samples from each lot were kept on the base of this instrument for testing while the samples were still warm (50 °C). A two cycle compression force versus time program was used to compress the samples to 40% of the original cooked grain thickness, which were returned to the original position and compressed again. A 4 mm diameter probe was used to compress, with pre-test and post-test speeds of 2 mm/min and a test speed of 2 mm/min. Hardness, adhesiveness, and cohesiveness were recorded from the test curves.
Reducing sugars
The dinitrosalycilic acid (DNS) colorimetric method was used to determine the amount of reducing sugars released by hydrolysis [14]. Each sample (1 g) was added to 9 mL of distilled water and homogenized for 30 s. DNS reagent (3 mL) was added to 1 mL of the homogenated in a test tube. The tubes were placed in a water bath (100 °C) for 5 min, transferred to ice to cool down, and then brought to room temperature in a water bath. Absorbance was measured at 550 nm using a spectrophotometer.
Lipid oxidation
Lipid oxidation was modified by 2-thiobarbituric acid reactive substance (TBARS) values of Yong et al. [10]. Plasma-treated sample (3 g) was inserted to 9 mL of distilled water and 50 μL of 7.2% ethanolic butylated hydroxytoluene solution in a tube (50 mL) and homogenized at 16,000 rpm for 30 s. A homogenated sample (1 mL) was transferred to another tube (15 mL) and added to 2 mL of 20 mM thiobarbituric acid in 15% trichloroacetic acid solution. The tubes were heated in a water bath at 90 °C for 30 min, cooled in cold water, and centrifugation at 3000 rpm for 10 min. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (UV Spectrophotometer 1601; Shimadzu Co., Kyoto, Japan) and lipid oxidation was reported as mg malondialdehyde per kg sample.
Statistical analyses
All the experiments were carried out in triplicate with three observations per trials. The statistical analyses were performed using one-way analysis of variance using SPSS software (ver. 18.0; SPSS Inc., Chicago, IL, USA). When significant differences were detected, the differences among the mean values were determined using the Duncan’s multiple comparison test at a p value < 0.05.
Results and discussion
Pathogen inactivation
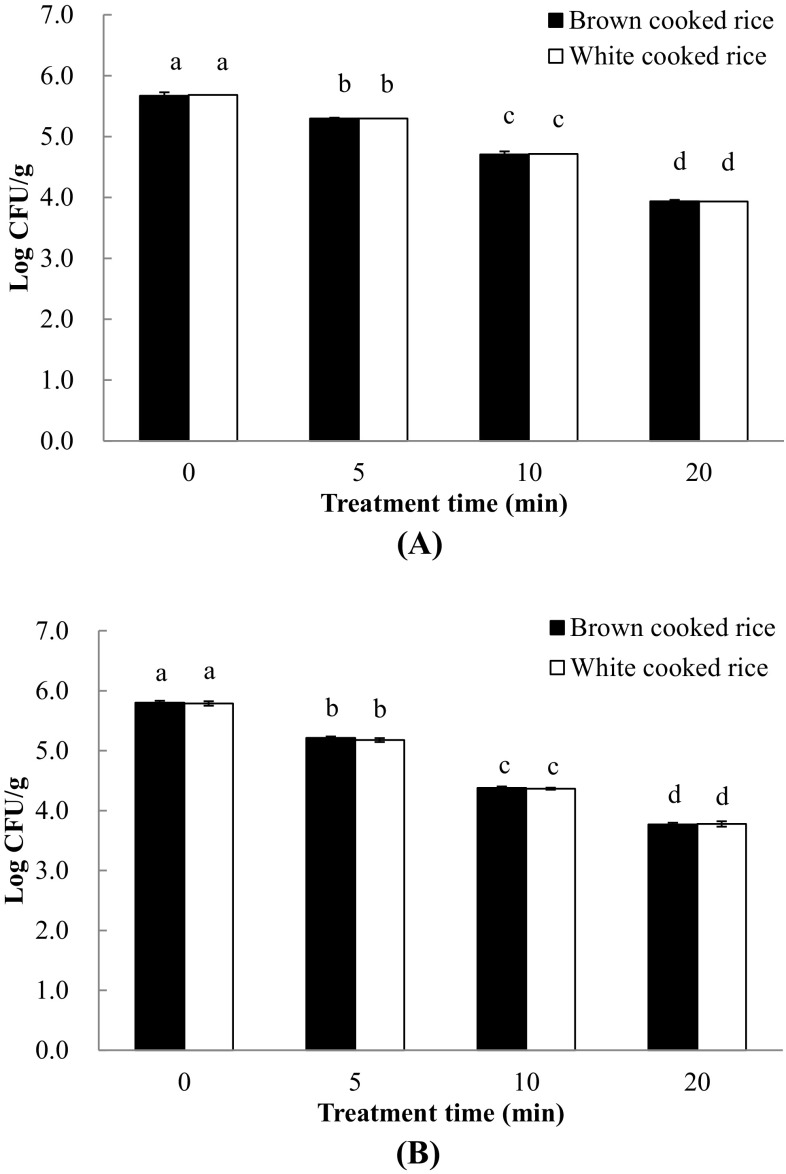
Escherichia coli O157:H7 was initially loaded on brown and white cooked rice at levels of 5.79 and 5.80 log CFU/g, respectively. APP decreased the number of pathogens to 2.01 and 2.03 log CFU/g on the brown and white cooked rice, respectively (Fig. 1). Bacillus cereus was initially loaded on the brown and white cooked rice at levels of 5.68 and 5.67 log CFU/g, respectively. After APP for 5, 10, and 20 min, B. cereus on brown and white cooked rice decreased significantly (p < 0.05) from 5.30 to 3.39 and from 5.29 to 0.05 log CFU/g, respectively (Fig. 1). Total aerobic bacteria and coliform bacterial counts on brown and white cooked rice decreased significantly (p < 0.05) by approximately 2 log CFU/g after the APP treatment (data not shown).
Fig. 1.
Inactivation of pathogens inoculated on brown and white cooked rice using atmospheric pressure plasma: (A) Bacillus cereus, (B) Escherichia coli O157:H7; a–dDifferent letters indicate significant differences among treatments (p < 0.05)
Lee et al. [11] showed plasma-induced inactivation of pathogens inoculated on brown rice. They demonstrated that the populations of B. cereus and E. coli O157:H7 inoculated on brown rice decreased significantly (p < 0.05) when treated with APP; log-reductions in B. cereus and E. coli O157:H7 counts increased from 0.32 to 1.29 log CFU/g and from 1.03 to 2.30 log CFU/g with increased APP exposure time from 5 to 20 min, respectively. Several plasma agents can be generated when plasma is discharged, including the electric field, UV photons, charged particles, and reactive species, which contribute to inactivation of pathogens. However, the electric field and UV are not major factors in plasma sterilization [15]. When sufficiently charged particles accumulate on the surface of pathogens, they are able to form an electric field, which alters protein structure. This change in protein structure results in the creation of pores in the pathogen membrane or inhibits enzymatic activities [16]. Joshi et al. [17] showed that reactive species generated by plasma cause oxidative DNA damage and membrane lipid peroxidation.
pH
The pH values of brown and white cooked rice decreased slightly after the APP treatment (Table 1). pH values also decreased in distilled water and orange juice with the extended APP treatment [18, 19]. The change in pH and acidity of plasma-treated produce is closely related to the dynamics of plasma chemistry. Tang et al. [20] reported that the concentration of NO3 –, which originates from HNO3 in the discharge, increases with plasma exposure, resulting in acidification of the liquid. The changes in pH are a stress element for bacteria and can cause cell death. Korachi and Aslan [18] indicated that the increase in H+, which is dissociated from bacterial molecules or H2O, also contributes to the reduction in pH observed by plasma.
Table 1.
Physicochemical properties in brown and white cooked rice following treatment with atmospheric pressure plasma
| Treatment time (min) | Brown cooked rice | White cooked rice | |
|---|---|---|---|
| pH | |||
| 0 | 7.18 ± 0.02a | 6.94 ± 0.03a | |
| 5 | 7.15 ± 0.02a | 6.84 ± 0.05b | |
| 10 | 7.10 ± 0.01b | 6.72 ± 0.03c | |
| 20 | 7.07 ± 0.02b | 6.68 ± 0.02c | |
| SEM1 | 0.012 | 0.026 | |
| Reducing sugar (%) | |||
| 0 | 2.36 ± 0.16c | 1.18 ± 0.03a | |
| 5 | 3.41 ± 0.21a | 1.09 ± 0.00b | |
| 10 | 2.96 ± 0.11b | 1.09 ± 0.03b | |
| 20 | 3.05 ± 0.06b | 0.98 ± 0.02c | |
| SEM1 | 0.012 | 0.019 | |
| TBARS value (mg malondialdehyde/kg) | |||
| 0 | 0.49 ± 0.01d | 0.04 ± 0.01d | |
| 5 | 0.54 ± 0.01c | 0.15 ± 0.01c | |
| 10 | 0.56 ± 0.01b | 0.20 ± 0.01b | |
| 20 | 0.59 ± 0.01a | 0.25 ± 0.01a | |
| SEM1 | 0.01 | 0.01 | |
a-dDifferent letters within same column differ significantly (p < 0.05)
1Standard errors of the mean (n = 3)
Pasting properties
The breakdown viscosity, which is the difference between peak viscosity and trough viscosity, represents the resistance to heat and shear force during starch gelatinization. It is also negatively correlated with amylose content and indicates the stability of the cooking process. As treatment time increased, the breakdown value tended to decrease in brown cooked rice, whereas the breakdown value tended to increase in white cooked rice (Table 2). The final viscosity occurs during the cooling phase when heating is stopped, and starch particles, such as amylose, are recombined and viscosity increases [13]. The final viscosity differed significantly between the two treatments. The setback viscosity is associated with starch retrogradation and is positively correlated with retrogradation speed [13]. As a result, plasma-exposed brown and white cooked rice showed different trends in RVA viscosity. The setback viscosities of all samples were similar. However, the setback value of white cooked rice after APP treatment decreased slightly and then decreased significantly (p < 0.05) after the 20-min treatment. Therefore, when white cooked rice is treated with APP, cooking stability increases and taste can be maintained for a long time.
Table 2.
Pasting characteristics of brown and white cooked rice following treatment with atmospheric pressure plasma
| Samples | Treatment time (min) | Pasting characteristics (unit RVU1) | ||||
|---|---|---|---|---|---|---|
| Peak viscosity | Trough viscosity | Break down2 | Final viscosity | Set back3 | ||
| Brown cooked rice | 0 | 158.40 ± 0.53a | 106.17 ± 2.15a | 52.23 ± 1.78a | 190.07 ± 1.64a | 31.67 ± 1.175 |
| 5 | 151.50 ± 5.30b | 99.90 ± 5.10b | 51.53 ± 0.25ab | 182.73 ± 3.05b | 31.27 ± 2.25 | |
| 10 | 152.53 ± 0.97ab | 102.53 ± 0.61ab | 50.00 ± 0.36bc | 183.40 ± 1.35b | 30.87 ± 0.49 | |
| 20 | 155.13 ± 3.02ab | 106.97 ± 2.75a | 48.23 ± 0.85c | 187.67 ± 2.35a | 32.50 ± 0.82 | |
| SEM4 | 2.53 | 2.54 | 0.82 | 1.80 | 1.11 | |
| White cooked rice |
0 | 194.73 ± 1.89b | 156.93 ± 0.65a | 37.80 ± 1.82c | 236.43 ± 2.56b | 41.73 ± 1.72a |
| 5 | 189.80 ± 1.04c | 150.27 ± 1.50b | 39.53 ± 2.21bc | 229.87 ± 1.79c | 40.13 ± 1.50a | |
| 10 | 201.40 ± 1.04a | 158.53 ± 2.22a | 42.83 ± 1.25b | 241.47 ± 2.61a | 40.10 ± 1.67a | |
| 20 | 201.43 ± 2.57a | 151.37 ± 2.00b | 50.03 ± 2.47a | 234.60 ± 2.88b | 33.17 ± 2.46b | |
| SEM | 1.43 | 1.39 | 1.63 | 2.04 | 1.53 | |
1Rapid Visco Units
2Peak viscosity minus trough viscosity
3Final viscosity minus peak viscosity
4Standard errors of the mean (n = 10)
5Any means in the same column followed by the same letter are not significantly (p < 0.05) different by Duncan’s multiple range test
Textural analysis
APP affected the textural properties of brown and white cooked rice (Table 3). Hardness is important for texture evaluation and consumer acceptability [21]. The results show that the hardness values of brown and white cooked rice were slightly lower after APP than those in the untreated samples (Table 3), but were not significantly different. Thirumdas et al. [22] showed that hardness of brown rice decreases with increased time and power of plasma treatment. Our previous study showed that water absorption rate and α-amylase activity both decrease the hardness of brown rice, which is often prevented despite adding nutritional advantage [11]. The cohesiveness of brown and white cooked rice was significantly (p < 0.05) lower after 20 min of exposure than the control sample. Szczsniak [23] defined cohesiveness as the strength of the internal bonds of food. It is the ratio of the second peak area to the first peak area of the compression cycle. Thirumdas et al. [22] reported that brown rice treated with plasma has decreased cohesiveness. Chewiness of white cooked rice was significantly (p < 0.05) lower than that in the control sample. Chewiness is the number of chews required for cooked rice to be suitable for swallowing during mastication [24]. The decrease in hardness is directly correlated to chewiness, which shows that less work is required to chew the rice [22].
Table 3.
Textural profile analysis of brown and white cooked rice following treatment with atmospheric pressure plasma
| Samples | Treatment time (min) | Hardness (N) | Adhesiveness (mJ) | Springiness | Gumminess (N) | Chewiness (N) | Cohesiveness |
|---|---|---|---|---|---|---|---|
| Brown cooked rice | 0 | 2.14 ± 0.26ns2 | 0.02 ± 0.01ns | 0.77 ± 0.02ns | 0.88 ± 0.12ns | 0.69 ± 0.11ns | 0.41 ± 0.03a |
| 5 | 2.18 ± 0.34 | 0.02 ± 0.01 | 0.79 ± 0.03 | 0.95 ± 0.16 | 0.76 ± 0.15 | 0.44 ± 0.04a | |
| 10 | 2.04 ± 0.44 | 0.02 ± 0.01 | 0.76 ± 0.03 | 0.83 ± 0.21 | 0.63 ± 0.16 | 0.41 ± 0.05a | |
| 20 | 2.00 ± 0.34 | 0.02 ± 0.01 | 0.75 ± 0.05 | 0.80 ± 0.20 | 0.60 ± 0.18 | 0.39 ± 0.05b | |
| SEM1 | 0.22 | 0.01 | 0.01 | 0.09 | 0.07 | 0.02 | |
| White cooked rice | 0 | 0.87 ± 0.09ns | 0.01 ± 0.01ab | 0.80 ± 0.03a | 0.41 ± 0.06a | 0.34 ± 0.05a | 0.48 ± 0.04b |
| 5 | 0.81 ± 0.13 | 0.01 ± 0.01b | 0.79 ± 0.04a | 0.41 ± 0.09a | 0.33 ± 0.09a | 0.50 ± 0.07ab | |
| 10 | 0.81 ± 0.06 | 0.01 ± 0.01ab | 0.82 ± 0.02a | 0.44 ± 0.05a | 0.36 ± 0.05a | 0.54 ± .04a | |
| 20 | 0.82 ± 0.08 | 0.02 ± 0.01a | 0.73 ± 0.05b | 0.34 ± 0.07b | 0.25 ± 0.06b | 0.41 ± 0.06c | |
| SEM | 0.04 | 0.01 | 0.02 | 0.03 | 0.03 | 0.023 |
a–cDifferent letters within same column differ significantly (p < 0.05)
1Standard errors of the mean (n = 10)
2Any means in the same column followed by the same letter are not significantly (p < 0.05) different by Duncan’s multiple range test
Reducing sugars
The reducing sugar contents showed brown cooked rice revealed significantly higher values treated with APP (p < 0.05) than those in the untreated sample (Table 1). Reducing sugar contents of brown cooked rice increased significantly after 5, 10, and 20 min of APP treatment, with a maximum value 1.44-fold greater than the untreated samples after the 5 min treatment. On the other hand, the reducing sugar content measured in white cooked rice was significantly smaller (p < 0.05) than that of the untreated samples. Sarangapani et al. [25] reported that reducing sugar content of parboiled rice flour increase after treatment with low pressure plasma. This increase in reducing sugars occurs due to dextrinization of starch with plasma [26].
Lipid oxidation
The TBARS values of untreated brown and white cooked rice were 0.49 and 0.04 mg malondialdehyde/kg sample, respectively (Table 1). The TBARS values of brown and white cooked rice increased slightly in response to APP, compared to those of the untreated samples. The TBARS value of brown cooked rice was higher than that of white cooked rice because brown rice has higher fat content than white rice. The lipid oxidation occurring in brown and white cooked rice treated by APP, may possible affect the sensory characteristics (data not shown). Some studies have also found increases in TBARS of animal-originating products, such as cheese and meat products treated with APP [9, 10]. However, very few studies have examined lipid oxidation of rice treated with APP. Radicals generated by APP accelerate the production of peroxides, this may be the cause of the increase of TBARS value under these conditions [17, 27] reported that reactive oxygen species are produced by plasma-induced oxidative stress; their study proved that intact E. coli cells and isolated membrane-rich fractions undergo lipid peroxidation in a manner that is proportional to the amount of plasma.
In conclusion, APP can improve the microbial safety of brown and white cooked rice. However, the APP protocol must be refined further to minimize the minor changes it makes in the quality attributes of brown and white cooked rice. Thus, further researches are proposed to assess its quality, such as a sensory characteristic of brown and white cooked rice after APP, and its suitability for industrial applications.
Acknowledgements
This study was supported by a Grant (PJ01255601) from the AGENDA Program, Rural Development Administration, Republic of Korea.
References
- 1.Luo K, Wang J, Kim SY, Kim SH, Oh DH. Experimental studies and modelling the behaviour of anaerobic growth of Clostridium perfrigens in cooked rice under non-isothermal condition. Food Control. 2017;71:137–142. doi: 10.1016/j.foodcont.2016.06.029. [DOI] [Google Scholar]
- 2.Little CL, Barnes J, Mitchell RT. Microbiological quality of takeaway cooked rice and chicken sandwiches: effectiveness food hygiene training of the management. Commun. Dis. Public Health. 2002;5:289–298. [PubMed] [Google Scholar]
- 3.Ha JH, Kim HJ, Ha SD. Effect of combined radiation and NaOCl/ultrasonication on reduction of Bacillus cereus spores in rice. Radiat. Phys. Chem. 2012;81:1177–1180. doi: 10.1016/j.radphyschem.2012.01.026. [DOI] [Google Scholar]
- 4.Fangio MF, Roura SI, Fritz R. Isolation and identification of Bacillus spp. and related genera from different starchy foods. J. Food Sci. 2010;75:218–221. doi: 10.1111/j.1750-3841.2010.01566.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Lee YK, Yang JY. Analysis on hazard microorganisms in raw materials and processing environment for Sunsik manufacture. J. Food Hyg. Safety. 2011;26:410–416. [Google Scholar]
- 6.Fernández A, Noriega E, Thompson A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013;33:24–29. doi: 10.1016/j.fm.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Ramazzina I, Berardinelli A, Rizzi F, Tappi S, Ragni L, Sacchetti G, Rocculi P. Effect of cold plasma treatment on physico-chemical parameters and antioxidant activity of minimally processed kiwifruit. Postharvest Biol. Technol. 2015;107:55–65. doi: 10.1016/j.postharvbio.2015.04.008. [DOI] [Google Scholar]
- 8.Moreau M, Orange N, Feuilloley MGJ. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol. Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Jayasena DD, Kim HJ, Yong HI, Park S, Kim K, Choe W, Jo C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attibutes. Food Microbiol. 2015;46:51–57. doi: 10.1016/j.fm.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Yong HI, Kim HJ, Park S, Kim K, Choe W, Yoo SJ, Jo C. Pathogen inactivation and quality changes in sliced cheddar cheese treated using flexible thin-layer dielectric barrier discharge plasma. Food Res. Int. 2015;69:57–63. doi: 10.1016/j.foodres.2014.12.008. [DOI] [Google Scholar]
- 11.Lee KH, Kim HJ, Woo KS, Jo C, Kim JK, Kim SH, Park HY, Oh SK, Kim WH. Evaluation of cold plasma treatments for improved microbial and physicochemical qualities of brown rice. Food Sci. Technol. 2016;73:442–447. [Google Scholar]
- 12.Kim HJ, Yong HI, Park S, Kim K, Kim TH, Choe W, Jo C. Effect of atmospheric pressure dielectric barrier discharge plasma on the biological activity of naringin. Food Chem. 2014;160:241–245. doi: 10.1016/j.foodchem.2014.03.101. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Oh SK, Lee JH, Yoon MR, Choi IS, Lee DH, Kim YG. Changes in quality properties of brown rice after germination. Korean J. Food Sci. Technol. 2012;44:300–305. doi: 10.9721/KJFST.2012.44.3.300. [DOI] [Google Scholar]
- 14.Lee KH, Choi HS, Hwang KA, Song J. Changes in Biological Qualities of Soy Grits Cheonggukjang by Fermentation with β-Glucosidase-Producing Bacillus Strains. J. Korean Soc. Food Sci. Nutr. 2016;45:702–710. doi: 10.3746/jkfn.2016.45.5.702. [DOI] [Google Scholar]
- 15.Lee H, Yong HI, Kim HJ, Choe W, Yoo SJ, Jang EJ, Jo C. Evaluation of the microbiological safety, quality changes, and genotoxicity of chicken breast treated with flexible thin-layer dielectric barrier discharge plasma. Food Sci. Biotechnol. 2016;25:1189–1195. doi: 10.1007/s10068-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J, Huang K, Wang J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control. 2015;50:482–490. doi: 10.1016/j.foodcont.2014.09.037. [DOI] [Google Scholar]
- 17.Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011;55:1053–1062. doi: 10.1128/AAC.01002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korachi M, Aslan N. The effect of atmospheric pressure plasma corona discharge on pH, lipid content and DNA of bacterial cells. Plasma Sci. Technol. 2011;13:99. doi: 10.1088/1009-0630/13/1/20. [DOI] [Google Scholar]
- 19.Shi XM, Zhang GJ, Wu XL, Li YX, Ma Y, Shao XJ. Effect of low-temperature plasma on microorganism inactivation and quality of freshly squeezed orange juice. IEEE T. Plasma Sci. 2011;39:1591–1597. doi: 10.1109/TPS.2011.2142012. [DOI] [Google Scholar]
- 20.Tang YZ, Lu XP, Laroussi M, Dobbs FC. Sublethal and killing effects of atmospheric-pressure, nonthermal plasma on eukaryotic microalgae in aqueous media. Plasma Process. Polym. 2008;5:552–558. doi: 10.1002/ppap.200800014. [DOI] [Google Scholar]
- 21.Chen HH, Chen Y, Chang CH. Evaluation of physicochemical properties of plasma treated brown rice. Food Chem. 2012;135:74–79. doi: 10.1016/j.foodchem.2012.04.092. [DOI] [Google Scholar]
- 22.Thirumdas R, Saragapani C, Ajinkya MT, Deshmukh RR, Annapure US. Influence of low pressure cold plasma on cooking and textural properties of brown rice. Innov. Food Sci. Emerg. 2016;37:53–60. doi: 10.1016/j.ifset.2016.08.009. [DOI] [Google Scholar]
- 23.Szczesniak AS. Classification of textural characteristics. J. Food Sci. 1963;28:385–389. doi: 10.1111/j.1365-2621.1963.tb00215.x. [DOI] [Google Scholar]
- 24.Prasert W, Suwannaporn P. Optimization of instant jasmin rice process and its physicochemical properties. J. Food Eng. 2009;95:54–61. doi: 10.1016/j.jfoodeng.2009.04.008. [DOI] [Google Scholar]
- 25.Sarangapani C, Thirumdas R, Devi Y, Trimukhe A, Deshmukh RR, Annapure US. Effect of low-pressure plasma on physico-chemical and functional properties of parboiled rice flour. Food Sci. Technol. 2016;69:482–489. [Google Scholar]
- 26.Lii CY, Liao CD, Stobinski L, Tomasik P. Behaviour of granular starches in low-pressure glow plasma. Carbohydr. Polym. 2002;49:499–507. doi: 10.1016/S0144-8617(01)00351-4. [DOI] [Google Scholar]
- 27.Kim HJ, Yong HI, Park S, Kim K, Choe W, Jo C. Microbial safety and quality attributes of milk following treatment with atmospheric pressure encapsulated dielectric barrier discharge plasma. Food Control. 2015;47:451–456. doi: 10.1016/j.foodcont.2014.07.053. [DOI] [Google Scholar]