Abstract
The present study is focused on probiotic characterization of four yeasts viz. Pichia barkeri VIT-SJSN01, Yarrowia lipolytica VIT-ASN04, Wickerhamomyces anomalus VIT-ASN01 and Saccharomyces cerevisiae VIT-ASN03 isolated from food samples based on their auto-aggregation, co-aggregation ability and haemolytic activity. All the yeast strains showed good self-adhering and co-adhering potentiality with a value index of greater than 85%. None of the strains exhibited haemolysis which confirmed their non-pathogenic nature. Yeast strains were encapsulated in sodium alginate, sodium alginate coated with chitosan and sodium alginate-gelatinized with starch. Size and morphology of the beads and capsules were determined using SEM analysis. Encapsulation output and viability under storage condition was investigated. It was found that probiotic yeasts encapsulated in sodium alginate beads, chitosan coated beads and microcapsules showed better survival to simulated gastrointestinal conditions compared to free cells.
Keywords: Auto-aggregation, Co-aggregation, Microencapsulation, Probiotic yeast, Sodium alginate
Introduction
Probiotics are supplements of live viable micro-organisms along with other beneficial substances that once administrated to human or animal produce physiological effects and help in the colonization of the intestinal region, which is beneficial to the host as well as harmful to pathogenic bacteria [1]. Most of the commercialized probiotics contain bacteria, such as Lactobacillus, Bifidobacterium and Bacillus. [2]. Very few probiotics contain yeast in its composition. Saccharomyces boulardii is the only yeast commercialized as probiotic for human so far.
Yeasts have already been reported as GRAS and QPS (Qualified presumption of safety) due to many attractive properties such as (a) rich in nutrition, (b) resist low pH and bile conditions, (c) antagonistic effects along with inhibition of many bacterial toxins, (d) antibiotic resistant ability, (e) immune modulation, (f) cholesterol assimilation and better antioxidant status [3]. There are many prerequisite conditions that must be met for microorganisms to be considered as a true probiotic, the most important of which is the survival of the potentially beneficial microorganisms in the human gut [4]. Important criteria used for selection of potential probiotic organisms include their survival in the gastrointestinal environment and they must be able to present beneficial functions as colony resistance, immunomodulation or nutritional contribution of the normal gastrointestinal microbiota when ingested by human and animal hosts. A criterion of utmost importance in selecting a potential candidate is the capability to adhere to epithelial cells. Auto-aggregation is the ability of the cells to self-adhere to cells of the same kind and colonize the environment. Co-aggregation is the interaction of the cells with different pathogenic bacteria [5]. Investigation of these adherence abilities is required to understand the mechanism behind colonization on the gut cells. Microbial adhesion to hydrocarbons (MATH) test is important to determine the surface hydrophobicity of the microbial cells. A very few studies have been evaluated in terms of hydrocarbon-microbe interaction in yeast cells [6]. Hemolysis is normally related to pathogenesis and yeast is generally considered as non-hemolytic.
Probiotics are susceptible to various environmental stresses such as acidity, O2 stress, heat and storage conditions in probiotic starter cultures and fermented dairy products. A probiotic simply cannot benefit human health, but should also fulfill the requirements of being stable, safe and viable in food products [7]. Encapsulation process is a promising technique for probiotic protection against adverse conditions to which probiotics are exposed. Microencapsulation with hydrocolloids as one of the most modern methods has remarkable effects on probiotic survival. Probiotic encapsulation technology (PET) is one of the promising methodologies which will help in neutralizing the problem of short shelf-life of the product and increased cell viability [8]. Alginate is the most commonly used biomaterial for microencapsulation. Other materials include carrageenan/locust bean gum [9, 10], chitosan [11], whey protein [12], cellulose acetate phthalate [13], gelatin [14], and starch [15]. The use of alginate has shown a remarkable effect on probiotic survival. Alginate has several advantages such as easy to handle and prepare, non-toxic, cost effective. Gelatinized starch can be used as a filler material in alginate capsules. The integration of starch can provide a uniform mixture to alginate providing good adhering capacity; facilitate capsule formation and improving the viability of the probiotics [16]. The mechanical strength of the alginate matrix needs to be enhanced with the aid of coating materials. Chitosan is a widely used coating agent other than poly-l-lysine. In the present study, the potentiality of probiotic yeasts has been evaluated based on their capacity of colonization, adherence capability and hemolytic activity. Survival of encapsulated probiotic yeast strains under simulated gastro intestinal condition has also been tested.
Materials and methods
Microorganisms
Yeast strains P. barkeri VIT-SJSN01, Y. lipolytica VIT-ASN04, W. anomalus VIT-ASN01 and S. cerevisiae VIT-ASN03 were isolated from different food sources viz. avocado, curd, mosambi, sweet lime and pineapple fruit and cultured in yeast extract peptone dextrose medium. They were purified and stored at 4 °C for further study.
Chemicals
Sodium alginate, pepsin, pancreatin, yeast extract, peptone, dextrose and agar (Himedia, Mumbai, Maharashtra, India) Chitosan (low molecular weight) (Sigma-Aldrich, USA) n-hexadecane, diethyl ether and chloroform (SRL chemicals, Chennai, Tamil Nadu, India) were purchased.
Preparation of cell suspension
The yeast strains obtained in logarithmic phase were grown in YEPD medium at 25 °C for 36 h. The cell biomass was harvested by centrifugation (Remi, C-24 BL, Mumbai, Maharashtra, India) at 5000 rpm/10 min followed by washing the cells twice with 0.9% sterile saline or phosphate buffer saline (0.01 M, pH 6.8) and then used for further experiments.
Auto-aggregation test
The analysis of auto-aggregation was carried out following the method of Lohith and Anu [17] with minor modifications. The cell pellets were obtained after washing and resuspending the cells with PBS to obtain a final cell density of around 1 × 109 CFU/ml at 600 nm (UV-2450, Shimadzu, Japan). Each yeast suspension (4 ml) was divided into sterile test tubes. The tubes were vortexed and incubated for 3, 5 and 24 h respectively. Absorbance was read at 600 nm against the blank solution. The auto-aggregation (%) was calculated using the following formula.
where At—Absorbance readings at different time points (t = 3, t = 5 and t = 24); A0—Absorbance readings taken initially.
Co-aggregation test
Co-aggregation ability of yeast stains with bacterial pathogens was evaluated following the method of Jancovic et al. [18] with modifications. Bacterial pathogens viz. Escherichia coli and Staphylococcus aureus were obtained in log phase culture. The yeast and the pathogenic cell suspension were prepared with the final density of 1 × 109 CFU/ml at 600 nm. 2 ml each pathogen and the yeast cells were dispensed into sterile tubes. The tubes were thoroughly mixed and incubated for 60 min. The absorbance was read at 600 nm. Control tubes each of pathogens and the yeast cells were prepared and absorbance was read individually. The percentage of co-aggregation was determined according to the formula
where Ax—represents absorbance of the yeast strain, Ay—represents absorbance of the pathogen under study; A (x + y)—represents absorbance of the mixture of both.
Adhesion to hydrophobic solvent
The cell surface hydrophobicity of yeast strains was measured by measuring microbial adhesion to hydrocarbons as described by Sica et al. [19] with minor modifications. Four milliliter of yeast cell suspension (OD 1.0 at wave length 600 nm) were added to 1 ml of each organic solvent, viz., n-hexadecane, diethyl ether and chloroform separately. The tubes were vortexed for 2 min to ensure mixing and the mixture was allowed to stand for 60 min to confirm the complete separation of two phases. The aqueous phase was gently separated out and the OD was read at 600 nm. Decrease in the OD of aqueous phase was taken as measurement of cellular surface hydrophobicity (H %) and the percentage of cells bound to the organic phase was calculated according to the formula as follows:
where ODb is optical density of cell suspension before mixing and ODa is optical density after mixing.
Hemolysis test
The experiment was carried out to determine the hemolytic activity of the yeast cells. The strains were streaked on blood agar plates containing 5% sheep blood and incubated at 37 °C for 24 h. Later hemolysis was checked following the method of Manns et al. [20].
Encapsulation procedure
Encapsulation of yeast strains was done following the standard methodologies. All glassware and solutions used in the protocol were sterilized at 121 °C for 15 min.
Yeast encapsulation in sodium alginate beads
The beads were prepared by extrusion method. The cell suspension was prepared in 0.9% (w/v) saline solution. An equal volume mixture of cell suspension and 4% (w/v) sodium alginate were mixed properly. The suspending mixture was then taken up in a syringe and then dropped slowly into a solution containing 1.5% (w/v) CaCl2. The beads were then left to harden for 45 min. The beads were washed with distilled water to remove excess calcium deposited and then stored in sterile vials at 4 °C.
Yeast encapsulation in sodium alginate beads coated with chitosan
Chitosan (1%, low molecular weight, Sigma Aldrich, India) was used as an additional coating. Chitosan (1%) was acidified with 0.4 ml of glacial acetic acid. The pH was adjusted in the range of 5.7–6.0 using 1 N NaOH. The solution was filtered using whatman filter paper and steam sterilized. The yeast entrapped sodium alginate beads prepared by extrusion technique were then suspended in the chitosan solution and shaken at 100 rpm for 30 min using an orbital shaker. These coated beads were then separated, washed and stored in sterile vials at 4 °C.
Yeast entrapment using emulsification technique
Microencapsulation of yeast isolates was done following the method of Mohammad et al. [21] with minor modifications. Gelatinized starch (2%, Himedia, India) was prepared and boiled until a gel was formed. An equal mixture of 4% (w/v) sodium alginate and probiotic yeast cultures were mixed and starch gel was added into the carrier solution which was then suspended into 250 ml of vegetable oil containing 0.2% (v/v) Tween 80. The whole mixture was shaken for 30 min unless it appeared creamy. One hundred microliter of CaCl2 (1.5%) was added into oil mixture and allowed to stand for 30 min. The capsules formed started settling at the bottom of the flask. The oil layer was then separated and the microcapsules were obtained by centrifuging at 350 rpm for 10 min. The capsules obtained were stored in peptone solution (0.1%) at 4 °C.
Determination of size and morphology of microencapsulated beads
The size and the shape of the encapsulated beads/capsules were analyzed using SEM (FEI Sirion, Eindhoven, Netherlands) and optical microscope. Samples were dehydrated using glutaraldehyde and kept at 4 °C for 3 h and then washed in a series of ethanol (50, 70, 90 and 100%) for 15 min. The samples were dried overnight and then placed in a specimen aluminum tub with the help of double sticky tape and were coated by sputter coater for 2 min at an accelerating voltage of 15 kV.
Encapsulation efficiency
The encapsulation efficiency (EE) was calculated by the following expression:
where Xt is the total amount of probiotic loaded in alginate beads and Xi represents the initial amount of probiotic added in the preparation process.
Gastrointestinal tract (GIT) simulation
Yeast tolerance was tested under simulated GIT conditions following the method of Ayama et al. [22] with modifications. Yeast cells (microencapsulated and free cells) were placed in a tube containing simulated gastric juice (phosphate buffer saline (PBS) pH 2.0 containing 3 mg/l pepsin (Sigma, USA) and incubated at 37 °C at a shaking speed of 50 rpm for 3 h. After incubation, the cells were removed for counting surviving cells and then placed in sterile simulated intestinal juice (PBS pH 8.0 containing 3 mg/ml pancreatic and 1% bile salt (Sigma, USA). The tubes were then incubated at 37 °C for 4 h. After incubation, 1 ml of each isolate was removed and the survival rate was counted by plating the contents of the beads on YEPD agar medium using pour plate method.
Release of the encapsulated cells
The probiotic yeast cells from the beads and capsules were released after treating the beads in 0.1 M PBS (pH 7.6) for 15 min after shaking in an orbital shaker (Remi, India). The cells were serially diluted and then plated using pour plate technique to determine CFU/ml of the untreated beads and capsules [23].
Determination of viability of microencapsulated cells at 4 °C
The entrapped cells were stored at 4 °C for 30 d. The viability of the cells was determined by releasing the contents, further diluting and plating them on YEPD agar (Himedia, India) medium using pour plate method [24].
Evaluation of survival rate of the treated cells
The survival counts of the treated microencapsulated and nonencapsulated cells were determined using the plate count method. The percentage of the survival in CFU/ml was calculated using the standard formula [25].
β-Glucuronidase activity
The β-Glucuronidase activity was performed using the API-ZYM kit (BioMerieux, India) and reading were made according to the manufacture indications [26].
Statistical analysis
Statistical analysis was performed using SPSS software system (Version 21, SPSS Institute Inc., Cary, NC, USA). The results were reported as average values (SD±) from three independent repetitions.
Result and discussion
Auto-aggregation ability
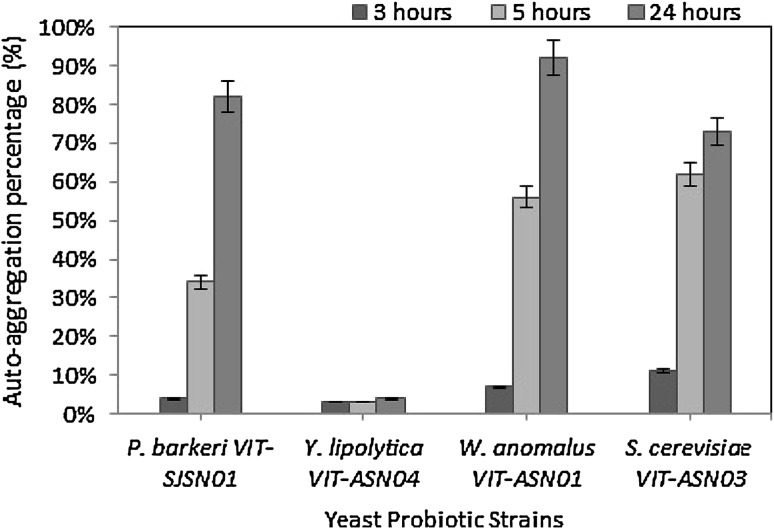
The auto-aggregation ability of the yeast strains is shown in Fig. 1. The isolates showed significant difference in their aggregation properties and most of them showed more than 50% auto-aggregation. W. anomalus VIT-ASN01 showed the highest auto-aggregation (91%) whereas lowest auto-aggregation was noted in Y. lipolytica VIT-ASN04 (4%). Therefore, the majority of the yeast isolates showed greater than 50% auto-aggregation capacity, which can prevent the invasion of various other pathogenic micro-organisms through biofilm formation. This quality of adherence can also cause increased persistence in the gastrointestinal tract. Such adherence capacity is only possible through surface proteins and they are usually strain specific [27].
Fig. 1.
Auto-aggregation ability of yeast strains
Co-aggregation assay
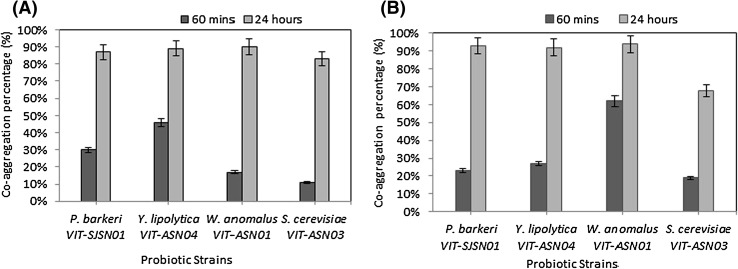
The co-aggregation assay is a reliable method to evaluate the close interaction between probiotic microbes and pathogenic bacteria. The co-aggregation ability of the yeast strains associated with E. coli is shown in Fig. 2(A). All the strains showed aggregation ability more than 80% after 24 h. The yeast strains W. anomalus VIT-ASN01 and Y. lipolytica VIT-ASN04 showed the highest co-aggregation ability (90%) followed by P. barkeri VIT-SJSN01 (87%) and S. cerevisiae VIT-ASN03 (83%) with E. coli. The percentage of co-aggregation ability of yeast strains with S. aureus shown in Fig. 2(B). Three strains have proved to have binding capacity greater than 90%. W. anomalus VIT-ASN01 showed the highest with 94%, followed by P. barkeri VIT-SJSN01 (93%), Y. lipolytica VIT-ASN04 (92%) and the least by S. cerevisiae VIT-ASN03 (68%). This study showed the ability of yeast cells to co-aggregate with other bacteria with a capability of greater than 85%. So, these yeast strains can serve as potential probiotic candidates who can help in preventing bacterial colonization and secreting antimicrobial substances [28].
Fig. 2.
(A) Co-aggregation ability of yeast strains with E. coli, (B) co-aggregation ability of yeast strains with S. aureus
Adhesion to hydrophobic solvent
Hydrophobicity has been considered as one of the main physical interactions during microbial adhesion to epithelial cells. MATH (Modified Adhesion to Hydrocarbons) assay was employed to evaluate the hydrophobic character of the yeast cell surface as shown in Table 1. Cell surface hydrophobicity was measured based on their adhesion to a hydrophobic substratum. The yeast strains showed variable degrees of hydrophobicity. Maximum hydrophobicity was noted in chloroform and diethyl ether compared to n-hexadecane. Y. lipolytica VIT-ASN04 and P. barkeri VIT-SJSN01 showed the maximum hydrophobicity in diethyl ether followed by P. barkeri VIT-SJSN01 in chloroform with 63%. Therefore, P. barkeri VIT-SJSN01 has been proved to be highly hydrophobic in nature due to its very high hydrophobicity values (> 50%) followed by W. anomalus VIT-ASN01 and Y. lipolytica VIT-ASN04. S. cerevisiae VIT-ASN03 showed the least hydrophobicity nature. High percentage of adhesion in chloroform signifies that the yeast strains may serve as electron donor while those with high values in diethyl may act as electron acceptor [29].
Table 1.
Hydrophobicity of yeast strains to different hydrocarbons
| S. no. | Yeast strains | Hydrophobicity (%) | ||
|---|---|---|---|---|
| Chloroform | n-hexadecane | Diethyl ether | ||
| 1 | P. barkeri VIT-SJSN01 | 63 ± 0.7 | 46 ± 0.9 | 74 ± 0.8 |
| 2 | Y. lipolytica VIT-ASN04 | 13 ± 0.9 | 22 ± 0.7 | 96 ± 0.9 |
| 3 | W. anomalus VIT-ASN01 | 59 ± 0.9 | 22 ± 0.8 | 42 ± 0.9 |
| 4 | S. cerevisiae VIT-ASN03 | 28 ± 0.8 | 04 ± 0.03 | 22 ± 0.8 |
Average values (SD±) from three independent repetitions are presented
Hemolysis test
The yeast strains were tested for haemolytic activity. None of the isolates exhibited haemolysis (clear blood lysis zones) in blood agar plates which proved that the yeast strains under study are non-pathogenic.
Encapsulation of probiotic yeasts
Size, morphology and encapsulation efficiency of alginate beads/capsules
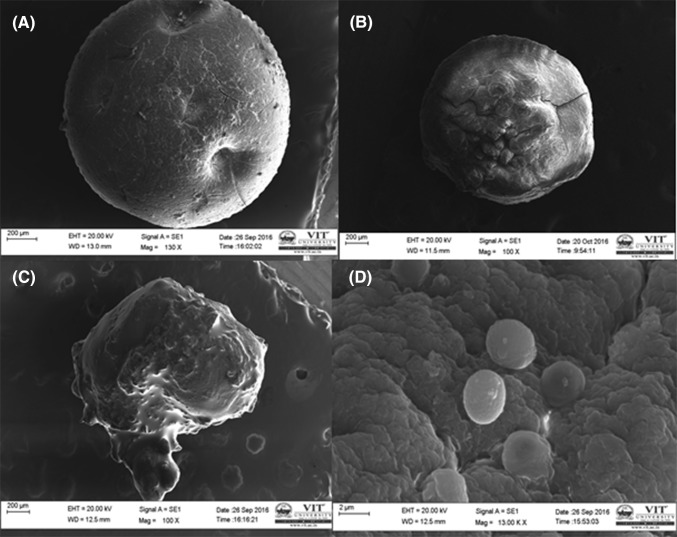
The size of the beads/capsules along with the encapsulation efficiency of alginate beads/capsules are shown in Table 2. No significant difference in bead size was noted in case of sodium alginate beads as well as beads coated with chitosan. The microcapsules made from emulsion technique showed smaller size. Encapsulation efficiency of alginate beads was found to be maximum (94.6%) in case of sodium alginate beads coated with chitosan. The shape of the beads and capsules help in understanding the surface texture of the encapsulated material. Figure 3(A–C) shows the SEM images of the beads and microcapsules. Significant the difference in morphology was noted between sodium alginate beads, chitosan coated bead, and microcapsule. The shape of sodium alginate bead was found to be more spherical and uniform in nature [Fig. 3(A)]. The shape of the chitosan coated sodium alginate bead was found to be rough in texture [Fig. 3(B)]. The image of sodium alginate microcapsule gelatinized with starch is shown in Fig. 3(C). These microcapsules are rough and not uniform in size. The successful entrapment of yeast cells in sodium alginate layer which defines the efficiency and accuracy of the process [Fig. 3(D)].
Table 2.
Size, encapsulation efficiency of alginate beads/capsules and the viability of encapsulated yeast cells stored at 4 °C for 30 days
| S. no. | Encapsulated beads/capsules | Size (mm) | Encapsulation efficiency (%) | Dose of yeast strain (CFU/ml) | Dose of yeast strain after 30 days (CFU/ml) | Viability (%) |
|---|---|---|---|---|---|---|
| 1 | Sodium alginate beads | 2.94 | 93 ± 0.8 | 1.35 × 106 | 1.05 × 106 | 77 ± 0.9 |
| 2 | Sodium alginate beads coated with chitosan | 2.96 | 94 ± 0.9 | 1.41 × 106 | 1.34 × 106 | 95 ± 0.7 |
| 3 | Sodium alginate-gelatinized starch microcapsules | 1.70 | 90 ± 0.9 | 3.26 × 106 | 2.96 × 106 | 91 ± 0.8 |
The CFU/ml indicates the average of the four strains taken together
Average values (SD±) from three independent repetitions are presented
Fig. 3.
Scanning electron photomicrograph: (A) yeast encapsulated in sodium alginate bead, (B) chitosan coated sodium alginate bead containing yeast, (C) microcapsules containing yeast made from emulsification technique, (D) yeast cells entrapped in sodium alginate matrices
Viability of the encapsulated yeast cells
The viability of the encapsulated yeast cells stored at 4 °C was tested. Based on the recommendation of International Dairy Federation (IDF), the minimum number of probiotic cells (CFU/ml) in an appropriate food product should have an index of ≥ 106 CFU/ml at the time of consumption [8]. Table 2 shows the viability of yeast cells after 30 d of storage at 4 °C. No contamination was noted when plated on appropriate media. This ensures the reliability and the efficiency of the technique used. The results indicated that beads coated with chitosan showed the highest percent viability (95%) followed by microcapsules (91%) and then sodium alginate beads (77%). Chitosan acted as a good barrier to prevent cell release and the carboxylate groups of alginate and the ammonium groups of chitosan when mixed with each other, electrostatic interaction was formed between both of them which lead to suppression of cell release [30]. The microcapsules formed through emulsification technique involved the use of starch as a carrier and supplemented nutrient to the embedded yeast cells. These yeast cells utilized the starch and hence lead to better preservation [31].
Survival of free and encapsulated yeast probiotics in simulated gastro-intestinal conditions
The survival of encapsulated probiotic strains depends on the capability of surviving and resisting in gastric fluid juice and release of the cells into the intestinal fluid. In the present study, immobilized cells have shown greater viability compared to free cells with 30–40% reduction in cell survival (Table 3). Additional coating with chitosan did not improve the viability of all the strains when compared to immobilized sodium alginate beads. The increase in cell survival (2%) was noted in case of P. barkeri VIT-SJSN01 and W. anomalus VIT-ASN01 while S. cerevisiae VIT-ASN03 showed reduction (5%) in survival rate. This results show that chitosan is not mechanically resistant to gastrointestinal conditions. There is report on the inefficiency of chitosan as a coating material in cell survival [32]. Chitosan is known to increase mechanical strength of sodium alginate that leads to zero cell loss which hinders the cell release completely into the medium leading to loss of viability. Microcapsules prepared from emulsification technique showed lowest viability when compared to sodium alginate and chitosan coated beads. In case of W. anomalus VIT-ASN01, microcapsules showed high viability (80%) compared to other strains. Overall, the extrusion technology showed much higher survival of encapsulated yeast cells compared to emulsification technology. The use of emulsifiers and oil can be toxic to the cells and hence can interfere. These capsules when tasted can also give a bad mouth feel and therefore cannot be considered to get incorporated [33].
Table 3.
Survival of yeast strains under simulated gastro-intestinal conditions
| Yeast strains | Type of encapsulation | CFU/ml of the treated encapsulation | CFU/ml of the untreated non encapsulation | Cell survival (%) |
|---|---|---|---|---|
| P. barkeri VIT-SJSN01 | Sodium alginate | 2.07 × 106 | 3.04 × 106 | 69 ± 0.7 |
| Chitosan coated sodium alginate | 1.65 × 106 | 2.03 × 106 | 71 ± 0.9 | |
| Sodium alginate-gelatinized starch | 2.45 × 106 | 3.9 × 106 | 62 ± 0.9 | |
| Free cells | 1.56 × 106 | 3.20 × 106 | 48 ± 0.8 | |
| Y. lipolytica VIT-ASN04 | Sodium alginate | 2.34 × 106 | 2.98 × 106 | 78 ± 0.7 |
| Chitosan coated sodium alginate | 1.12 × 106 | 1.55 × 106 | 72 ± 0.8 | |
| Sodium alginate-gelatinized starch | 2.31 × 106 | 3.99 × 106 | 58 ± 0.8 | |
| Free | 1.67 × 106 | 3.20 × 106 | 52 ± 0.9 | |
| W. anomalus VIT-ASN01 | Sodium alginate | 1.97 × 106 | 2.51 × 106 | 78 ± 0.9 |
| Chitosan coated sodium alginate | 1.96 × 106 | 2.48 × 106 | 81 ± 0.9 | |
| Sodium alginate-gelatinized starch | 1.20 × 106 | 1.52 × 106 | 80 ± 0.8 | |
| Free | 2.1 × 106 | 4.07 × 106 | 52 ± 0.8 | |
| S. cerevisiae VIT-ASN03 | Sodium alginate | 2.7 × 106 | 3.64 × 106 | 75 ± 0.8 |
| Chitosan coated sodium alginate | 3.4 × 106 | 4.47 × 106 | 70 ± 0.9 | |
| Sodium alginate-gelatinized starch | 4.54 × 106 | 7.3 × 106 | 63 ± 0.7 | |
| Free | 3.20 × 106 | 5.44 × 106 | 59 ± 0.9 |
Average values (SD±) from three independent repetitions are presented
β-Glucuronidase activity
None of the yeast strains showed β-Glucuronidase activity, as determined by the API-ZYM test (data not shown). All the yeast strains are identified as a safe bio resources.
It can be concluded that 4 yeast strains viz. P. barkeri VIT-SJSN01, Y. lipolytica VIT-ASN04, W. anomalus VIT-ASN01 and S. cerevisiae VIT-ASN03 were found to possess the desirable in vitro probiotic properties based on their auto-aggregation, co-aggregation ability and hemolytic activity. Therefore, the present approach of using encapsulated yeast probiotics may be important for the delivery of probiotic cultures in human system. Among the three types of encapsulation, sodium alginate beads and chitosan coated sodium alginate beads formed through extrusion technique showed better results under simulated gastrointestinal condition compared to microcapsules formed by emulsion technique.
Acknowledgements
The authors are grateful to VIT University for providing the necessary laboratory facilities and instrumental facilities.
References
- 1.Juliana C, Augusto J, Paulo M, Gustavo V, Jose N. Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express. 2013;3:31–42. doi: 10.1186/2191-0855-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obradovic NS, Krunic TC, Trifkovic KT, Bulatovic ML, Rakin MP, Bugarski BM. Influence of chitosan coating on mechanical stability of biopolymer carriers with probiotic starter culture in fermented whey beverages. Int. J. Polym. Sci. 2015;5:1–8. doi: 10.1155/2015/732858. [DOI] [Google Scholar]
- 3.Pennacchia C, Blaiotta G, Pepe O, Villani F. Isolation of Saccharomyces cerevisiae strains from different food matrices and their preliminary selection for a potential use as probiotics. J. Appl. Microbiol. 2008;6:1919–1928. doi: 10.1111/j.1365-2672.2008.03968.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 5.Jankovic T, Frece J, Abram M, Gobin I. Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int. J. Sanit. Eng. Res. 2012;6:1–9. [Google Scholar]
- 6.Gaurav S. Bacterial Hydrophobicity: Assessment Techniques, Applications and Extension to Colloids. An Abstract of Dissertation, Oregon State University (2010)
- 7.Gbassi GK, Vandamme T. Probiotic encapsulation technology: from microencapsulation to release into the gut. Pharm. J. 2012;4:149–163. doi: 10.3390/pharmaceutics4010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Etchepare MDA, Barin JS, Cichoski AJ, Jacob-Lopes E, Wagner R, Fries LLM, de Menezes CR. Microencapsulation of probiotics using sodium alginate. Ciênc. Rural. 2015;45:1319–1326. doi: 10.1590/0103-8478cr20140938. [DOI] [Google Scholar]
- 9.Trindade F, Grosso CRF. Microencapsulation of L. acidophilus (La-05) and B. Lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. J. Microencapsul. 2002;19:485–494. doi: 10.1080/02652040210140715. [DOI] [PubMed] [Google Scholar]
- 10.Doleyres Y, Fliss I, Lacroix C. Continuous production of mixed lactic starters containing probiotics using immobilized cell technology. Biotechnol. Progr. 2004;20:145–150. doi: 10.1021/bp020096w. [DOI] [PubMed] [Google Scholar]
- 11.Yonekura L, Sun H, Soukoulis C, Fisk I. Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fibre by spray drying: technological characterization, storage stability and survival after in vitro digestion. J. Funct. Foods. 2014;6:205–214. doi: 10.1016/j.jff.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dolly P, Anishaparvin A, Joseph GS, Anandharamakrishnan C. Microencapsulation of Lactobacillus plantrum by spray-freeze-drying method and evaluation of survival in simulated gastrointestinal conditions. J. Microencapsul. 2011;28:568–574. doi: 10.3109/02652048.2011.599435. [DOI] [PubMed] [Google Scholar]
- 13.Adhikari K, Mustapha A, Grun IU. Survival and metabolic activity of microencapsulated Bifidobacterium longum in stirred yogurt. J. Food Sci. 2003;68:275–280. doi: 10.1111/j.1365-2621.2003.tb14152.x. [DOI] [Google Scholar]
- 14.Rokka S, Rantamaki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur. Food Res. Technol. 2010;231:1–12. doi: 10.1007/s00217-010-1246-2. [DOI] [Google Scholar]
- 15.Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 2000;62:47–55. doi: 10.1016/S0168-1605(00)00380-9. [DOI] [PubMed] [Google Scholar]
- 16.Pedroso DL, Dogenski M, Thomazini M, Heinemann RJB, Favaro-Trindade CS. Microencapsulation of Bifidobacterium animalis subsp. lactis and Lactobacillus acidophilus in cocoa butter using spray chilling technology. Braz. J. Microbiol. 2013;44:777–783. doi: 10.1590/S1517-83822013000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maria C, Izaskun M, Maria V. Encapsulation technology to protect probiotic bacteria. Spain: Tecnalia Parque Tecologico de Alava; 2012. [Google Scholar]
- 18.Lohith K, Anu A. In vitro probiotic characterization of yeasts of food and environmental origin. Int. J. Probio. Prebio. 2014;9:1–6. [Google Scholar]
- 19.Jankovic T, Frece J, Abram M, Gobin I. Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int. J. Sanit. Eng. Res. 2012;6:19–24. [Google Scholar]
- 20.Sica MG, Brugnoni LI, Marucci PL, Cubitto MA. Characterization of probiotic properties of lactic acid bacteria isolated from an estuarine environment for application in rainbow trout (Oncorhynchus mykiss, Walbaum) farming. Antonie Van Leeuwenhoek. 2012;101:869–879. doi: 10.1007/s10482-012-9703-5. [DOI] [PubMed] [Google Scholar]
- 21.Manns JM, Mosser DM, Buckley HR. Production of a hemolytic factor by Candida albicans. Infect. Immun. 1994;62:5154–5156. doi: 10.1128/iai.62.11.5154-5156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammad Z, Babak T, Anousheh S, Nima M. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in stimulated human gastro-intestinal condition. Iran. J. Pharm. Res. 2014;13:843–852. [PMC free article] [PubMed] [Google Scholar]
- 23.Ayama H, Sumpavapol P, Chanthachum S. Effect of encapsulation of selected probiotic cell on survival in simulated gastrointestinal tract condition. J. Sci. Technol. 2014;36(3):291–299. [Google Scholar]
- 24.Jobanputra AH, Karode BA, Chincholkar SB. Calcium alginate as supporting material for the immobilization of rifamycin oxidase from Chryseobacterium species. Biotechnol. Bioinf. Bioeng. 2011;1:529–535. [Google Scholar]
- 25.Călinescu I, Chipurici P, Trifan A, Bădoiu C. Immobilization of Saccharomyces cerevisiae for the production of bioethanol. Sci. Bull. Ser. B. 2012;74:33–40. [Google Scholar]
- 26.Zago M, Fornasari ME, Carminati D, Burns P, Suarez V, Vinderola G, Reinheimer J, Giraffa G. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 2011;28:1033–1040. doi: 10.1016/j.fm.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Huiyi S, Weiting Y, Meng G, Xiudong L, Xiaojun M. Microencapsulated probiotics using emulsification technique coupled with internal or external gelation process. Carbohydr. Polym. 2013;96:181–189. doi: 10.1016/j.carbpol.2013.03.068. [DOI] [PubMed] [Google Scholar]
- 28.Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008;226:1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- 29.Del Re B, Busetto A, Vignola G, Sgorbati B, Palenzona DL. Autoaggregation and adhesion ability in a Bifidobacterium suis strain. Lett. Appl. Microbiol. 1998;27:307–310. doi: 10.1046/j.1472-765X.1998.00422.x. [DOI] [PubMed] [Google Scholar]
- 30.Bellon-Fontaine MN, Rault J, Van Oss CJ. Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces. 1996;7:47–53. doi: 10.1016/0927-7765(96)01272-6. [DOI] [Google Scholar]
- 31.Kamalian N, Mirhosseini H, Mustafa S, Manap MYA. Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr. Polym. 2014;111:700–706. doi: 10.1016/j.carbpol.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Dash I, Barik J, Nayak A, Sahoo M, Dethose A, Jhonson E, Kumar S, Jayabalan R. Comparative studies of ethanol production and cell viability: free cells versus immobilized cells. Res. J. Pharm. Biol. Chem. Sci. 2015;6:1708–1714. [Google Scholar]
- 33.Chávarri M, Marañón I, Ares R, Ibáñez FC, Marzo F, del Carmen Villarán M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010;142:185–189. doi: 10.1016/j.ijfoodmicro.2010.06.022. [DOI] [PubMed] [Google Scholar]