Abstract
The phytochemical content of acorn (Quercus aegilops) products (nuts and flour) and by products (shells and leaching waters) regarding their content in total phenols, fatty acids, sodium, potassium and calcium was investigated. Antioxidant activity was also examined. Acorn materials presented high total phenol content (up to 47.6 ± 0.6 mg gallic acid equivalents (GAE)/g dry material), with a substantial amount remaining after leaching (11.6 ± 0.7 mg GAE/g flour), and high DPPH radical scavenging and ferric reducing activity. Their content in potassium, calcium, oleic and linoleic acids was considered significant. Molecular weight distribution of proteins and peptides was also studied and found between 7 and 45 kDa; only for acorn shells a band > 250 kDa appeared. Leaching parameters (time, material size, material to water ratio, temperature, NaCl presence) significantly affected the phytochemical content of the remained leached material.
Electronic supplementary material
The online version of this article (10.1007/s10068-017-0293-x) contains supplementary material, which is available to authorized users.
Keywords: Acorn, Phenols, Antioxidant activity, Fatty acids, Macroelements
Introduction
Use of acorn has a worldwide long history in human nutrition, folk medicine (astringents, anti-diarrhoeals, anti-ulcers and antidotes), animal feed and tanneries [1–6]. Presently, it is used on a small scale as feedstuff, resulting in meat products of high quality (Iberian pigs), and for traditional leather tanning. Since there is potential for many other routes of its exploitation, there emerged a scientific interest for the quality attributes of acorn products such as flour, starch and oil [2, 4, 5, 7]. Recently, acorn extracts have been reported to protect chicken patties against oxidative damage, and preserve organoleptic and quality attributes during chilled storage and reheating [8].
Acorn bears compounds, such as essential amino acids, fatty acids, vitamins, polyphenols and minerals, with well established health, nutritional and functional benefits [1, 9, 10]. Moreover, it is a gluten free source that could be efficiently used towards the development of relevant gluten free products with continuously expanding demand [11]. Additionally, it is a natural material with a long history of nutritional use. Acorns from genus Lithocarpus once comprised more than 50% of the traditional diets of native Americans, while those of genus Quercus (Q.) appear as dietary constituents of diets all over the world [3, 12]. In Europe, up to the early twentieth century, acorns were widely used in the Mediterranean region (mainly in Spain and Italy) furnishing up to 25% of the food consumed by the poorer classes, mainly for bread making or as a coffee substitute [3]. Acorns were also used in Serbia (nineteenth century) with records for their beneficial influence on human health [10]. In addition, Portuguese legislation includes acorn oil in the category of directly alimentary oils [5]. Consequently, acorn is considered to stand out among other natural materials and worth attention in favor of its nutritionally promising content and potency of secure circulation in the markets. It is known that “natural” does not always entail “safety”, when the latter is related to the history of nutritional human use of a natural product.
Numerous species and hybrids, varieties and cultivars exist and the chemical composition of their tissues and respective products are known to vary widely among them [7]. Genetic conditions, environment, clime and sampling parameters are known to significantly differentiate composition and characteristics of a plant material [7, 13]. However for the exploitation of a natural material, knowledge on the variability of its components is vital as industry is interested for standardized resources of ensured quality. Furthermore, most acorn varieties are bitter and need to be leached, similarly to many cereals, legumes and fruits [14], to become more pleasant and less astringent. Leaching practices are empirically carried out [15] and there is no specific procedure or optimized process [16]. Therefore, leaching parameters may significantly differentiate the bioactive content of the final product.
To our knowledge, Q. aegilops (Ithburiensis or Macrolepis), a deciduous drought-resistant Oak, capable of growing on shallow and poor soils [17], has been scarcely studied regarding ethnobotanical, physiological and other matters, whereas information concerning its chemical and nutritional characteristics is missing. The objective of the present study is to examine selective quality characteristics of Q. aegilops products and relevant by-products in view of their exploitation for food, feed, health and biological sectors. In specific, the phenol content, the antioxidant activity, the mineral content (Na, K and Ca), as well as fatty acid profile and molecular weight distribution of proteins and peptides of acorn nuts, acorn flour, and acorn by products such as shells and leaching waters were examined. The effect of leaching parameters on the phytochemical content of acorn material was also studied.
Materials and methods
Materials
Caffeic acid (98%, CAF) was purchased from Riedel de Haën (Seelze, Germany). Gallic acid (99.5%), DPPH· radical (1,1-diphenyl-2-picrylhydrazyl, 90%), 2,4,6-tripyridyl-s-triazine (TPTZ), FeCl3 ·6H2O were from Sigma Chemical Co. (St. Louis, MO, USA). Folin-Ciocalteu reagent and Νa2CO3 (99.8%) were from Panreac Quimica (Barcelona, Spain). Potassium (K) was from Merk (Darmstadt, Germany), whereas sodium (Na) and calcium (Ca) were from Polyscience Niles (Illinois, USA). A prestained broad range protein standard (10–250 kDa) and 4–15% precast linear gradient polyacrylamide gels were obtained from Bio-Rad Laboratories (Hercules, CA, USA). Sodium dodecyl sulfate (SDS) and β-mercaptoethanol were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other common reagents and solvents were of the appropriate purity from various suppliers and the water used was deionized obtained by an ion-exchange resin system (ZALION 2000) with a minimum resistance of 800,000 Ω/cm.
Raw acorn material
Acorn material used for the scope of the study was kindly supplied by the owners of the Red Tractor Farm of Kea Island, Greece. Acorns were collected in October 2011 from Kea. In specific, the following samples were employed in the study: healthy large size unleached acorn nuts (UAN), partially infested smaller size unleached acorn nuts (UIAN), germinated unleached acorn nuts (UGAN), acorn shells (AS), and acorn flour (AF) obtained from healthy large size unleached acorn nuts. Acorn materials (UAN, UIAN, UGAN, AS) were crushed in a kitchen type multi chopper. AF was prepared by the suppliers: after the collection, caps were removed from acorns and acorns were allowed to completely dry outdoors before being stored in net bags. Dry acorns were dehulled and then allowed to stand in water for 20 h (350 L of water for every 50 kg of acorn). The softened acorns were then sliced with a commercial vegetable rotary blade slicer and slices were rinsed and allowed to soak overnight in water (50:1). Water was then drained off. Acorn meal was left to stand in water for 2 days, stirring occasionally and changing water every ~ 20 h. Acorn meal was subsequently dehydrated (40 °C) in a tunnel dryer to ~ 12% w/w moisture content and finally grinded with a hammer mill into flour.
Preliminary screening of acorn extracts
For the selection of the appropriate extraction parameters, acorn material was obtained by grinding acorn nuts (UIAN) with a hammer mill (ΑPEX, Chemical Engineers) and sieving to obtain 0.5–1.0 mm. Extracts were always prepared in triplicate.
Extract by sonication
0.5 g of acorn material was extracted in an ultrasonic bath (Elmas 30H Elmasonic, Singen, Germany; ultrasonic power effective 80 W, ultrasonic frequency 37 kHz) either in 25 mL water or in 25 mL methanol for 30 min at room temperature.
Extract by maceration
0.5 g of acorn material was macerated either in 25 mL water or in 25 mL methanol for 24 h at room temperature under magnetic agitation.
Extract by microwaves
0.5 g of acorn material was extracted upon microwaves (Morris K80201 MW, P.R.C., Energy output 800 W) either in 25 mL water for 1 or 1.5 min or in 25 mL methanol for 1 min. 100 mL Erlenmeyer flasks covered with parafilm were used as extraction vessels. Further increase of extraction time beyond 1.5 and 1 min for water and methanol respectively was not employed to avoid solvent loss.
Acorns leaching process
Leaching was employed on acorn material prepared from acorn nuts (UIAN) either as a whole, crushed (with a kitchen type multi chopper) or milled with a hammer mill (ΑPEX, Chemical Engineers and sieved to obtain 0.5–1.0 mm).
Effect of acorn material (size)
An appropriate amount of whole, crushed or milled material was leached in water (5% w/v acorn to water ratio) for 3 days. The mixture was periodically agitated and the water was renewed every day.
Effect of material to solvent ratio
An appropriate amount (5, 15 or 30% w/v acorn to water ratio) of crushed material was leached in water for 2 days. The mixture was periodically agitated and the water was renewed every day.
Effect of leaching medium
An appropriate amount (5% w/v acorn in water) of crushed material was leached for 2 days in (a) water at room temperature (RT), (b) water at 60 °C and (c) aqueous NaCl solution (3.5% w/v in order to simulate salt content of the seawater) at RT. The mixture was periodically agitated and the water was renewed every day.
Leached materials resulted from the above mentioned leaching procedures were subjected to microwave assisted aqueous extraction. Briefly, 0.5 g of leached material was extracted upon microwaves (Morris K80201MW, P.R.C., Energy output 800 W) in 25 mL water for 1.5 min.
Content in total phenols (TP) determined via the Folin–Ciocalteu assay
Suitable aliquots of acorn extracts or acorn leaching waters were transferred into a 10 mL volumetric flask. Subsequently, 5 mL of water and 0.5 mL of the Folin–Ciocalteu reagent were added. After 3 min, 1 mL of saturated (35% w/v) sodium carbonate solution was added and the mixture was then diluted with water to 10 mL. One hour latter the absorbance was measured at 725 nm against a blank solution. Calibration curves were constructed using standard solutions of gallic acid (15–300 μg/10 mL). TP content was expressed as gallic acid equivalents (GAE).
Antioxidant activity determined via the DPPH· assay
Acorn aqueous extracts were firstly freeze dried (Freeze dryer Gamma 1–20 LMC2, Martin Christ, Osterode, Germany) and the dry residue was then re-dissolved in MeOH (1000 mg/L dry extract weight basis). 2.9 mL of a DPPH· solution (0.1 mM in MeOH) was mixed with 0.1 mL of the methanolic extract. The absorption at 516 nm (A516) was recorded at the beginning of the reaction (t = 0) and after 30 min (t = 30). The results were expressed as % inhibition = [(A516 (t = 0) − A516 (t = 30)) × 100/A516 (t = 0)].
Antioxidant activity determined via the FRAP assay
The FRAP assay was carried out according to Benzie and Strain [18] with some modifications. A mixture containing 3 mL of freshly prepared and pre-warmed (at 37 °C) FRAP reagent and 100 μL of extract was incubated at 37 °C for 20 min and the absorbance was then recorded at 593 nm. The same was applied for CAF solutions (10–300 mg/L) to construct a standard curve. The ferric reducing ability of the examined extracts was expressed as CAF equivalents.
Na, K and Ca content determination via emission flame photometry
The content of the extracts in Na, K and Ca was determined with the aid of a single channel emission flame photometer (model PFP7 Jenway, Essex, England) after selection of the appropriate optical filter for the element being assessed. In each set of measurements under the same experimental conditions (fuel and air flow, sensitivity adjustments) along with the extracts, respective standard solutions of known concentrations (Na: 1–60 mg/L; K: 1–150 mg/L; Ca: 150–1000 mg/L) were also analyzed. The concentration of tested extracts was determined via the constructed calibration curves, depicting light emitted from the flame (direct reading digital instrument) versus concentration of the respective standard. Measurement repeatability was determined in every set of measurements for a standard and an extract and was always satisfactory (CV < 10%, n = 5).
Fatty acid profile
Fatty acid methyl esters (FAMEs) were prepared using n-hexane as an extraction solvent. Lipids were extracted from all samples under investigation following the procedure of Bligh and Dyer [19] as modified by Hanson and Olley [20]. For the determination of fatty acids, 1 μL of the extracted FAMEs was injected with a TriPlus autosampler into a Finnigan Focus gas chromatograph (Thermo Electron, S.p.A., Rodano, Milan, Italy) operating in the splitless mode. The gas chromatograph was equipped with a flame ionization detector and a 60 m × 0.25 mm (i.d.) capillary column (AT-Aquawax; Alltech Associates, Inc., Deerfield, IL, USA) with helium as carrier gas (flow rate: 1.2 mL/min). Injection and detection temperatures were set at 220 and 250 °C, respectively. The oven temperature program was as follows: initial oven temperature 150 °C, holding for 1 min, then raising to 220 °C with a scanning rate of 2 °C/min and holding for 46 min. Retention times of FAMEs standards were used to identify the chromatographic peaks of the sample using the ChromQuest Software (version 4.2.34; Thermo Electron S.p.A., Rodano, Milan, Italy).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Molecular weight (MW) distribution of proteins and peptides was studied with SDS-PAGE. Twenty milligrams of each sample were accurately weighed in Eppendorf tubes and mixed with 500 μL of double-concentrated sample buffer (4 g/100 mL SDS, 20 mL/100 mL glycerol, 10 mL/100 mL β-mercaptoethanol, 0.004 g/100 mL bromophenol blue, 0.125 mol/L Tris–HCl, pH 6.8). The tubes were placed in a boiling waterbath (WB-6; Witeg Labortechnik GmbH, Wertheim, Germany) for 5 min in order to solubilize proteins and subsequently cooled at room temperature. Thirty microliters of each sample were then loaded onto the wells of a 4–15% precast linear gradient polyacrylamide gel (Bio-Rad, Hercules, CA, USA). Electrophoresis was carried out in a Criterion gel electrophoresis cell (Bio-Rad, Hercules, CA, USA) equipped with a power supply unit (E861; Consort nv, Turnhout, Belgium) at a constant amperage of 45 mA for 50 min and room temperature. Staining and destaining of recovered gel was performed as previously described [21]. Images of the destained gel were captured with an Olympus digital camera (C-5060 wide zoom; Olympus Corporation, Center Valley, PA, USA). The MW of separated proteins and peptides was identified using the VisionWorksLS Image Acquisition and Analysis Software (UVP, Inc., Upland, CA, USA) based on the MWs of a prestained protein standard (10–250 kDa).
Statistical analysis
Experiments were carried out in triplicate and results were expressed as mean ± SD. Fatty acid analysis, total phenol content and antioxidant activity data were analyzed using one-way analysis of variance (ANOVA); whenever significant effects were detected (p < 0.05), Tukey’s honestly significant difference (HSD) test was performed. Data were normalized when necessary in order to conform to the requirements (normal distribution and equal variances) for applying this parametric test. Statistical analysis was carried out using the Minitab Statistical Software Package (version 16, Minitab, Inc., State College, PA, USA).
Results and discussion
Preliminary screening for the selection of optimal extraction parameters
Since the extraction parameters may crucially affect the determined phytochemical content of a material [22] various extracts were preliminary prepared and examined as regards their phenol, Na, K and Ca contents (Fig. SI1, Supporting Information section). Different acorn extracts presented TP content in the range of 19–48 mg GAE/g dry material, with the aqueous one prepared upon 1.5 min microwaves to contain almost double the content of TP in comparison to the rest. Regarding mineral content, aqueous extracts showed a significantly better performance in relation to the respective methanolic ones. Aqueous preparations upon 24 h maceration and 1.5 min microwaves presented the highest contents in the examined macroelements (Na: 1 and 2, K: 14 and 11, Ca: 23 and 18 mg/g dry material, for maceration and microwaves, respectively). Therefore, taking into consideration matters of efficacy, cost, convenience, time and the fact that water is an environmental friendly and safe solvent, aqueous extracts prepared upon microwaves for 1.5 min were further employed in the study.
Phenolic content of acorn material
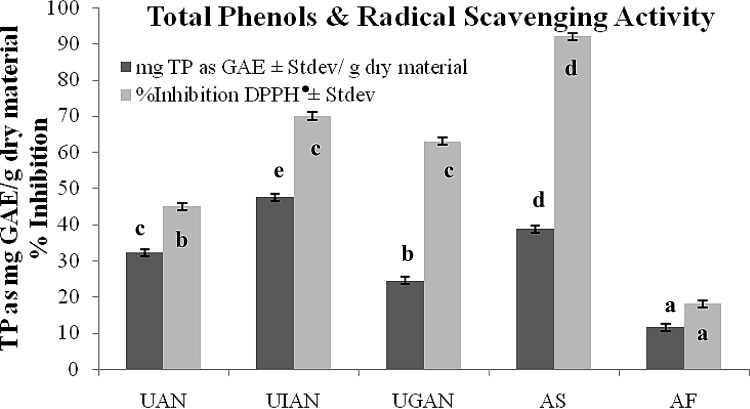
The phenol content of a natural product is amongst its most important quality attributes due to the beneficial role of phenols to human health [23]. Findings presented in Fig. 1 reveal that all studied acorn materials (nuts, flour, shells) are good phenolic sources. UAN and UIAN corresponding to different nut size and degree of infestation presented 32.3 ± 0.5 and 47.6 ± 0.6 mg GAE/g dry material, respectively. Such differences, although statistically significant, are expected for natural products [13]. UGAN presented a lower TP content (Fig. 1) when compared to UAN and UIAN. According to the literature, germination that involves enzymatic activity is used for improving nutritional value, mobilizing nutrients, and decreasing the antinutritional content in cereals and may lead to phenol reduction [14, 24]. AF resulting from leached acorn nuts contained less phenols (11.6 ± 0.7 mg GAE/g dry material). Leaching is a practice employed to remove tannins, bitter phenols and other bitter compounds [25] and therefore is expected to cause phenol decrease. This is in accordance with published data reporting the decrease of phenols and other phytochemicals of natural products after soaking [25]. Finally, the acorn shells (AS) which is a by product, contained 38.7 ± 0.8 mg GAE/g dry shell, implying its potency for efficient exploitation as phenol source. Cantos et al. [1] also reported that in Q. suber, the contribution of skin to TP content was relatively high, whereas acorn shell extracts from Q. acutissima Carruth have been also reported to be a good phenolic and antioxidant source [26].
Fig. 1.
Total phenol content (TP) and DPPH· scavenging activity of acorn material; values in columns of same grey shade with different letters are considered significantly different (p < 0.05)
Current findings are in line with literature presenting acorn to have a high phenolic content, holding a significant amount even after leaching and drying [1, 9, 10, 12, 27, 28]. According to Kobs [28], Q. velutina and Q. alba prior to leaching contained ~ 39 and 27 mg GAE/g, and after leaching ~ 12 and 11 mg GAE/g, respectively. In the study of Custódio et al. [27] the TP content of Q. extracts was of similar size too (~ 10 and 7 mg GAE/g for cork and holm oak, respectively). Acorn material, even in its leached form, can be regarded as an advantageous phenolic source compared to other natural products praised for their phenolic dynamic. Acorn is richer in phenols in comparison to various cereal (wheat, whole wheat, barley, millet, rye, oat, sorghum) flours reported to hold no more than ~ 4 mg GAE/g, which is the case of sorghum [29–32]. Moreover, from the over 100 different foodstuffs examined by Wu et al. [33], only ground cinnamon holds significantly higher amount of TP (~ 157 mg GAE/g) in comparison to acorn; fresh and dried fruits, vegetables, nuts and grain products reach ~ 7 (cranberries), 12 (prunes), 12 (red kidney beans), 20 (pecans) and 8 (corn flakes) mg GAE/g, respectively.
Antioxidant activity of acorn material
Natural antioxidants have drawn extreme attention since apart from extending the shelf life of a product, may also prevent or delay oxidation which damages biological tissues and generates diseases [34]. DPPH is of the most popular assays in antioxidant activity studies due to its simplicity and accuracy [34]. The ability of acorn material to scavenge the DPPH radical was tested on several extracts. Initially, methanol acorn extracts were prepared upon sonication, maceration and microwaves, as well as methanol extracts (prepared upon microwaves) from unleached acorn nuts (UAN, UIAN, UGAN), AF and AS and have been proved very efficient inhibitors (94–96%). As the activity was very high and did not allow comparison between samples aqueous extracts prepared upon microwaves for 1.5 min were freeze dried and subsequently re-dissolved in methanol in the same extract weight basis to further examine their scavenging activity. As shown in Fig. 1, AS presented the highest antioxidant activity, followed by unleached acorn nuts and finally AF. The antioxidant potency of acorn shells has been also reported by Youn et al. [26], where the examined polar extracts (of lower concentration) presented similar with the present study % DPPH inhibition values. The 60% of the initial antioxidant activity of acorn nut (UAN) is lost during the leaching procedure for the preparation of edible flour. % Inhibitions recorded are considered high and comparable to that of other materials regarded as potent radical scavengers. Methanol olive leaf extracts (of triple extract weight basis) are shown to possess up to 90% inhibition under similar experimental conditions [13]. Antioxidant activity findings are in line with published data that report high DPPH scavenging activity for acorn tissues [9]. Cantos et al. [1] have studied the DPPH inhibition of extracts prepared from skin and endosperm of Q. suber, rotundifolia and ilex and report that acorn extracts are potent scavengers and that skin may be either more or less potent depending on the specie. Direct quantitative comparisons cannot be made due to differences in the experimental conditions employed and in the expression of results.
Na, K and Ca content of acorn material
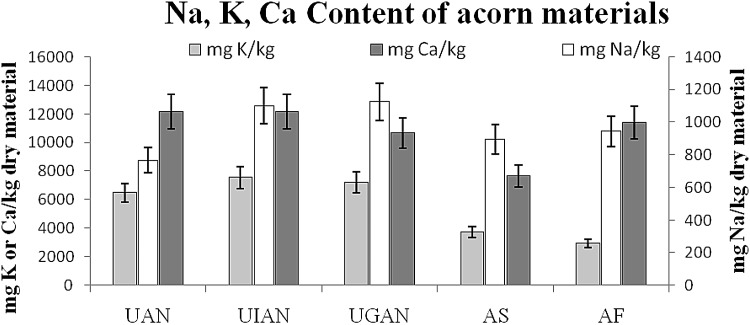
Acorn content in the studied minerals is shown in Fig. 2. The material has a promising dynamic for the examined macroelements whose content was found higher than that published for most cereals [10, 31, 35, 36]. Mineral content (mg/kg dry material) of acorn materials ranged from 767 (UAN) to 1125 (UGAN) for Na, from 2938 (AF) to 7549 (UIAN) for K and from 7670 (AS) to ~ 12,150 (UAN, UIAN) for Ca. K levels of acorn material of the present study are of the same magnitude to that presented by Rakić and collaborators [10] for Q. robur, while Ca content is 10 times higher. Moreover, acorn flour of the present study if compared to that studied by Rybicka and Gliszczyńska-Świgło [37] presents ~ 55 times more Na, similar content in K and ~ 7 times more Ca. Considerable differences are noticed in literature also between cereal and grain products of the same type, even when examined under the same experimental conditions [37]. Mineral content of grains may vary due to genetic, environmental, climatic, and cultivation differences and thus concentrations cover a broad range [35, 38].
Fig. 2.
Content in Na, K and Ca (mg/kg) of acorn materials
Fatty acid profile of acorn nuts and flour
The fatty acid profile of the various acorn samples is shown Table 1. The predominant fatty acid component of all samples studied was oleic acid (C18:1ω9) accounting for about 50%, followed by palmitic (C16:0) and linoleic (C18:2ω6) acids, which represented about 23% each of the total fatty acids detected. These findings were similar with those previously reported by Charef et al. [2] regarding the fatty acid composition of Q. ilex and Q. suber acorn species grown in Algeria. Similarly, PUFA/SFA (0.9) and ω6/ω3 ratios of acorn flour of the present study are of the same size to those reported by Silva and collaborators [16] (~ 0.9–1.0 and 19–25 respectively), whereas the MUFA/SFA ratio is lower (1.9 as opposed to 3.3–3.7). The unsaturated to saturated ratio of the samples were ~ 3, slightly lower than that reported by the former scientific groups for the examined Q. ilex, suber and rotundifolia species (~ 4–5%). Other fatty acid components detected in the lipid fraction of the samples were the omega-7, palmitoleic (C16:1ω7) and vaccenic (C18:1ω7) acids, as well as the saturated stearic (C18:0) acid and the polyunsaturated linolenic (C18:3ω3) acid. Since acorn samples exhibited different lipid contents, with average values of 2.94, 4.53, 6.82 and 6.97% for UAN and UIAN, AF and UGAN, respectively, it was expected that their fatty acid composition, expressed as g fatty acid per 100 g sample, would also differ. Therefore, UAN with the lowest lipid content had the lowest concentrations of individual fatty acids. Overall, acorn oil rich in oleic and linoleic acids, of significant importance to human health and nutrition, and with higher amount of unsaturated compared to saturated fatty acids is considered to present an interesting lipidic profile. Moreover and although it cannot be regarded as an oil-rich material [2], its oil content (~ 3–7%) is in the range of other plant materials (e.g. wheat germs) that are used for their bioactive content from pharmaceutical, cosmetic and food sectors [39].
Table 1.
Fatty acid composition (mg fatty acid/100 g sample) of acorn materials
| UAN | UIAN | UGAN | AF | |
|---|---|---|---|---|
| C16:0 | 671.35 ± 11.43c | 1029.22 ± 3.84b | 1652.24 ± 115.82a | 1618.73 ± 24.59a |
| C16:1ω7 | 11.61 ± 0.62b | 16.53 ± 0.96ab | 20.91 ± 1.97a | 15.69 ± 1.93ab |
| C18:0 | 61.89 ± 1.04b | 88.34 ± 3.84c | 134.52 ± 5.91a | 118.33 ± 4.34a |
| C18:1ω9 | 1422.08 ± 17.05c | 2175.99 ± 4.80b | 3324.34 ± 34.01a | 3251.78 ± 23.15a |
| C18:1ω7 | 22.79 ± 1.46b | 35.11 ± 2.24a | 45.31 ± 3.94a | 36.15 ± 2.89a |
| C18:2ω6 | 676.64 ± 3.95b | 1027.40 ± 0.64c | 1610.77 ± 33.51a | 1585.65 ± 42.44a |
| C18:3ω3 | 46.89 ± 2.29c | 69.31 ± 2.56b | 97.23 ± 7.39a | 100.94 ± 3.86a |
| SFA | 733.24 ± 12.47c | 1117.55 ± 0.00b | 1786.76 ± 109.91a | 1737.05 ± 20.25a |
| MUFA | 1456.48 ± 19.13c | 2227.63 ± 1.60b | 3390.56 ± 39.32a | 3303.61 ± 24.11a |
| PUFA | 723.53 ± 1.66c | 1096.71 ± 3.20b | 1708.00 ± 40.91a | 1686.59 ± 46.30a |
Values are presented as mean ± SD of triplicate determinations (n = 3)
Means across the same row with different superscripts are statistically significant (p < 0.05)
SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids
Protein molecular weight distribution of acorn material
The MW of proteins and peptides of the various acorn samples, as well as of shells were examined. All samples had protein profiles that consisted of similar bands, but with different intensities, probably due to the different protein contents (Fig. SI2, Supporting Information section). The MWs of the bands detected were of 7, 10, 15, 21, 25, 37 and 45 kDa. Furthermore, in all samples some of the proteins did not enter the gel suggesting that high MW components are also present. Regarding the protein profile of the shells, only three bands were detected with MWs of 20 and > 250 kDa. Acorn nuts and shells have been reported to contain all the amino-acids commonly found in plant proteins. Moreover, the acorn nut, which is consumed by the Iberian pig during the final fattening period, has been found to be easily digestible but to be lacking the high protein content besides requiring dietary supplementation with lysine [10].
TP content of leached acorn material and leaching wastewaters
For the preparation of palatable acorn products leaching practices are usually employed. Still, such practices are known to influence the extraction of phytochemicals from a natural material [23]. Therefore, the effect of leaching parameters (material to solvent ratio, time of extraction, size of the material, temperature and presence of NaCl) on the phytochemical content of acorn was examined and findings for TP content are presented in Tables 2 and 3.
Table 2.
Effect of leaching parameters on total phenol content (TP) of leaching wastewaters (LW)
| TP as mg GAE ± SD/L of LW | |
|---|---|
| Material form (water RT, 5% w/v) | |
| Whole | |
| 1st day | 600.9 ± 111.5c |
| 2nd day | 359.7 ± 3.7b |
| 3rd day | 191.1 ± 8.0a |
| Crushed | |
| 1st day | 1023.1 ± 65.6d |
| 2nd day | 417.9 ± 2.4b |
| 3rd day | 106.8 ± 8.1a |
| Grinded | |
| 1st day | 1236.9 ± 76.5e |
| 2nd day | 594.1 ± 13.3c |
| 3rd day | 125.8 ± 12.1a |
| % w/v material (water RT, crushed nut) | |
| 5% | |
| 1st day | 1066.8 ± 43.9b |
| 2nd day | 406.1 ± 19.7a |
| 15% | |
| 1st day | 2497.5 ± 117.8d |
| 2nd day | 1178.5 ± 26.6b |
| 30% | |
| 1st day | 3503.9 ± 196.0e |
| 2nd day | 2234.8 ± 16.6c |
| Leaching medium (crushed nut, 5% w/v) | |
| Water (RT) | |
| 1st day | 2131.2 ± 74.1c |
| 2nd day | 389.9 ± 3.1a |
| Water (60 °C) | |
| 1st day | 4352.4 ± 63.1d |
| 2nd day | 386.9 ± 13.3a |
| NaCl (3.5% w/v) | |
| 1st day | 539.3 ± 41.8b |
| 2nd day | 453.2 ± 18.9a |
Values in the same column with different letters are considered significantly different (p < 0.05)
TP expressed as mg GAE/L
Table 3.
Total phenol content (TP) of extracts prepared from leached acorn material
| TP as mg GAE ± SD (g) | |
|---|---|
| Material form (RT water, 5% w/v, after 3 days leaching) | |
| Whole | 12.0 ± 1.1c |
| Crushed | 7.2 ± 0.5b |
| Grinded | 2.1 ± 0.2a |
| % w/v acorn (RT water, crushed nut, after 2 days leaching) | |
| 5 | 10.5 ± 0.4c |
| 15 | 3.9 ± 0.1b |
| 30 | 2.0 ± 0.1a |
| Leaching medium (crushed nut, 5% w/v, after 2 days leaching) | |
| Water (RT) | 7.4 ± 0.2c |
| Water (60 °C) | 1.9 ± 0.1a |
| NaCl (3.5% w/v) | 6.6 ± 0.1b |
Values in the same column with different letters are considered significantly different (p < 0.05)
TP is expressed as mg GAE ± SD/g; aqueous extracts (2% w/v) were prepared from leached acorn material (after the application of different leaching practices) upon 1.5 min microwaves
As depicted in Table 2 crushing and grinding acorn nuts significantly enhances the removal of phenols from the material with differences being more significant on the first day of leaching. As expected, the smaller the size of the material, the higher the amount of phenols leached out [23]. The major amount of phenols leached out on the first and second day of leaching, while on the third day differences were not significant between samples. Therefore, the rest parameters were examined only for 2 days leaching.
In addition, the higher the amount of material into the leaching water, the higher the amount of phenols leached in it. This was shown for the first and second day of leaching, as well as when the sum was calculated (Table 2). Still, the greater amount of TP was leached out on the first day. The increase in the amount of the material leached in water was not analogous to the increase of TP content of leaching waters. Literature reports that increase in solvent to material ratio initially enhances TP extraction [23, 40], and that a further increase in the ratio frequently adversely affects extraction [40, 41].
Moreover, as presented in Table 2, the amount of phenols leached out from acorn the first day of leaching was significantly affected from the medium. Higher temperature (60 °C) in comparison to room temperature doubled phenol removal. Temperature increase enhances the extraction of phenols from a plant material [42, 43] as it increases analyte solubility. The solvent can more easily reach the sample due to lower solvent viscosity and surface tension, resulting in a higher extraction rate. Still, under high temperatures hydrolysis, oxidation and degradation may take place, due to the thermosensitivity of phenols [23].
The presence of salt led to a lower degree of phenols leaching from the material. This was in contrast to findings of Habibi et al. [25] concerning debittering of table olives in brine or water alone, where phenol removal was similar in both cases. However, acorn presents different chemical composition and textural characteristics to olive fruit and thus salt may differently affect the leaching procedure.
The above findings were verified when the TP content of the leached acorn material was determined (Table 3). The whole nut held a higher phenolic content with respect to crushed and milled nut after being leached. Additionally, the higher the material to water ratio for leaching the lower the amount of phenols retained in the material. As regards the effect of leaching medium, high temperature deprived a significant amount of phenols from the nut. In the presence of salt the leached material retained a significant amount of TP. The latter indicates that leaching in sea water—a convenient, environmental friendly and no cost practice—could displace leaching with water for sites surrounded by sea.
Antioxidant activity of leached acorn material and leaching wastewaters
FRAP assay widely used for aqueous testing systems [34] was employed to assess the antioxidant activity of leaching waters resulting from acorn leached at RT, at 60 °C and at RT in the presence of NaCl. Aqueous extracts of the respective leached materials were also examined. As is shown in Table 4 leaching water of 60 °C was the most potent followed by leaching water at RT and the respective in the presence of NaCl. The leached materials, as expected, followed the reverse order, thus the highest potency was presented by leached nuts at RT with or without the presence of NaCl. The material leached at 60 °C presented lower activity, as the antioxidants moved to the leaching medium. Leaching waters of the second day of leaching and the respective leached materials did not present redox activity. This strengthens the suggestion that the leaching should be carefully conducted and not thoughtlessly extended so as the antioxidant dynamic of the material can be preserved. According to Kobs [28] the fifteen acorn varieties examined presented significant redox activity.
Table 4.
Redox (FRAP) antioxidant activity of 1st day leaching wastewaters and extracts prepared from respective leached acorn materials
| FRAP redox activity (caffeic acid equivalent) | |
|---|---|
| Leaching wastewaters | |
| Water (RT) | 23 ± 4a |
| Water (60 °C) | 85 ± 1b |
| NaCl (3.5% w/v) | 12 ± 2a |
| Acorn material | |
| Water (RT) | 110 ± 9c |
| Water (60 °C) | 15 ± 4a |
| NaCl (3.5% w/v) | 93 ± 4b |
Values in the same column with different letters are considered significantly different (p < 0.05)
Results are expressed as caffeic acid equivalents; aqueous extracts (2% w/v) were prepared from leached acorn material (after the application of different leaching practices) upon 1.5 min microwaves
Na, K and Ca content of leaching wastewaters
Concerning the removal of minerals during acorn leaching, it was shown that the studied minerals (Fig. SI3, Supporting Information section) were stepwise removed from acorn to leaching waters following the same trend. Minerals were mainly leached out from acorn on the first day, especially when the material was crushed or grinded. On the third day, the leaching waters were shown to contain almost no minerals. Moreover, and as was shown for TP content the higher the acorn to water ratio (30–15 and 5%) the larger the concentration of minerals into the leaching waters. It seems that irrespectively to the mineral measured, leaching waters coming from leaching of 30 and 15% w/v acorn hold ~ 4 and ~ 2.5 times more minerals respectively in comparison to those resulting from the leaching of 5% w/v acorn. These findings are in accordance with published data regarding minerals leaching out from plant products; the extraction process (method, temperature, time) may significantly affect the mineral percentage extracted and this is affected by the type of plant used [44, 45]. Therefore, the procedure should be carefully designed so that the final leached material will not be devoid of minerals.
Concluding remarks
Results of the present study are expected to be useful in making important advances in industrial and scientific fields and set the grounds for establishing Quercus aegilops tissues as a profitable and competitive resource for the development of high added value products in view of their exploitation in food, feed, medical and cosmetic sector. Acorn products (nuts and flour) and by products (shells and leaching waters) is of phytochemical interest with respect to their content in phenolic antioxidants, minerals and fatty acids. Leached acorn flour could find an interesting place in gluten free formulations enhancing nutritional and technological deficiencies. Findings suggest that the leaching procedure for the preparation of palatable acorn flour should be carefully designed and applied so as not to deprive the material of its bioactive (phenols, antioxidants, minerals) potential. Leaching in the sea water seems to be an effective, no cost, environmental friendly practice that leads to nutritionally rich acorn meals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge Mrs. Marcie Mayer Maroulis and the Red Tractor Farm (P.O. Box 24, Korissia, Cycladic Islands 84002, Greece) for providing acorn materials and sharing ideas.
References
- 1.Cantos E, Espín JC, López-Bote C, de la Hoz L, Ordóñez JA, Tomás-Barberán FA. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food Chem. 2003;51:6248–6255. doi: 10.1021/jf030216v. [DOI] [PubMed] [Google Scholar]
- 2.Charef M, Yousfi M, Saidi M, Stocker PJ. Determination of the Fatty Acid Composition of Acorn (Quercus), Pistacia lentiscus Seeds Growing in Algeria. Amer. Oil Chem. Soc. 2008;85:921–924. doi: 10.1007/s11746-008-1283-1. [DOI] [Google Scholar]
- 3.Claudia P. Acorn bread: A traditional food of the past in Sardinia (Italy) J. Cult. Herit. 2013;14:S71–S74. doi: 10.1016/j.culher.2012.11.012. [DOI] [Google Scholar]
- 4.Correia PR, Beirão-da-Costa ML. Effect of Drying Temperatures on Starch-Related Functional and Thermal Properties of Acorn Flours. Journal of Food Science. 2011;76:E196–E202. doi: 10.1111/j.1750-3841.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopes IM, Bernardo-Gil MG. Characterisation of acorn oils extracted by hexane and by supercritical carbon dioxide. Eur. J. Lipid Sci. Technol. 2005;107:12–19. doi: 10.1002/ejlt.200401039. [DOI] [Google Scholar]
- 6.Nieto R, Rivera M. García MA, Aguilera JF. Amino acid availability and energy value of acorn in the Iberian pig. Livest. Prod. Sci. 2002;77:227–239. doi: 10.1016/S0301-6226(02)00040-4. [DOI] [Google Scholar]
- 7.Özcan TJ. Characterization of Turkish Quercus L. taxa based on fatty acid compositions of the acorns. Amer. Oil Chem. Soc. 84: 653–662 (2007)
- 8.Ferreira VCS, Morcuende D, Hérnandez-López SH, Madruga MS, Silva FAP, Estévez MJ. Antioxidant extracts from acorns (Quercus ilex L.) effectively protect ready-to-eat (RTE) chicken patties irrespective of packaging atmosphere. Food Sci. 82: 622–631 (2017) [DOI] [PubMed]
- 9.Rakić S, Petrović S, Kukić J, Jadranin M, Tešević V, Povrenović D, Šiler-Marinković S. Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 2007;104:830–834. doi: 10.1016/j.foodchem.2007.01.025. [DOI] [Google Scholar]
- 10.Rakić S, Povrenović D, Tešević V, Simić M, Maletić R. Oak acorn, polyphenols and antioxidant activity in functional food. J. Food Eng. 2006;74:416–423. doi: 10.1016/j.jfoodeng.2005.03.057. [DOI] [Google Scholar]
- 11.Molavi H, Keramat J, Raisee B. Evaluation of the Cake Quality Made from Acorn-Wheat Flour Blends as a Functional Food. Journal of Food Biosciences and Technology. 2015;5(2):53–60. [Google Scholar]
- 12.Meyers KJ, Swiecki TJ, Mitchell AE. Understanding the native Californian diet: Identification of condensed and hydrolyzable tannins in tanoak acorns (Lithocarpus densiflorus) J. Agric. Food Chem. 2006;54:7686–7691. doi: 10.1021/jf061264t. [DOI] [PubMed] [Google Scholar]
- 13.Papoti VT, Tsimidou MZ. Impact of sampling parameters on the radical scavenging potential of olive (Olea europaea L.) leaves. J. Agric. Food Chem. 2009;57:3470–3477. doi: 10.1021/jf900171d. [DOI] [PubMed] [Google Scholar]
- 14.Osman MA. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem. 2004;88:129–134. doi: 10.1016/j.foodchem.2003.12.038. [DOI] [Google Scholar]
- 15.Harris MA. Acorns. AAOOB Storable Foods (2000)
- 16.Capparucci C, Gironi F, Piemonte V. Tannins extraction from walnuts residues. Chem. Eng. Trans. 2011;24:469–474. [Google Scholar]
- 17.Fotelli MN, Radoglou KM, Constantinidou HI. Water stress responses of seedlings of four Mediterranean oak species. Tree Physiol. 2000;20:1065–1075. doi: 10.1093/treephys/20.16.1065. [DOI] [PubMed] [Google Scholar]
- 18.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 19.Bligh EG, Dyer WJ. A rapid method of total lipid oxidation and purification. Can J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 20.Hanson SWF, Olley J. Application of the Bligh and Dyer method of lipid extraction to tissue homogenates. Biochem. J. 1963;89:101–102. [Google Scholar]
- 21.Karayannakidis PD, Chatziantoniou SE, Zotos A. Effects of selected process parameters on physical and sensorial properties of yellowfin tuna (Thunnus albacares) skin gelatin. J. Food Process. Eng. 2014;37:461–473. doi: 10.1111/jfpe.12103. [DOI] [Google Scholar]
- 22.Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyarisiima CC, Okot MW, Svihus B. Use of wood ash extract and germination to improve the feeding value of Ugandan Sekedo sorghum (Sorghum bicolor) for broiler chicks. Anim. Feed Sci. Technol. 2005;120:67–77. doi: 10.1016/j.anifeedsci.2004.10.008. [DOI] [Google Scholar]
- 25.Habibi M, Golmakani MT, Farahnaky A, Mesbahi G, Majzoobi M. NaOH-free debittering of table olives using power ultrasound. Food Chem. 2016;192:775–781. doi: 10.1016/j.foodchem.2015.07.086. [DOI] [PubMed] [Google Scholar]
- 26.Youn UY, Shon MS, Kim GN, Katagiri R, Harata K, Kamegai M, Ishida Y, Lee SC. Antioxidant and anti-adipogenic activities of acorn shells. Food Sci. Biotechnol. 2016;25:1183–1187. doi: 10.1007/s10068-016-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Custódio L, Patarra J, Alberício F. Neng NdR, Nogueira JMF, Romano A. Phenolic composition, antioxidant potential and in vitro inhibitory activity of leaves and acorns of Quercus suber on key enzymes relevant for hyperglycemia and Alzheimer’s disease. Ind. Crop. Prod. 2015;64:45–51. [Google Scholar]
- 28.Kobs LM. Dietary polyphenolic intake from acorns and acorn meal. Thesis: University of Georgia, Athens, GA; 2008. [Google Scholar]
- 29.Yu L, Haley S, Perret J, Harris M. Comparison of wheat flours grown at different locations for their antioxidant properties. Food Chem. 2004;86:11–16. doi: 10.1016/j.foodchem.2003.08.037. [DOI] [Google Scholar]
- 30.Revanappa SB, Salimath PV. Phenolic acid profiles and antioxidant activities of different wheat (Triticum aestivum L.) varieties. J. Food Biochem. 2011;35:759–775. doi: 10.1111/j.1745-4514.2010.00415.x. [DOI] [Google Scholar]
- 31.Ragaee S. Abdel-Aal ESM., Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006;98:32–38. doi: 10.1016/j.foodchem.2005.04.039. [DOI] [Google Scholar]
- 32.Ryan L, Thondre PS, Henry CJK. Oat-based breakfast cereals are a rich source of polyphenols and high in antioxidant potential. J. Food Comp. Anal. 2011;24:929–934. doi: 10.1016/j.jfca.2011.02.002. [DOI] [Google Scholar]
- 33.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004;52:4026–4037. doi: 10.1021/jf049696w. [DOI] [PubMed] [Google Scholar]
- 34.Moon JK, Shibamoto T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 35.Ekholm P, Reinivuo H, Mattila P, Pakkala H, Koponen J, Happonen A, Hellström J, Ovaskainen ML. Changes in the mineral and trace element contents of cereals, fruits and vegetables in Finland. J. Food Comp. Anal. 2007;20:487–495. doi: 10.1016/j.jfca.2007.02.007. [DOI] [Google Scholar]
- 36.Hager AS, Wolter A, Jacob F, Zannini E, Arendt EK. Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J. Cereal Sci. 2012;56:239–247. doi: 10.1016/j.jcs.2012.06.005. [DOI] [Google Scholar]
- 37.Rybicka I, Gliszczyńska-Świgło AJ. Minerals in grain gluten-free products. The content of calcium, potassium, magnesium, sodium, copper, iron, manganese, and zinc. Food Comp. Anal. 59: 61–67 (2017)
- 38.MacEvilly C. Cereals, contribution to the diet. In: Encyclopedia of food sciences and nutrition (2nd Edition). (Oxford: Academic Press) pp 1008–1014 (2003)
- 39.Sonntag NOV, Composition and characteristics of individual fats and oils. In: Bailey’s industrial oil and fat products vol 1, Ed By Swern D (Ath edn. Wiley, New York) pp 289–478 (1979)
- 40.Wang X, Wu Y, Chen G, Yue W, Liang Q, Wu Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013;20:846–854. doi: 10.1016/j.ultsonch.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Devi RR, Arumughan C. Phytochemical characterization of defatted rice bran and optimization of a process for their extraction and enrichment. Bioresour. Technol. 2007;98:3037–3043. doi: 10.1016/j.biortech.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Gironi F, Piemonte V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011;89:857–862. doi: 10.1016/j.cherd.2010.11.003. [DOI] [Google Scholar]
- 43.Harbourne N, Jacquier JC, O’Riordan D. Optimisation of the aqueous extraction conditions of phenols from meadowsweet (Filipendula ulmaria L.) for incorporation into beverages. Food Chem. 2009;116:722–727. doi: 10.1016/j.foodchem.2009.03.017. [DOI] [Google Scholar]
- 44.Ong CC. Direct analysis of plant minerals and comparison of extraction processes using ICP-AES. Food Chem. 1992;45:145–149. doi: 10.1016/0308-8146(92)90026-X. [DOI] [Google Scholar]
- 45.Lestienne I, Icard-Vernière C, Mouquet C, Picq C, Trèche S. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005;89:421–425. doi: 10.1016/j.foodchem.2004.03.040. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.