Abstract
The effects of Chios mastic gum (Pistacia lentiscus var. Chia) and exercise on physical characteristics, blood lipid markers, insulin resistance, and hepatic function were investigated in healthy, non-smoking Japanese men aged ≥ 40 years. Participants were allocated to control (C, 5 g/day placebo powder, n = 7), mastic (M, 5 g/day mastic powder, n = 7), or mastic plus physical activity groups (M + PA, 5 g/day mastic powder and 30-min exercise three times/week, n = 7), and measurements were taken at baseline, 3 and 6 months. Serum triglycerides were significantly reduced at 3 months in M and M + PA compared with C (P < 0.05). Serum insulin and homeostatic model assessment of insulin resistance values were significantly reduced at 3 and 6 months in M + PA, and at 6 months in M, compared with C (P < 0.05). These results indicate that Chios mastic gum intake for 6 months reduced serum triglyceride and insulin concentrations, and additional exercise enhanced the effect on insulin.
Keywords: Chios mastic gum, Physical activity, Triglycerides, Insulin, HOMA-IR
Introduction
Chios mastic gum (CMG) is a popular functional food and has been widely researched in recent years. It plays an important role in the treatment of several modern lifestyle-related diseases in Western countries, such as Greece [1]. However, little is known about its functional and medical efficacy in Asia, including Japan. Medicinal plants and functional foods are currently in high demand in Japan, for the treatment of the same lifestyle-related diseases that affect Western society [1].
Mastic (Pistacia lentiscus var. Chia) is a common evergreen shrub found in eastern Mediterranean areas [2]. However, the best quality mastic plants are unique to the southern part of Chios, a Greek island in the Aegean Sea. The natural tree sap, collected by making incisions in the branches and trunks of the mastic trees, has been prized since ancient times as a natural chewing gum, and a luxury product for cleaning teeth and eliminating bad breath [3]. CMG has been used in traditional Greek medicine for over 2500 years, and famous Ancient Greek physicians, such as Hippocrates (460–370 BC), Dioscorides, Theophrastos, and Galen, as well as ancient Babylonians, Egyptians, and Arabs, have referred to its healing properties [3].
CMG has been the focus of many recent studies, which have confirmed its efficacy as a functional food for gastrointestinal disturbances [4–6] and oral hygiene [7, 8]. It has strong antimicrobial [9], anti-inflammatory [10], and antioxidative activities [11], and has been shown to have hepatoprotective and cardioprotective effects [12]. Moreover, it is known to have diabetes prevention and treatment effects [13]. Triantafyllou et al. [12] reported mastic-related improvements in total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), total cholesterol/high-density lipoprotein cholesterol (HDL-C) ratio, apolipoprotein A1, apolipoprotein B, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and γ-glutamyl transpeptidase (γ-GTP) in Greek subjects. The same group reported that the anti-inflammatory activity of mastic occurs via control of tumor necrosis factor-alpha, which induces oxidative stress [10]. Moreover, in their screen of natural products in the Chinese Natural Database, Petersen et al. [13] reported the presence and activity of oleanolic acid, an effective antidiabetic compound, in CMG.
While the effects of CMG observed in these studies should also apply to Japanese people, this remains to be confirmed. In addition, the Ministry of Health, Labour and Welfare in Japan has proposed that lifestyle-related diseases are closely related to eating habits, smoking, and physical activity [14]. Therefore, the aim of this study was to clarify the effects of CMG and exercise on physical characteristics, blood lipid markers, insulin resistance, and hepatic function in healthy, non-smoking Japanese men aged ≥ 40 years.
Materials and methods
We recruited 21 men aged ≥ 40 years who were non-smokers, in good health with no cardiovascular or metabolic diseases, not receiving drug therapy, and not participating in any other clinical studies at the time of this study. Informed consent was obtained from all participants after the experiment was described to them in detail. Participants were assigned at random to the following three groups ensuring that there were no significant differences between groups in age or body composition (age and height values are mean ± standard error): control (C, n = 7, age 50.3 ± 11.3 years, height 170.7 ± 5.8 cm), mastic (M, n = 7, age 50.1 ± 7.7 years, height 169.2 ± 4.5 cm), mastic plus physical activity (M + PA, n = 7, age 43.0 ± 7.0 years, height 168.7 ± 4.0 cm). During the study period, participants in groups C and M were asked to maintain the same lifestyle habits. The physical characteristics of the participants are shown in Table 1. Participants were asked to complete simple questionnaires about their current lifestyle, such as physical activity, alcohol consumption, and previous smoking habits, before starting the experiment.
Table 1.
Physical characteristics, physical activity, and energy intake at baseline, 3 months, and 6 months
| Parameter | Timepoint | Groupa | P value | |||
|---|---|---|---|---|---|---|
| C (n = 7) | M (n = 7) | M + PA (n = 7) | (Time, interaction) | |||
| Body mass (kg) | Baseline | 75.3 ± 6.8 | 70.2 ± 10.9 | 73.5 ± 12.8 | 0.217 | 0.361 |
| 3 months | 76.9 ± 6.9 | 70.8 ± 11.0 | 73.9 ± 12.5 | |||
| 6 months | 76.7 ± 6.8 | 70.3 ± 11.0 | 73.2 ± 13.2 | |||
| Body mass index (kg/cm2) | Baseline | 25.8 ± 2.2 | 24.4 ± 2.8 | 25.8 ± 4.6 | 0.217 | 0.361 |
| 3 months | 26.2 ± 2.3 | 24.4 ± 2.9 | 25.9 ± 4.6 | |||
| 6 months | 26.1 ± 2.2 | 24.3 ± 2.9 | 25.5 ± 4.5 | |||
| Waist circumference (cm) | Baseline | 90.5 ± 4.1 | 86.0 ± 8.5 | 88.7 ± 10.5 | 0.005 | 0.528 |
| 3 months | 90.9 ± 4.8 | 88.4 ± 9.5 | 89.9 ± 11.0 | |||
| 6 months | 92.0 ± 5.3 | 87.8 ± 10.1 | 90.1 ± 10.3 | |||
| Body fat percentage (%) | Baseline | 23.9 ± 3.7 | 21.3 ± 5.8 | 23.2 ± 7.1 | 0.165 | 0.331 |
| 3 months | 24.6 ± 3.8 | 22.3 ± 5.6 | 23.5 ± 7.0 | |||
| 6 months | 23.4 ± 3.5 | 21.0 ± 6.2 | 22.6 ± 6.9 | |||
| Systolic blood pressure (mmHg) | Baseline | 134.3 ± 8.4 | 137.0 ± 24.1 | 118.7 ± 10.3 | 0.359 | 0.77 |
| 3 months | 134.7 ± 11.7 | 135.8 ± 15.8 | 122.6 ± 15.7 | |||
| 6 months | 133.7 ± 11.8 | 129.5 ± 18.3 | 118.3 ± 11.3 | |||
| Diastolic blood pressure (mmHg) | Baseline | 89.6 ± 9.3 | 89.0 ± 13.5 | 80.3 ± 11.2 | 0.004 | 0.134 |
| 3 months | 92.3 ± 9.0 | 94.2 ± 15.1 | 80.0 ± 9.9 | |||
| 6 months | 90.2 ± 10.1 | 88.0 ± 15.1 | 72.2 ± 5.4 | |||
| Physical activity (steps/day) | Baseline | 9344 ± 1822 | 10,900 ± 2910 | 9398 ± 1911 | < 0.001 | < 0.001 |
| 3 months | 9461 ± 2307 | 11,046 ± 3435 | 12,745 ± 1257 | |||
| 6 months | 9237 ± 2060 | 11,222 ± 3508 | 13,036 ± 1575 | |||
| Energy intake (kcal/day) | Baseline | 2154 ± 94 | 2333 ± 166 | 2227 ± 280 | 0.003 | 0.964 |
| 3 months | 2179 ± 85 | 2377 ± 160 | 2288 ± 231 | |||
| 6 months | 2215 ± 152 | 2404 ± 163 | 2314 ± 277 | |||
All data are presented as mean ± standard error
Physical activity values for M + PA at 3 months and 6 months are inclusive of the prescribed thrice weekly 30 min walk
aGroups: C, control group; M, mastic intake group; M + PA, mastic intake plus physical activity group
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committees of Waseda University (No. 2012-028).
Capsules were prepared for use in the experiments from imported powdered mastic, made by grinding mastic resin obtained directly from the Chios Gum Mastic Growers Association. Each mastic capsule was composed of 334.0 mg of mastic powder, 8.2 mg of cornstarch, 30.4 mg of peppermint and menthol powder (4.7 mg peppermint powder, 25.7 mg menthol powder), and 0.4 mg of silicon dioxide powder. Each placebo capsule was composed of 283.0 mg of microcrystalline cellulose and 17.0 mg of peppermint and menthol powder (0.24 mg peppermint powder, 16.76 mg menthol powder). Capsule shells were water-soluble and made from porcine gelatin. Both mastic and placebo capsules were prepared by Toyo Capsule Co., Ltd. (Shizuoka, Japan).
Regarding the chemical composition of CMG, poly-β-myrcene (an adhesive and insoluble polymer) constitutes approximately 25% of CMG by weight, and several triterpenoids have also been isolated. Once poly-β-myrcene is removed, the extract can be further separated into an acidic fraction, which contains major triterpenoid acids (e.g. masticadienonic acid, isomasticadienonic oleanonic acid, moronic acid, masticadienolic acid, isomasticadienolic acid, oleanolic acid), and a neutral fraction, which contains neutral triterpenic compounds (e.g. oleanolic aldehyde, 28-norolean-17-en-3-one, tirucallol, β-amyrone, isomasticadienolic aldehyde, dammaradienone). Trace components include verbenone, α-terpinolene, and linalool—which contribute to the mastic oil’s antibacterial activity—and camphene, which has fat-reducing activity [15]. Georgiadis et al. [16] remarked that CMG’s antibacterial, fat-reducing, and anti-inflammatory activities suggest synergistic effects: “a combination of several compounds is more potent than any single compound on its own.”
This study was a double-blind, placebo-controlled, randomized trial. Group C participants consumed five placebo capsules with 200 mL of water three times per day, before breakfast, lunch, and dinner. Likewise, participants in groups M and M + PA consumed five mastic capsules with 200 mL of water, three times per day (total dose, 5010 mg/day) before meals. The dose was determined according to the study of Triantafyllou et al. [10]. Participants in group M + PA also participated in 30-min walks three times per week, in addition to their normal everyday activities. Participants were asked to walk briskly at a pace that increased their respiratory rate. The study period was 6 months for all groups. All participants were provided with a calendar in which they were required to tick-mark boxes when they took the capsules, and to note any missed doses. The participants of group M + PA were also required to note down the duration of each walk.
To assess their physical activity, all participants were asked to wear a uniaxial accelerometer (Lifecoder-EX; Suzuken Co. Ltd., Nagoya, Aichi, Japan) for 7 continuous days (at baseline and at 3 and 6 months, including Saturdays and Sundays) from rising until bedtime (except when undressing or bathing). We collected accelerometer data from all participants simultaneously to show that seasonality was not an issue. When assessing differences in physical activity between groups, we accounted for the exercise prescribed to group M + PA by subtracting 1285 steps/day from the 3- and 6-month values (assuming 100 steps/min of exercise, 100 steps × 30 min × 3 days = 9000 steps; 9000 steps/7 days = 1285 steps/day).
To assess dietary habits, all participants were interviewed using the Food Frequency Questionnaire based on food groups (Kenpakusha, Tokyo, Japan) at three timepoints (baseline, and 3 and 6 months). Diets were analyzed using the computerized nutritional analysis system of the Food Frequency Questionnaire [17].
We measured physical characteristics and collected blood samples at baseline (before starting the experiment), and 3 and 6 months after starting the experiment. Body mass was measured to the nearest 0.1 kg using a digital balance (Inner Scan 50; Tanita Corporation, Tokyo, Japan). Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer (YS-OA; As One Corporation, Osaka, Japan). BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured to the nearest 0.1 cm at the level of the umbilicus using a flexible plastic tape measure. Arterial blood pressure was measured from the right arm using a standard mercury sphygmomanometer (605P, Yagami Co. Ltd., Nagoya, Aichi, Japan) with the participant in a seated position. Each participant was seated in a chair for 5 min before the measurements were taken. Three blood pressure measurements were obtained at each time point, and the mean of these values was recorded. Percent body fat was measured using bioelectrical impedance analysis with an Inner Scan 50 (Tanita Corporation, Tokyo, Japan).
After fasting (overnight for at least 12 h) and avoiding physical activity for 24 h, venous blood samples were taken from the antecubital vein. Blood samples for serum were immobilized for 30 min at room temperature and centrifuged at 4 °C, and those for plasma were immediately centrifuged after collection. Serum and plasma samples were stored at − 80 °C until analysis. The following were measured: blood lipid markers [TC, LDL-C, HDL-C, triglycerides (TG), and lecithin–cholesterol acyltransferase (LCAT)], insulin resistance (glucose, insulin), and hepatic function (SGOT, SGPT, and γ-GTP). Serum TC concentration was determined by timed endpoint enzyme colorimetry with the cholesterol dehydrogenase-ultraviolet method. Serum HDL-C and LDL-C concentrations were assayed by the timed endpoint direct method. Serum TG were estimated according to timed endpoint enzyme colorimetry with the GK-GPO glycerol blanked method. Serum LCAT was measured by the dipalmitoyl lecithin substrate method. Fasting blood glucose was quantitated by hexokinase ultraviolet absorption spectrophotometry. And serum insulin was measured by a chemiluminescent enzyme immunoassay. The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as follows: HOMA-IR = fasting insulin (μU/mL) × fasting blood glucose level (mg/dL)/405. SGOT, SGPT, and γ-GTP concentrations were measured by the method recommended by the JSCC.
Data were analyzed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA). Two-way analysis of variance for repeated measures (6 trials) with Bonferroni post-hoc analysis was used to assess main effects and the group by time interaction. For variables with a significant group by time statistical interaction, change from baseline values were calculated for each participant by subtracting their individual baseline value from their 3- and 6-month values. Group differences in change from baseline values were analyzed at 3 and 6 months using one-way analysis of variance. All data are presented as mean ± standard error, and P values less than 0.05 were considered significant.
Results and discussion
Physical characteristics, physical activity, and energy intake of the participants at baseline, 3, and 6 months are summarized by group in Table 1. There were no significant group by time statistical interactions for body mass, BMI, waist circumference, percent body fat, systolic or diastolic blood pressure, or energy intake (Table 1), indicating that intake of mastic gum with or without physical activity for 6 months had no effect on physical characteristics or energy intake. There was a significant group by time statistical interaction for physical activity, with M + PA showing a trend for an increase in physical activity at 3 months (P = 0.073), and a significant increase in physical activity at 6 months (P = 0.034), compared with baseline. This result shows that the thrice weekly 30 min walks prescribed to the M + PA group significantly increased their overall physical activity during the 6-month study period.
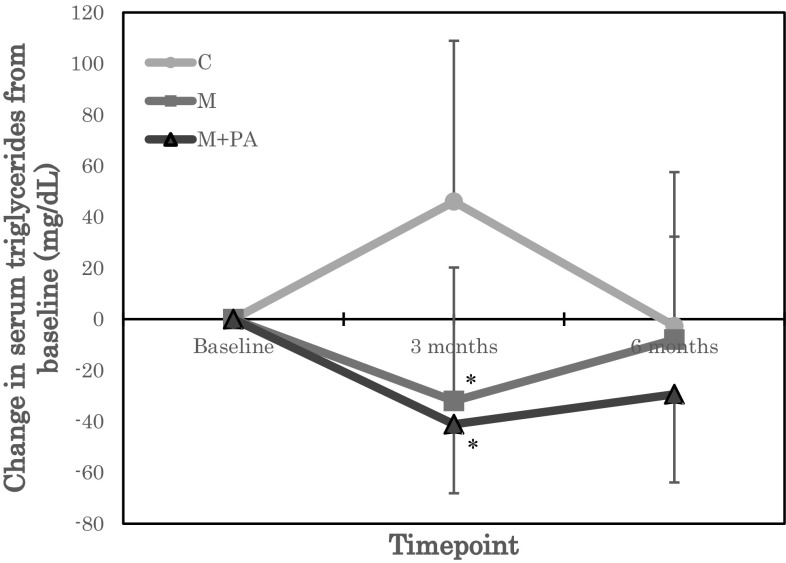
Biochemical parameters of the participants at baseline, 3 and 6 months are summarized by group in Table 2. For the blood lipid markers, a significant group by time statistical interaction was observed for TG (Table 2). A post-hoc test of unadjusted TG values showed a trend for TG to be reduced at 3 months in M compared with C (P = 0.074). A post-hoc test of TG values adjusted for baseline showed that TG was significantly reduced at 3 months in M (P = 0.023) and M + PA (P = 0.011) compared with C (Fig. 1). No significant group by time statistical interactions were observed for TC, HDL-C, LDL-C, or LCAT (Table 2).
Table 2.
Biochemical parameters at baseline, 3 months, and 6 months
| Parameter | Timepoint | Groupa | P value | |||
|---|---|---|---|---|---|---|
| C (n = 7) | M (n = 7) | M + PA (n = 7) | (Time, interaction) | |||
| Triglycerides (mg/dL) | Baseline | 116.3 ± 72.4 | 129.6 ± 89.7 | 113.0 ± 58.5 | 0.487 | 0.016 |
| 3 months | 162.3 ± 126.8 | 97.6 ± 49.6 | 71.9 ± 42.4 | |||
| 6 months | 113.6 ± 63.9 | 102.1 ± 10.4 | 83.6 ± 43.9 | |||
| Total cholesterol (mg/dL) | Baseline | 201.1 ± 19.0 | 200.9 ± 31.6 | 214.6 ± 42.4 | 0.32 | 0.591 |
| 3 months | 214.9 ± 24.2 | 202.7 ± 20.2 | 208.9 ± 46.8 | |||
| 6 months | 203.7 ± 16.1 | 194.4 ± 21.0 | 206.1 ± 36.8 | |||
| HDL cholesterol (mg/dL) | Baseline | 53.6 ± 8.2 | 61.4 ± 9.9 | 59.0 ± 14.7 | 0.281 | 0.495 |
| 3 months | 54.1 ± 13.5 | 56.9 ± 10.3 | 58.9 ± 13.3 | |||
| 6 months | 52.3 ± 10.5 | 58.3 ± 8.5 | 57.6 ± 15.2 | |||
| LDL cholesterol (mg/dL) | Baseline | 119.9 ± 15.7 | 105.1 ± 19.3 | 133.6 ± 45.2 | 0.165 | 0.331 |
| 3 months | 125.1 ± 17.8 | 109.7 ± 15.4 | 132.4 ± 48.7 | |||
| 6 months | 121.6 ± 15.8 | 103.7 ± 15.2 | 129.4 ± 45.1 | |||
| LCAT (U) | Baseline | 107.0 ± 13.7 | 103.0 ± 20.8 | 112.1 ± 26.6 | 0.395 | 0.223 |
| 3 months | 110.6 ± 17.0 | 98.5 ± 10.3 | 95.8 ± 19.5 | |||
| 6 months | 104.9 ± 15.3 | 109.5 ± 29.1 | 102.0 ± 25.0 | |||
| Glucose (mg/dL) | Baseline | 100.3 ± 11.7 | 103.0 ± 5.8 | 99.9 ± 7.2 | 0.163 | 0.097 |
| 3 months | 102.9 ± 9.5 | 97.6 ± 6.1 | 93.7 ± 4.5 | |||
| 6 months | 100.7 ± 10.8 | 88.0 ± 15.1 | 93.0 ± 6.8 | |||
| Insulin (μIU/mL) | Baseline | 4.9 ± 1.4 | 6.4 ± 3.1 | 9.4 ± 6.6 | 0.474 | 0.003 |
| 3 months | 6.4 ± 1.6 | 5.4 ± 2.4 | 7.5 ± 6.3 | |||
| 6 months | 7.0 ± 3.0 | 5.4 ± 2.1 | 6.8 ± 6.5 | |||
| HOMA-IR | Baseline | 1.2 ± 0.4 | 1.6 ± 0.8 | 2.3 ± 1.5 | 0.395 | 0.007 |
| 3 months | 1.6 ± 0.33 | 1.3 ± 0.6 | 1.8 ± 1.5 | |||
| 6 months | 1.7 ± 0.7 | 1.4 ± 0.6 | 1.6 ± 1.6 | |||
| SGOT (U/L) | Baseline | 24.6 ± 4.8 | 26.7 ± 10.8 | 28.9 ± 13.5 | 0.086 | 0.435 |
| 3 months | 24.6 ± 6.1 | 23.1 ± 11.4 | 23.1 ± 5.1 | |||
| 6 months | 23.3 ± 4.2 | 25.1 ± 13.5 | 21.7 ± 6.2 | |||
| SGPT (U/L) | Baseline | 25.6 ± 7.6 | 21.6 ± 7.7 | 40.6 ± 30.1 | 0.006 | 0.111 |
| 3 months | 21.6 ± 6.1 | 13.4 ± 5.3 | 20.1 ± 9.9 | |||
| 6 months | 23.3 ± 8.3 | 23.1 ± 21.1 | 23.7 ± 17.9 | |||
| γ-GTP (U/L) | Baseline | 36.7 ± 14.4 | 60.9 ± 47.4 | 43.1 ± 19.9 | 0.042 | 0.332 |
| 3 months | 37.6 ± 17.0 | 52.1 ± 42.3 | 34.3 ± 13.9 | |||
| 6 months | 36.3 ± 13.9 | 56.5 ± 45.9 | 35.9 ± 16.9 | |||
All data are presented as mean ± standard error
γ-GTP γ-glutamyl transpeptidase, HDL high-density lipoprotein, HOMA-IR homeostatic model assessment of insulin resistance, LCAT lecithin–cholesterol acyltransferase, LDL low-density lipoprotein, SGOT serum glutamic oxaloacetic transaminase, SGPT serum glutamate pyruvate transaminase
aGroups: C, control group; M, mastic intake group; M + PA, mastic intake with physical activity group
Fig. 1.
Change in serum triglycerides from baseline. Groups: C, control group; M, mastic intake group; M + PA, mastic intake with physical activity group. *P < 0.05 versus C
Studies in rodents have also shown reductions in TG and other lipids with CMG intake. Vallianou et al. [15] reported that the administration of CMG essential oil to hyperlipidemic rats reduced TG, TC, and LDL-C concentrations by 65.4, 59.9, and 72.5%, respectively, and attributed this result to the camphene content of CMG. Georgiadis et al. [18] fed diabetic mice with low or high doses of mastic powder for 8 weeks, and showed that TG was significantly reduced in both groups, and TC and LDL-C were significantly reduced in the low-dose group. In human studies, the effects of CMG on TG and other lipids are less consistent. In a study by Kartalis et al. [19], 179 healthy Greek male subjects received a daily dose of placebo, 1 g CMG, 1 g polymer-free CMG, or 2 g polymer-free CMG. While TC concentrations were reduced in the 1 g CMG group, the were no group effects on serum LDL-C or TG. Importantly, the CMG doses used in this study (1 or 2 g CMG/day) were lower than that used for the present study (5 g CMG/day). CMG dose may thus be a factor in how CMG affects blood lipid markers, and warrants further study.
Research suggests that the effects of CMG on lipid metabolism could be mediated by peroxisome proliferator-activated receptors (PPARs). Oleanolic acid, oleanonic acid, and gallic acid (components of CMG) are known to act on PPARs. PPARs regulate lipid metabolism, cell differentiation and proliferation, and immune responses, and are structurally similar to steroid hormone receptors [20]. PPARs also function as transcription factors, aiding lipid synthesis and oxidation, glucose uptake and insulin sensitivity, and inflammatory and immunoregulatory gene expression via activation of cell signaling pathways [21]. To date, three types of PPARs have been identified: PPARα, PPARγ, and PPARβ/δ. PPARα inhibits apolipoprotein C-III gene expression and reduces the formation of TG [21]. PPARβ/δ improves serum lipid profiles and increases insulin sensitivity [22, 23]. PPARγ is expressed on adipocytes, regulates the expression of genes that mediate adipogenesis, energy metabolism, and the actions of insulin [24, 25], and is a pivotal regulatory factor in adipogenesis [26]. According to Dedoussis et al. [27], the restoration of intracellular glutathione levels and downregulation of CD36 expression are pathways through which mastic triterpenes exert their antioxidant and antiatherogenic effects. This supports the idea that the overall effect of CMG on lipid metabolism is mediated via its function as a PPARα agonist. Furthermore, the activation of PPARβ/δ improves the serum lipid profile, which seems to support the notion that CMG exerts beneficial effects on lipid metabolism [16].
Physical activity has also been shown to affect lipid metabolism. Sakamoto et al. [28] reported that aerobic and continuous exercise regimes are needed to improve lipid metabolism. Holloszy et al. [29] showed that after 6 months of training, 15 male subjects (who did not exercise at all prior to the study) showed a reduction in TG from 208 to 125 mg/dL. Physical activity may thus have contributed to the reduction in TG shown by M + PA in the present study. However, there did not appear to be any significant additional benefit of physical activity, with no differences in TG observed between M + PA and M.
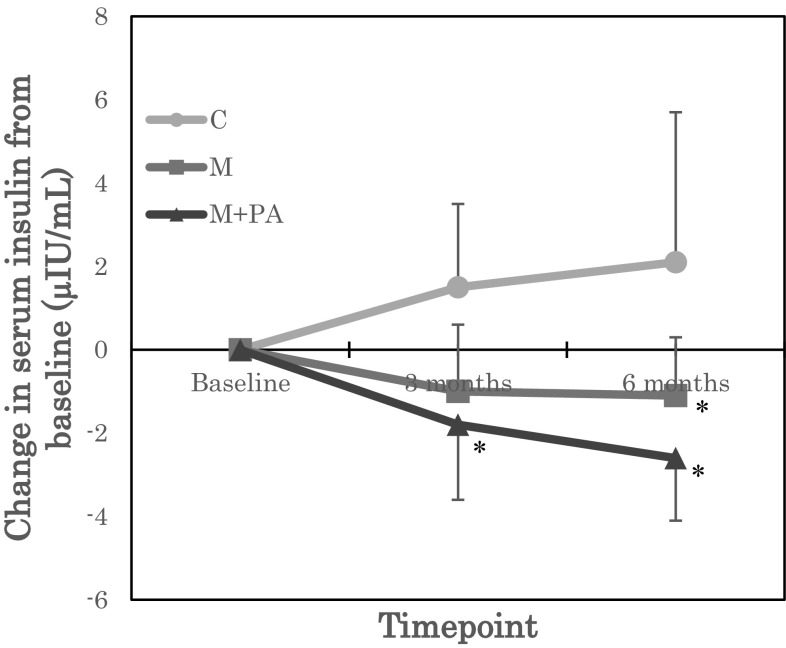
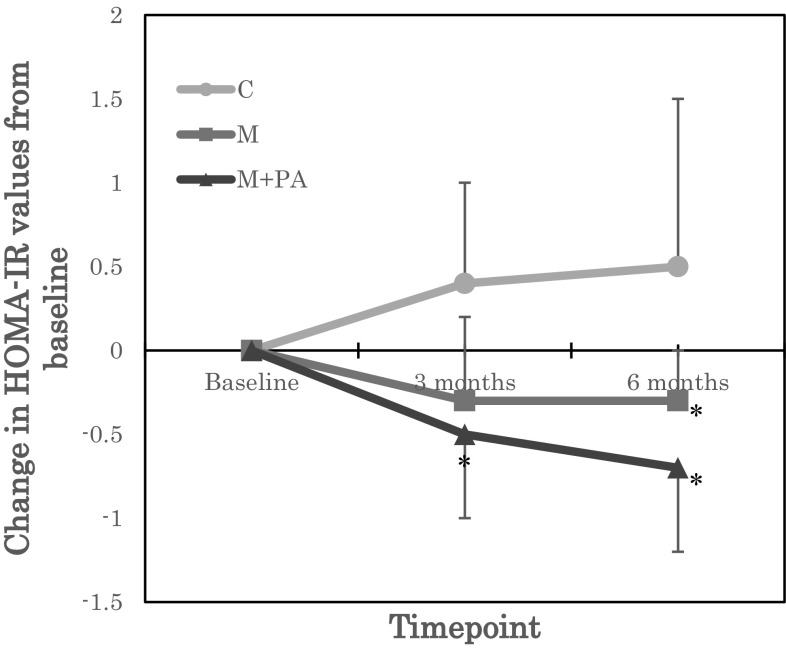
Regarding glucose metabolism, while there were no effects on fasting glucose, there were significant group by time statistical interactions for insulin and HOMA-IR (Table 2). Post-hoc tests of unadjusted insulin and HOMA-IR values showed that insulin and HOMA-IR were significantly reduced in M + PA at 3 months (insulin P = 0.04, HOMA-IR P = 0.07) and 6 months (insulin P = 0.032, HOMA-IR P = 0.041), compared with C. Post-hoc tests of insulin and HOMA-IR values adjusted for baseline showed that insulin and HOMA-IR were significantly reduced in M + PA at 3 months (insulin P = 0.007, HOMA-IR P = 0.017) and 6 months (insulin P = 0.005, HOMA-IR P = 0.009), and in M at 3 months (insulin P = 0.039, HOMA-IR P = 0.049), compared with C (Figs. 2 and 3). To our knowledge, this is the first report to show an association between CMG intake and insulin resistance.
Fig. 2.
Change in serum insulin from baseline. Groups: C, control group; M, mastic intake group; M + PA, mastic intake with physical activity group. *P < 0.05 versus C
Fig. 3.
Change in homeostatic model assessment of insulin resistance (HOMA-IR) values from baseline. Groups: C, control group; M, mastic intake group; M + PA, mastic intake with physical activity group. *P < 0.05 versus C
Previous studies on humans suggest that the effects of CMG on glucose metabolism may be dose dependent. For instance, Triantafyllou et al. [12] showed that for Greek male subjects, ingestion of a low daily CMG dose resulted in decreased glucose levels, whereas a high daily dose (5 g CMG/day, i.e. the same dose as the present study) did not affect glucose levels. In the aforementioned study of 179 healthy Greek male subjects, Kartalis et al. [19] showed that glucose levels were reduced in the group that received the relatively low daily dose of 1 g CMG. Conversely, in diabetic mice, both low and high doses of mastic powder (fed for 8 weeks) substantially reduced blood glucose levels [18].
There are several mechanisms by which CMG might affect insulin sensitivity. Oleanonic acid, a component of CMG, has been identified as a PPARγ agonist, and may therefore partly regulate insulin-mediated gene expression via the activation of PPARγ [13]. The decrease in HOMA-IR values in the mastic gum groups in our study may thus have occurred via PPARγ. In addition, the activation of PPARβ/δ has been shown to increase insulin sensitivity [22, 30]. Triterpenes (another component of CMG) have been shown to have beneficial effects on pancreatic β-cells that include promoting the action of insulin by increasing insulin secretion and inhibiting protein tyrosine phosphatase-1B [31].
Physical activity has also been shown to have beneficial effects on insulin sensitivity in healthy and insulin-resistant individuals [32, 33]. It induces various metabolic adaptations such as upregulation of muscle glucose transporter type 4 protein [34, 35], increases in enzyme activity, and muscle capillarization. Moreover, physical activity decreases basal glucose production and further inhibits hepatic glucose production by insulin [32].
None of the three measures of hepatic function (SGOT, SGPT, γ-GTP) showed a significant group by time statistical interaction in the present study, suggesting that hepatic function was not affected by 6 months of CMG intake or combined CMG intake and physical activity.
This study had some limitations. First, the number of participants was small; however, our results may still be compared with those of the Greek studies. Second, we investigated only healthy men over 40 years of age. According to Karataris et al. [19], the effect of CMG is not as apparent in this group. Moreover, our results cannot be generalized to women, obese individuals, or individuals with disease. Third, the study design did not include a “physical activity only” group; thus, the contribution of physical activity to the effects shown by M + PA is unclear. Fourth, we were unable to measure PPARs as a means of investigating the underlying mechanisms. Nevertheless, our study contains several useful methodological points, such as confirming the capsule intake in all groups, implementing physical activity three times per week in the M + PA group, and assisting eating habits based on caloric intake. Further, we found decreases in TG, insulin, and HOMA-IR with a combination of CMG intake and exercise.
In conclusion, we found that CMG intake affected lipid and glucose metabolism, consistent with earlier reports on Greek subjects. In particular, CMG intake alone reduced TG levels at 3 months, and insulin and HOMA-IR values at 6 months. CMG intake combined with exercise reduced TG levels at 3 months, and insulin and HOMA-IR values at 3 and 6 months. To our knowledge, this is the first study to report beneficial effects of CMG intake on blood lipid markers and insulin resistance in healthy Asian subjects, specifically in a Japanese population. Future studies should examine the effects of CMG intake and physical activity on groups not examined here, such as women, obese individuals, individuals with disease, and other ethnic groups.
Acknowledgements
We thank the General Manager of the Greek-Japanese Chamber of Commerce in Athens, Ms. Katerina Katopis, for initiating this study. We also thank the Chios Mastic Growers Association in Greece, particularly its President Mr. George Toumpos, for providing moral support for this study, and the following people for their role in supporting academic and cultural exchange between Japan and Greece: Ambassador of Greece to Japan, Ambassador Loukas Karatsolis; the former Ambassador of Greece to Japan, Ambassador Nikolaos Tsamados; the Counsellor of the Greek Embassy in Tokyo, Mr. Dionyssios Protopapas; the Ambassador of Japan to Greece, Ambassador Masuo Nishibayashi; and the former Ambassador of Japan to Greece, Ambassador Hiroshi Toda. The authors received no financial or material support for this research.
Compliance with ethical standards
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fukazawa T, Smyrniodis I, Konishi M, Takahashi M, Kim H, Numao S, Sakamoto S. Comparative study between Western and Asian pharmaceutical plants: the efficacy of the Greek mastic and the Japanese ume. Deltos. 2014;43:31–38. [Google Scholar]
- 2.Lemonakis N, Magiatis P, Kostomitsopoulos N, Skaltsounis AL, Tamvakopoulos C. Oral administration of chios mastic gum or extracts in mice: quantification of triterpenic acids by liquid chromatography-tandem mass spectrometry. Planta Med. 2011;77:1916–1923. doi: 10.1055/s-0031-1279996. [DOI] [PubMed] [Google Scholar]
- 3.Zacharopoulos C, Barbikas E. Chios Mastiha Historic and Folkloric references. Chios: The Chios Mastiha Growers Association; 2008. pp. 2–37. [Google Scholar]
- 4.Wellmann M. Pedanii ioscuridis Anazarbei de Materia Medica Libri Quinque. Berlin: Weidmann; 1907. [Google Scholar]
- 5.Huwez FU, Thirwell D, Cockayne A, Ala’Aldeen DA. Mastic gum kills Helicobacter pylori. New Engl. J. Med. 1998;339:1946. doi: 10.1056/NEJM199812243392618. [DOI] [PubMed] [Google Scholar]
- 6.Dabos K, Sfika E, Vlatta LJ, Giannikopoulos G. The effect of mastic gum on Helicobacter pylori: a randomized pilot study. Phytomedicine. 2010;17:296–299. doi: 10.1016/j.phymed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Topitsoglou-Themeli V, Kolokotronis A, Dangalis P, Lambrou D. Chios mastiha and oral hygiene: differentiation in microbial plaque formation. Pedodontia. 1985;2:56–59. [Google Scholar]
- 8.Takahashi K, Fukazawa M, Motohira H, Ochiai K, Nishikawa H, Miyata T. A pilot study on antiplaque effects of mastic chewing gum in oral cavity. J. Periodontol. 2003;74:501–505. doi: 10.1902/jop.2003.74.4.501. [DOI] [PubMed] [Google Scholar]
- 9.Magiatis P, Melliou E, Skaltsounis AL, Chinou IB, Mitaku S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Planta Med. 1999;65:749–752. doi: 10.1055/s-2006-960856. [DOI] [PubMed] [Google Scholar]
- 10.Triantafyllou A, Bikineyeva A, Dikalova A, Nazarewicz R, Lerakis S, Dikalov S. Anti-inflammatory activity of chios mastiha gum is associated with inhibition of TNF-alpha induced oxidative stress. Nutr. J. 2011;10:64. doi: 10.1186/1475-2891-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Rahman AHY, Youssef SAM. Mastiha as an antioxidant. J. Am. Oil Chem. Soc. 1975;52:423. doi: 10.1007/BF02545280. [DOI] [Google Scholar]
- 12.Triantafyllou A, Chaviaras N, Sergentanis TN, Protopappa E, Tsaknis JS. Chios mastic gum modulates serum biochemical parameters in a human population. J. Ethnopharmacol. 2007;111:43–49. doi: 10.1016/j.jep.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RK, Christensen KB, Assimopoulou AN, Fretté X, Papageorgiou VP, Kristiansen K, Kouskoumvekaki I. Pharmacophore-driven identification of PPARγ agonists from natural sources. J. Comput. Aided Mol. Des. 2011;25:107–116. doi: 10.1007/s10822-010-9398-5. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama Y. Ministerial notification no. 430 of the Ministry of Health, Labour and Welfare: a basic direction for comprehensive implementation of national health promotion. Available from: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-enkoukyoku/0000047330.pdf. Accessed Dec 27, 2012.
- 15.Vallianou I, Peroulis N, Pantazis P, Hadzopoulou-Cladaras M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS One. 2011;6:e20516. doi: 10.1371/journal.pone.0020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgiadis I, Karatzas T, Korou M, Katsilambros N, Perrea D. Beneficial health effects of chios gum mastic and peroxisome proliferator-activates receptors: indications of common mechanisms. J. Med. Food. 2015;18:1–10. doi: 10.1089/jmf.2014.0021. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Yoshimura Y, Kaimoto T, Kunii D, Komatsu T, Yamamoto S. Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. Jpn. J. Nutr. 2001;59:221–232. doi: 10.5264/eiyogakuzashi.59.221. [DOI] [Google Scholar]
- 18.Georgiadis I, Karatzas T, Korou LM, Agrogiannis G, Vlachos IS, Pantopoulou A, Tzanetakou IP, Katsilambros N, Perrea DN. Evaluation of chios mastic gum on lipid and glucose metabolism in diabetic mice. J. Med. Food. 2014;17:393–399. doi: 10.1089/jmf.2013.0069. [DOI] [PubMed] [Google Scholar]
- 19.Kartalis A, Didagelos M, Georgiadis I, Benetos G, Smyrnioudis N, Marmaras H, Voutas P, Zotika C, Garoufalis S, Andrikopoulos G. Effect of chios mastic gum on cholesterol and glucose levels of healthy volunteers: a prospective, randomized, placebo-controlled, pilot study (CHIOS-MASTIHA) Eur. J. Prev. Cardiol. 2016;23:722–729. doi: 10.1177/2047487315603186. [DOI] [PubMed] [Google Scholar]
- 20.Berger J, Moller D. The mechanism of action of PPARs. Annu. Rev. Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 21.Nissen SE, Nicholls SJ, Wolski K, Howey DC, McErlean E, Wang MD, Gomez EV, Russo JM. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. J. Am. Med. Assoc. 2007;297:1362–1373. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- 22.Wagner K, Wagner N. Peroxisome proliferator-activated receptor beta/delta (PPAR-β/δ) acts as a regulator of metabolism linked to multiple cellular functions. Pharmacol. Ther. 2010;125:423–435. doi: 10.1016/j.pharmthera.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/S0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 24.Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alterations in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 25.Fajas L, Auboeuf D, Raspé E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis and expression of the human PPAR gamma gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 26.Kahara T, Takamura T, Hayakawa T, Nagai Y, Yamaguchi H, Katsuki T, Katsuki K, Katsuki M, Kobayashi K. PPARγ gene polymorphism is associated with exercise-mediated changes of insulin resistance in healthy men. Metabolism. 2003;52:209–212. doi: 10.1053/meta.2003.50038. [DOI] [PubMed] [Google Scholar]
- 27.Dedoussis GV, Kalioka AC, Psarras S, Chiou A, Mylona A, Papadopoulos NG, Andrikopoulos NK. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis. 2004;174:293–303. doi: 10.1016/j.atherosclerosis.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto S. Case study: exercise therapy. Tokyo: Kyorin-shoin; 2000. pp. 86–167. [Google Scholar]
- 29.Holloszy JO, Skinner JS, Toro G, Cureton TK. Effects of a six month program of endurance exercise on the serum lipids of middle-aged men. Am. J. Cardiol. 1964;14:753–760. doi: 10.1016/0002-9149(64)90004-9. [DOI] [PubMed] [Google Scholar]
- 30.Coll T, Rodriguez-Calvo R, Barroso E, Serrano L, Eyre E, Palomer X, Vazquez-Carrera M. Peroxisome proliferator-activated receptor (PPAR)β/δ: a new potential therapeutic target for the treatment of metabolic syndrome. Curr. Mol. Pharmacol. 2009;2:46–55. doi: 10.2174/1874467210902010046. [DOI] [PubMed] [Google Scholar]
- 31.Castellano J, Guinda A, Delgado T, Rada M, Cayuela JA. Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791–1799. doi: 10.2337/db12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int. Sports Med. 2000;21:1–12. doi: 10.1055/s-2000-8847. [DOI] [PubMed] [Google Scholar]
- 33.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Ann. Rev. Med. 1998;49:235–261. doi: 10.1146/annurev.med.49.1.235. [DOI] [PubMed] [Google Scholar]
- 34.Gulve EA, Spina RJ. Effect of 7–10 days of cycle ergometer exercise on skeletal muscle GLUT-4 protein content. J. Appl. Physiol. 1995;79:1562–1566. doi: 10.1152/jappl.1995.79.5.1562. [DOI] [PubMed] [Google Scholar]
- 35.Hardin DS, Azzarelli B, Edwards J, Wigglesworth J, Maianu L, Brechtel G, Johnson A, Baron A, Garvey WT. Mechanisms of enhanced insulin sensitivity in endurance-trained athletes: effects on blood flow and differential expression of GLUT-4 in skeletal muscles. J. Clin. Endocrinol. Metabolism. 1995;80:2437–2446. doi: 10.1210/jcem.80.8.7629239. [DOI] [PubMed] [Google Scholar]