Abstract
Physicochemical properties of six different varieties of barley and their β-glucans were evaluated along with in vitro bile acid binding and starch digestibility for health beneficial effects. β-Glucan concentrations in less-hulled, beer, black, waxy-naked, naked, and blue barley were 3.44, 3.46, 6.08, 6.75, 6.45, and 5.91%, respectively. Viscosity of waxy-naked barley flour was the highest. While the yield of β-glucan from waxy-naked barley after extraction was 95.49%, less-hulled barley was 70.09%. As the increase of β-glucan purification, in vitro bile acid binding was increased when compared with cholestyramine and cellulose. In vitro starch digestibility of barley flour and the mixture of potato starch with β-glucan were increased by heat and β-glucan concentration. Estimated glycemic index (GI) calculated based on in vitro starch digestibility was decreased by increasing β-glucan. These results suggest that the physicochemical properties of barley were dependent on the variety of barley and especially β-glucan was involved.
Keywords: Barley, β-glucan, In vitro bile acid binding, Starch digestion, Glycemic index
Introduction
Barley, an ancient and important cereal crop, was presumably first used as human food but evolved primarily into feeding, malting, and brewing. Barley can be classified into various types: spring or winter, two-row or six-row, hulled or hull-less, and malting or feeding. The different types of barley are various in their physicochemical properties and contain different concentration of dietary fiber including β-glucan [1, 2]. Hull-less barley requires removing hulls and is more suitable for food processing and human consumption than hulled barley. On the other hand, hulled barley is preferred for malting and brewing [3]. The hull-less barley was genetically developed to have waxy endosperm and high-amylose starch [4]. Waxy starch genotypes are usually associated with high β-glucan content [5].
β-Glucan, non-starch polysaccharide, is found in walls of endosperm and aleurone cells of barley. It constitutes 2–11% weight of the total kernel carbohydrates but usually ranges between 4 and 7%. Because β-glucans are enclosed with starch and protein matrix and lipid in the grain, it is difficult to extract β-glucan. The extraction and purification of β-glucans in barley can be affected by flour particle size, temperature, pH, and ionic strength [1, 6]. A typical extraction process involves: inactivation of endogenous enzymes, extraction of β-glucans, and purification-isolation stage [7]. Structurally, β-glucans are linear homopolymers of D-glucopyranosyl residues linked mostly via two or three consecutive β(1 → 4) linkages that are separated by a single β(1 → 3) linkage with the ratio of about 2.3–3.0 [8]. β-Glucans from different types of cereals showed the similar molecular structure but exhibited variation in the ratios of β(1 → 4) to β(1 → 3) linkages [9]. The chain conformation of β-glucans is attributed to their ability to form solution with high intrinsic viscosity [8]. The rheological properties of β-glucan solution depend mainly on the ability of β-glucan chains to associate, which can be determined by the proportion of cellotriosyl/cellotetraosyl units and their arrangements [10]. In addition, solubility of β-glucan is influenced by decreasing or increasing molar ratio of cellotriosyl/cellotetraosyl units [11].
The ability of β-glucan to form viscous solution is a key physicochemical property responsible for physiological effects of β-glucan [12]. When consumed barley, β-glucan as a soluble dietary fiber increases small intestinal viscosity because of its low molecular weight and tendency to form viscous solution and results in reducing cholesterol levels [13]. The molecular weight of β-glucans to be physiologically active varies between 2.65 × 104 and 3.24 × 106 g/mol [14]. The molecular weight of β-glucans depends on genetic and environmental factors, isolation, and purification process [15]. Frank et al. [16] reported that β-glucans with low molecular weight (2.17 × 105 g/mol) and high molecular weight (7.97 × 105 g/mol) had similar cholesterol-lowering effects in human. Serum cholesterol concentration can be reduced by barley β-glucans which effectively excretes bile acids. And bile acids are synthesized in the liver from cholesterol. Circulation by being converted from cholesterols to bile acids in the liver can effectively reduce cholesterol in serum [17, 18].
Along with cholesterol lowering effect of β-glucan, it can slow down the digestion of carbohydrates as showing low glycemic index (GI) [19–21]. The GI is a classification of the blood glucose-raising potential of carbohydrates in foods. In general, low-GI foods are defined as having a GI of less than 55, medium-GI foods a GI of 56–69, and high-GI foods a GI of over 70. Low-GI diets are associated with improvement of insulin sensitivity and increase of colonic fermentation. β-Glucans from barley have been shown to influence human glycemic control and help lower GI of foods. In this study, the physicochemical properties of β-glucans extracted from six different varieties of barley grown in Jeju were investigated along with the health beneficial characteristics of β-glucan for an attempt to better understand the characteristics of β-glucan in barley.
Materials and methods
Materials
Six different varieties of hulled and hull-less barley were obtained from a local market in Jeju (Jeju, Korea). Hulled barley varieties included less-hulled barley (Iksan168ho) and beer barley (Iksan173ho). Hull-less barley varieties included black barley (Iksan100ho), waxy-naked barley (Suwon236ho), naked barley (Erie36ho), and blue barley (Iksan479ho). All barley grains were ground into flour with a mill (MF10, Ika-Werke GmbH & Co., Staufen, Germany) through a 100 mesh screen.
Chemical composition of barley flours
Chemical composition of barley flours were determined by AOAC [22] and AACC [23] method. Moisture content of barley flours was measured with a moisture analyzer (MX-50, AND Ltd. Co., Tokyo, Japan) at 105 °C. Crude protein, fat, and ash were determined by Kjeldahl method, Soxhlet extractor, and ash incineration, respectively. Contents of β-glucan, starch, and total dietary fiber were analyzed by following the AACC method 32-23.01 and 32-76.13, and AOAC method 991.43 using assay kits (Megazyme International Co., Wicklow, Ireland). All analyses were run in triplicate and the averages were reported on a dry-weight basis.
Viscosity of barley suspension
Viscosity of barley suspension was determined by modification of the procedure of Jeong et al. [24]. Barley flour suspension was prepared by 1:8 (barley flour to water ratio) and the viscosity was measured by a viscosity analyzer (DV-I, Brookfield Co., Middleboro, MA, USA). A stirring speed was 100 rpm, spindle No.2 was used at room temperature (25 °C), and No.4 (less-hulled barley), No.5 (beer barley), No.6 (black barley), and No.7 (waxy-naked, naked, and blue barley) were used at 70 °C for heating.
Extraction of β-glucan
β-Glucan was extracted from barley using the procedure of Kim and White [25] with modifications. Barley flour (15.0 g) was suspended in 150 mL of 82% ethanol and refluxed to remove fat and inactive endogenous enzyme at 80 °C for 3 h. Barley suspension was then centrifuged at 3100×g for 20 min to remove supernatant. Precipitate was washed with 50 mL of 95% ethanol, centrifuged at 3100×g for 15 min twice, and dried at 40 °C for overnight. β-Glucan was extracted from the refluxed and dried flour (about 12.0 g) by using 240 mL distilled water at 65 °C for 3 h. After extraction, suspension was centrifuged at 3100×g for 20 min and the process was repeated for two more times. The supernatant was treated with 0.2 mL α-amylase (Sigma-Aldrich, St. Louis, MO, USA) and 36–40 mg calcium chloride (Sigma-Aldrich) in a shaking water bath (JSSB-30T, JS Research Inc., Gongju, Korea) at 90 °C for 2 h and centrifuged at 3100×g for 20 min. The supernatant was then reacted with 15 mg pancreatin (Sigma-Aldrich) and 0.2 mL 10% sodium azide (Sigma-Aldrich) at 40 °C for 3 h. Reactant was added with 2-fold 60% ethanol, stayed overnight at 4 °C, and centrifuged at 3100×g for 20 min. The precipitate was freeze dried. Total β-glucan contents in freeze-dried β-glucan extracts were measured for the calculation of extraction yield.
Molecular weight determination of β-glucan
The molecular weight (MW) of β-glucans extracted from different barley varieties was determined by size-exclusion ultra high-performance liquid chromatography (SE-UHPLC, Ultimate 3000, Dionex, Thermo Flasher Scientific Inc., Waltham, MA, USA) equipped with a refractive index detector (RI-101, Shodex Scientific Co., Ltd, Kanagawa, Japan). The SE-UHPLC was composed of a solvent delivery module, a 50 μL loop injection valve, a guard column (Ohpak SB-G, Shodex Showa Denko KK, Tokyo, Japan), and three sequentially connected columns (Ohpak SB-806 HQ, Ohpak-805 HQ, Ohpak-804 HQ, Shodex Showa Denko KK). The columns and detector were controlled at 40 °C. The mobile phase was MiliQ water (Milipore, Bedford, MA, USA) containing 0.02% sodium azide (Sigma-Aldrich) and the flow rate was 0.6 mL/min. β-Glucan samples were filtered through a 0.45 μm nylon syringe filter (Minisart RC 25, Sartorius Stedim Biotech, Goettingen, Germany). β-Glucan MW standards (P-MWBGS, Megazyme International Co.) with reported MW values, 3.59, 2.45, 1.93, 1.23, and 0.4 × 105 g/mol, were used to estimate β-glucan molecular weight. The peak MW and number-average MW (M n) were obtained by a first-order polynomial curve of log MW against retention time. The M n was calculated by the equation M n = ∑wi/∑(wi/MWi), where wi was the weight fraction of time × height derived from the SE-UHPLC chromatogram [21].
Resolubility of β-glucans extracted from barley
Resolubility of the extracted β-glucan in water was measured according to the method of Lee et al. [26] with modifications. The β-glucan dispersion in water (1%, w/v) was agitated at 50 °C for 12 h and centrifuged at 12,000×g for 10 min. Precipitate was dried and calculated as percentage (%) = [(weight of β-glucan dissolved in the supernatant)/(initial weight of β-glucan in the dispersion)] × 100.
Ratio of β(1 → 3) to β(1 → 4) linkages in extracted β-glucans
Ratio of β(1 → 3) to β(1 → 4) linkages was determined to compare the structure of extracted β-glucan [27]. The content of β(1 → 3) linked glucoses was determined as following: β-glucan (2.0 mg) was added to 1 mL sodium phosphate buffer (20 mM, pH 6.5) with 4U lichenase (Megazyme International Co.) which broke all β(1 → 4) linkages of 3-O-substituted glucose residues in β-glucan at 40 °C for 22 h. Reactant was added to 5 mL sodium acetate buffer (200 mM, pH 4.0) and centrifuged at 2000×g for 10 min. After obtained 0.1 mL supernatant, the content of β(1 → 3) linked glucoses was determined using d-glucose assay kit (K-GLUC, Megazyme International Co.). Total glucose was determined as followed: β-glucans (2.0 mg) was added to 1 mL sodium phosphate buffer (20 mM, pH 6.5) with 4U lichenase (Megazyme International Co.) and incubated at 40 °C for 22 h. Reactant was treated with β-glucosidase (0.2U, Megazyme International Co.) at 50 °C for 10 min. After the obtained 0.1 mL suspension, total glucose content was determined. The β(1 → 4) linked glucoses was calculated by subtracting β(1 → 3) linked glucoses from total glucoses and ratio of β(1 → 3) to β(1 → 4) linkages was calculated as the amounts of β(1 → 4) linked glucoses/β(1 → 3) linked glucoses.
In vitro bile acid binding
In vitro bile acid binding of β-glucans from barley was determined to investigate health beneficial effect of β-glucan [28]. The bile acid mixture was prepared with sodium cholate (35%, Sigma-Aldrich), sodium deoxycholate (35%, Sigma-Aldrich), sodium glycocholate (15%, Sigma-Aldrich), and sodium taurocholate (15%, Sigma-Aldrich) in 10 mL of sodium phosphate buffer (50 mM, pH 6.9). The total amount of bile acid was 11.2 µmol/100 mg of bile acid mixture. Cholestyramine (Sigma-Aldrich) as a positive control and cellulose (Sigma-Aldrich) as a negative control were used. The stimulated gastric digestion was performed that 50 mg of cholestyramin and cellulose and 10 mg of β-glucan were digested with 1 mL of 0.01 N HCl and incubated in a shaking water bath at 37 °C for 1 h. The pH of mixture was adjusted to 6.9 with 0.1 N sodium hydroxide. The bile acid mixture (4 mL) and porcine pancreatin (5 mL, Sigma-Aldrich; 6.25 mg/mL in a 50 mM phosphate buffer, pH 6.9) were added and incubated in a shaking water bath at 37 °C for 1 h. After centrifugation at 2700×g for 15 min, the supernatant was taken. The precipitate was mixed with 5 mL of sodium phosphate buffer (50 mM, pH 6.9) and centrifuged again to remove the residue. The supernatant was taken and combined with the former supernatant. The supernatant that contained unbound bile acid was analyzed by a Bile Acid Diagnostic Kit (Trinity Biotech, Bray Co., Wicklow, Ireland). The standard curve was developed from the different concentrations of bile acid mixture (1.4, 0.7, 0.35, 0.175, 0.07, 0.035 µmole/mL) and the concentration of the bile acid bound were calculated. The in vitro bile acid binding of β-glucan from different varieties of barely was calculated on the basis of 100% bile acid binding of cholestyramine.
Preparation of barley flour and mixtures of potato starch and β-glucans for in vitro starch digestibility
To evaluate in vitro starch digestibility of β-glucan, barley flours (5% in distilled water) and eight different mixtures of potato starch (OCI Company Ltd., Seoul, Korea) with β-glucan extracted from barley were prepared by the method of Kim and White [19] with modification. For the mixtures, potato starch (5%) and β-glucan (1%) extracted from six barley varieties were solubilized in water. Eight different mixtures were: (1) potato starch solution (S, non-heated), (2) potato starch solution with heat (S, heated), (3) potato starch and β-glucan from less-hulled barley (S + Less-hulled), (4) potato starch and β-glucan from beer barley (S + Beer), (5) potato starch and β-glucan from black barley (S + Black), (6) potato starch and β-glucan from waxy-naked barley (S + Waxy), (7) potato starch and β-glucan from naked barley (S + Naked), and (8) potato starch and β-glucan from blue barley (S + Blue). The ratio of potato starch and β-glucan was established after preliminary study. All solutions were adjusted to the same volume and were heated at 90 °C for 10 min to gelatinize starch and cooled to room temperature except the samples with no heat.
In vitro starch digestibility
In vitro starch digestibility of the barley flours (non-heated and heated) of six different varieties and the eight different mixtures of potato starch with β-glucans were determined [19]. The enzyme for digestion was prepared with 0.9 g of pancreatin (Sigma-Aldrich) in 8 mL of distilled water and centrifuged at 1500×g for 10 min. The supernatant (5.4 mL) was mixed with 0.8 mL of diluted amyloglucosidase (89 U/mL, Megazyme International Co.). The enzyme solution was added with 0.5 mL of distilled water.
The six different barley flours (non-heated and heated), the mixtures of potato starch with β-glucans (heated), potato starch without β-glucan (non-heated and heated), and white bread (control) were digested as following: the sample was mixed with 10 glass beads (5 mm diameter), 2 mL of 0.05 M hydrochloric acid, and 10 mg of pepsin in 50 mL tube. The samples were incubated at 37 °C in a shaking water bath for 30 min. Four milliliter of sodium acetate buffer (0.5 M, pH 5.2) and 1 mL of enzyme solution were added to the tube at 1 min interval while the samples were incubated at 37 °C in a shaking water bath. Aliquots (100 µL) from the tube were taken at 0, 10, 20, 30, 60, 90, 120, and 180 min and 1 mL of 50% ethanol was added to each tube. These tubes were centrifuged at 800×g for 5 min. The glucose concentration of the supernatants (100 µL) was measured using d-glucose assay kit (Megazyme International Co.). Total starch hydrolysis was calculated as following: Total starch hydrolysis (%) = ((released glucose weight × 160/182)/(total starch weight in prepared solutions)) × 100.
Estimated glycemic index
The estimated glycemic index (GI) was calculated by the equation of Goni et al. [29]. Hydrolysis curve for each product was described as following: where C is the concentration at time t (min) and is the equilibrium concentration estimated by above equation. The area under the hydrolysis curve (AUC) was calculated for all products. where is the final time (180 min) and is the initial time (0 min). A hydrolysis index (HI) was predicted by dividing the AUC of each treatment by the AUC of control (white bread). The GI was then estimated as followed:
Statistical analysis
All analyses were done in triplicate. Data were analyzed by the analysis of variance (ANOVA) and followed by the Duncan’s multiple range test (p < 0.05) using SPSS (Statistics Package for the Social Science, Ver. 18.0, SPSS Inc., Chicago, IL, USA).
Results and discussion
Chemical compositions of barley flour
Chemical compositions of six different varieties of barley flours are in Table 1. Protein contents of black barley were greater than other barley varieties as showing 14.88%. Fat contents of black barley, waxy-naked barley, naked barley, and blue barely were greater than those of less-hulled barley and beer barley. All six varieties of barley flours contained similar amounts of ash and starch as showing 1.05–1.31 and 69.53–76.53%, respectively. Total dietary fiber in barely flours was ranged from 10.07 to 15.64%, which was greater than those reported by Lee [30] that total dietary fiber in barley were 9–10%. The waxy-naked, naked, and blue barley contained high amount of total dietary fiber. β-Glucan contents of black, waxy-naked, naked, and blue barley flours were ranged from 5.91 to 6.75%, which were greater than those of less-hulled and beer barley flour. Lee [31] reported that total β-glucan contents of hulled barley and hull-less barley varieties were 3.27–5.55%. The β-glucan contents of hulled barley varieties in the current study were similar to the report of Lee [31] but hull-less barley varieties were greater than them. According to Skendi et al. [32] report, barley usually contained 5–11% of β-glucan. The less-hulled and beer barley contained low amounts of β-glucan because these barley varieties were genetically modified to reduce β-glucan for beer brewing [3]. The study of Baik [2] reported that barley was usually composed of 65–68% starch, 10–17% protein, 4–9% ββ-glucan, 2–3% fat, and 1.5–2.5% ash, which was a similar result with the current study.
Table 1.
Chemical composition of different varieties of barley flour
| Barley | Chemical composition (%) | |||||
|---|---|---|---|---|---|---|
| Protein1 | Fat | Ash | Starch | Total dietary fiber | β-Glucan | |
| Less-hulled | 9.22 ± 0.42b2,3 | 1.26 ± 0.23c | 1.05 ± 0.12a | 72.24 ± 2.77a | 10.07 ± 2.56a | 3.44 ± 0.14c |
| Beer | 9.41 ± 0.63b | 1.22 ± 0.10c | 1.15 ± 0.10a | 74.65 ± 3.92a | 10.74 ± 2.99a | 3.46 ± 0.06c |
| Black | 14.88 ± 0.84a | 3.98 ± 0.56a | 1.26 ± 0.21a | 70.60 ± 5.34a | 10.86 ± 2.24a | 6.08 ± 0.23b |
| Waxy-naked | 8.87 ± 0.87b | 2.70 ± 0.10b | 1.31 ± 0.09a | 76.53 ± 5.23a | 15.64 ± 2.82a | 6.75 ± 0.07a |
| Naked | 7.99 ± 0.70b | 4.08 ± 0.52a | 1.16 ± 0.07a | 69.53 ± 6.99a | 14.10 ± 2.26a | 6.45 ± 0.42ab |
| Blue | 9.32 ± 0.96b | 2.39 ± 0.95b | 1.22 ± 0.27a | 72.83 ± 5.80a | 14.09 ± 4.18a | 5.91 ± 0.53b |
1All chemical contents were calculated as dry basis, %
2Each value is mean ± standard deviation
3Means with the different letter in a column indicate significant difference (p < 0.05) by Duncan’s multiple range test
Viscosity of barley flour suspension
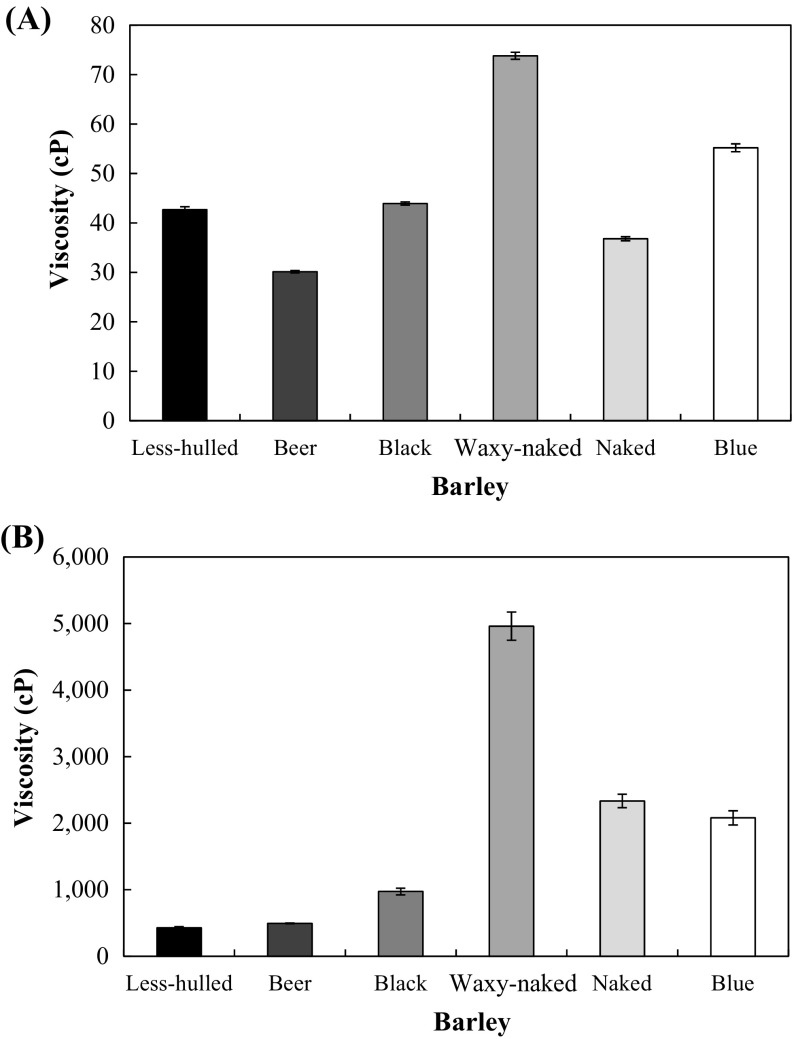
Viscosity of barley flour suspensions measured at room and heating temperature was shown in Fig. 1. The viscosity of waxy-naked barley at room temperature was 73.80 cP which was the highest among other varieties of barley. The viscosities of other barley varieties ranged from 30.13 to 55.20 cP. After heating, the viscosity of waxy-naked barley greatly increased to 4960 cP and black, naked, and blue barley were in the range of 973–2333 cP. These values were greater than those of less-hulled and beer barley suspensions (429–493 cP). Viscous solution generally contains water soluble polysaccharides including soluble dietary fiber [33]. Izydorczyk et al. [3] reported that viscosity, which determined the physical characteristics of barley, was affected by β-glucan concentration [3]. Therefore, in the current study, it was estimated that the viscosity of barley flour suspension at room temperature was affected by β-glucan. Wood [34] showed that β-glucan formed high viscous solution. Black, waxy-naked, naked, and blue barley containing high amount of β-glucan showed higher viscosity than less-hulled and beer barley did (Fig. 1). As heating, the viscosity of all barley flour suspensions was increased. This was possibly related to starch gelatinization as well [6]. In addition, the soluble dietary fiber was extruded to increase in viscosity because the insoluble cell walls of barley were destroyed by heating [35].
Fig. 1.
Viscosity of barley flour suspensions at room temperature (A) and heating (B)
Characteristics of β-glucan extracted from barley
β-Glucans extracted from six different varieties of barley showed the β-glucan concentration in the range of 70.09–95.46% (Table 2). β-Glucan in the less-hulled barley extract was the lowest as 70.09%. Izydorczyk et al. [36] reported that β-glucan extraction was affected by barley genotype and milling rate. Although barley mostly had high concentration of β-glucan, the extraction of β-glucan was difficult because β-glucans are structurally enclosed to starch, protein, and lipid [37]. The less-hulled barley was the variety which was not completely milled so that the yield of ββ-glucan was not possibly high. The β-glucan extract from beer barley was comparatively high in β-glucan concentration (85.47%) despite of its variety. Therefore, the extraction yield of β-glucan depended on not only the β-glucan content in barley flour but also other conditions, such as extraction processing, temperature, pH, and time [2]. The β-glucan extracts contained 2.57–7.48% of starch (Table 2). Likely, most of the starch in the barley flour was removed during enzyme treatment with α-amylase and pancreatin. β-Glucan extracts were mostly soluble β-glucan although other complex carbohydrates might not be measured by the methods of total starch and β-glucan analysis [38].
Table 2.
Chemical composition, molecular weight (MW) and characteristics of β-glucan extracted from different varieties of barley
| Barley (%) | Composition (%, dry wt basis) | β-Glucan MW (× 105 g/mol) | Characteristics | |||
|---|---|---|---|---|---|---|
| β-Glucan | Starch | Peak MW | Average MW | Resolubility | Ratio of β(1 → 3) and β(1 → 4) linkages | |
| Less-hulled | 70.09 ± 5.78c1,2 | 7.25 ± 0.39a | 9.14 ± 0.58a | 6.72 ± 0.69ab | 39.67 ± 1.44bc | 1.71 ± 0.11a |
| Beer | 85.47 ± 4.42b | 6.7 ± 0.78ab | 7.40 ± 1.48b | 6.03 ± 0.65bc | 29.89 ± 3.00d | 2.86 ± 1.47a |
| Black | 89.17 ± 2.95ab | 3.29 ± 1.29c | 6.79 ± 1.15b | 5.68 ± 0.33c | 45.93 ± 0.82b | 2.37 ± 0.02a |
| Waxy-naked | 95.46 ± 3.10a | 2.57 ± 1.38c | 8.92 ± 0.41a | 7.09 ± 0.63a | 56.56 ± 8.70a | 2.25 ± 0.34a |
| Naked | 88.85 ± 5.42ab | 4.32 ± 1.24bc | 9.30 ± 0.15a | 6.77 ± 0.39ab | 47.17 ± 3.85b | 2.02 ± 0.13a |
| Blue | 83.06 ± 1.53b | 7.48 ± 2.72a | 9.82 ± 0.39a | 7.29 ± 0.42a | 36.13 ± 1.04 cd | 2.15 ± 0.11a |
1Each value is mean ± standard deviation
2Means with the different letter in a column indicate significant difference (p < 0.05) by Duncan’s multiple range test
Peak and average molecular weight (MW) of β-glucan extracts are shown in Table 2. The peak MW of blue, naked, less-hulled, and waxy-naked barley were 9.82, 9.30, 9.14, and 8.92 × 105 g/mol, respectively, and beer and black barley were 7.40 and 6.79 × 105 g/mol, respectively. The average MW of barley flours ranged from 5.68 to 7.29 × 105 g/mol. This study indicated that the peak and average MW of hull-less barley varieties were similar to each other except black barley. The peak and average MW of black barley were more similar to beer barley. In addition, the MW of β-glucan was high when β-glucan content was low. Park et al. [39] reported that β-glucan MW was decreased as β-glucan purification was increased. Lazaridou and Biliaderis [15] were reported that β-glucan MW was different depending on barley varieties. Choi et al. [40] reported that the MW of β-glucan from non-waxy and waxy barley exhibited in the range of 2.0–9.3 × 105 and 5.4–9.7 × 105 g/mol, respectively. The molecular weight of β-glucan extracted from six different barley varieties depended on barley variety. Furthermore, the β-glucan molecular weight ranges could affect the biological functions of β-glucan when incorporated in food products.
Resolubility of the β-glucan extract from waxy-naked barley in water was the greatest as 56.56% and beer barley was the lowest as 29.89% (Table 2). According to Izydorczyk et al. [36], the β-glucan of waxy type barley showed slightly higher solubility than other types of barley. Although β-glucan concentration extracted from beer barley was high, its resolubility was low. Extracted β-glucans were mostly composed of β-glucan and starch. Therefore, resolubility of β-glucan extracted from different varieties of barley was affected by β-glucan structure, component, extraction conditions, and preprocessing [30]. In addition, the water solubility can influence blending of β-glucan into foods and more stable mixtures.
The ratio of β(1 → 3) and β(1 → 4) linkages in β-glucan extract to compare structural characteristics is shown in Table 2. The ratio of β(1 → 3) and β(1 → 4) linkages was as 1.71–2.86 to 1.0 which were similar to the results of Stone and Clarke [9] study showing as 2.3–3.0 to 1.0. The study of Phillip and Stone [40] indicated that the more β-glucan purified, the more the ratio of β(1 → 3) and β(1 → 4) linkages increased. The β-glucan extracted from less-hulled barley with low purification showed low ratio of β(1 → 3) and β(1 → 4) linkages.
In vitro bile acid binding
In vitro bile acid binding of β-glucan, cholestyramine, and cellulose are shown in Table 3. The positive control, cholestyramine, was bound to 8.96 µmol of bile acid/100 mg. The negative control, cellulose, was calculated as 0.66% binding when cholestyramine was bound to 100% of bile acids. These values of two controls were similar to the results shown in Kim and White [41]. The in vitro bile acid binding of β-glucan extracted from waxy-naked barley was the highest as 27.16%. This result was possibly attributed to the high amount of β-glucan in the extract. Other barley β-glucan extracts were calculated as 24.39-25.74% of bile-acid binding. Although the less-hulled barley contained low β-glucan content, the in vitro bile acid binding was measured as similar as other barley varieties. Kim and White [41] reported that the bile acid binding values was influenced by the β-glucan concentration. β-Glucan can lower the cholesterol level by removing bile acid [42]. Kahlon and Smith [18] showed that food fractions, such as dietary fiber, prevented the reabsorption of bile acid and stimulated plasma and liver cholesterol conversion to additional bile acids. Eliminating bile acids consumes cholesterol and reduces the serum cholesterol level. Kahlon and Smith [18] confirmed that bile acid binding was affected by the structure of carbohydrate and the presence of flavonoids and polyphenols. The bile acid binding of β-glucan from the black barley (the lowest bile-acid binding) was possibly influenced by the contents of flavonoids, such as anthocyanins [43]. In addition, the high bile-acid binding value of the less-hulled barley containing low β-glucan concentration might be suggested that the other types of dietary fiber present in the β-glucan extract possibly helped to bind bile acids.
Table 3.
In vitro bile acid binding of extracted β-glucan from different varieties of barley
| Barley | Bile acid bound | |
|---|---|---|
| Relative % to cholestyramine | µmol/100 mg total weight | |
| Less-hulled | 25.74 ± 0.18b1,2 | 2.31 ± 0.02b |
| Beer | 25.07 ± 0.28bc | 2.25 ± 0.02bc |
| Black | 24.39 ± 0.19c | 2.18 ± 0.11c |
| Waxy-naked | 27.16 ± 0.43a | 2.43 ± 0.04a |
| Naked | 25.40 ± 0.40bc | 2.27 ± 0.04bc |
| Blue | 25.26 ± 0.62bc | 2.26 ± 0.06bc |
| Cholestyramin | 100 | 8.96 ± 0.53 |
| Cellulose | 0.66 | 0.06 ± 0.02 |
1Each value is mean ± standard deviation
2Means with the different letter in a column indicate significant difference (p < 0.05) by Duncan’s multiple range test
In vitro starch digestibility
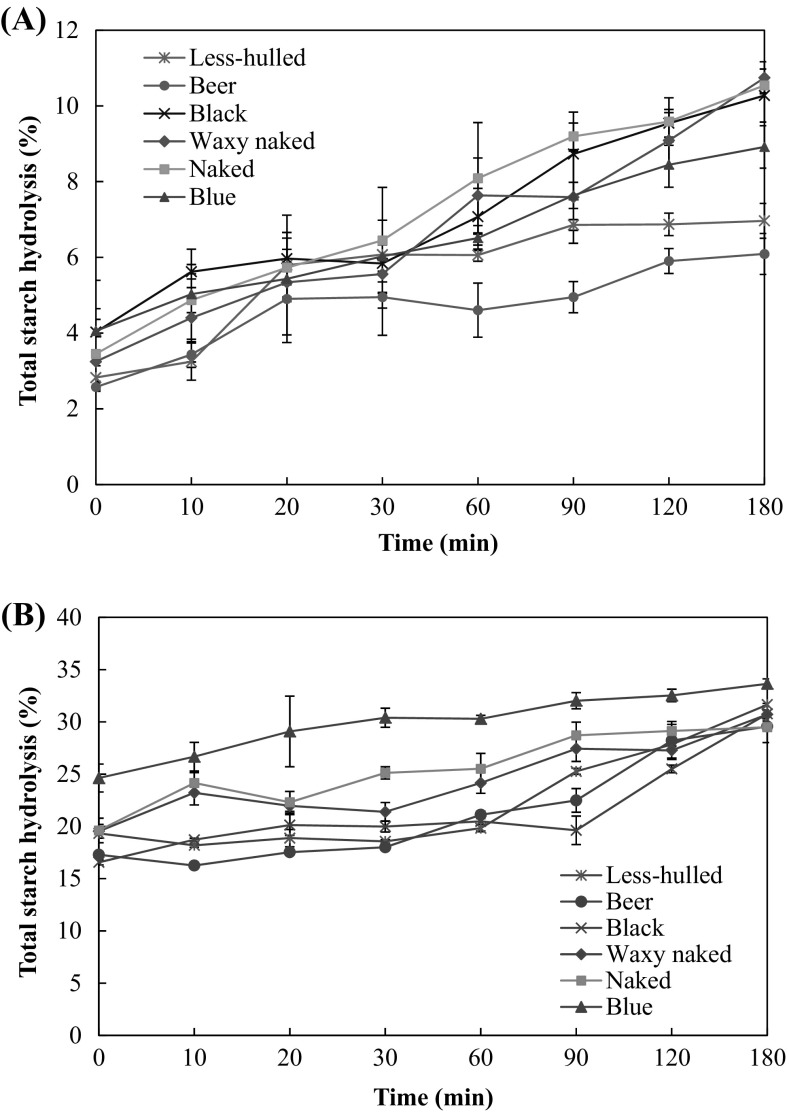
In vitro starch digestibility of barley flour is shown in Fig. 2. The starch hydrolysis of all barley flour increased as increasing the digestion time. The starch digestibility of all raw barley flour had similar patterns; however, barley that contained low β-glucan (i.e. less-hulled and beer barleys) was in low starch digestion. When heated barley flour, the starch digestibility greatly increased as shown in Fig. 2(B). The starch digestibility of blue barley flour was the highest when heated. Generally, the starch digestibility can be influenced by structure of starch, cultivar, β-glucan content, and sensitivity of enzyme [44]. Barley starch had usually low amylose content and rice, potato, and bean starch with high amylose content were more easily digested than barley did [29, 45].
Fig. 2.
In vitro starch hydrolysis of barley flour solution non-heated (A) and heated (B)
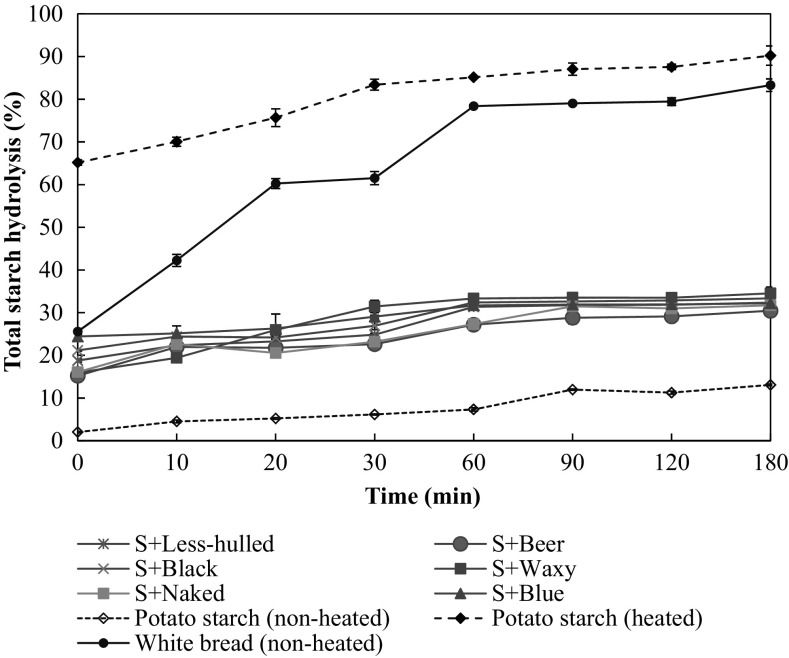
In vitro starch digestibility of potato starch (non-heated and heated) and the mixtures of potato starch with β-glucan extracted from different varieties of barley are shown in Fig. 3. The starch digestibility of the mixtures of potato starch and β-glucans were lower than those of heated potato starch and white bread as a control. These results indicated that β-glucan in the mixtures possibly retarded starch digestion. The mixture of potato starch and β-glucan extracted from waxy-naked barley (S + Waxy) tended to be high in starch digestibility when compared with other mixtures with β-glucan extracts. According to Kim and White [19], the β-glucan with low molecular weight impacted to reduce starch digestion. In addition, the rate of starch digestion can be slow by the incorporation of indigestible amylopectin of starch [46]. Therefore, structural features of starch in β-glucan extract should be investigated as a further study.
Fig. 3.
In vitro starch hydrolysis of potato starch (non-heated and heated), white bread, and the mixtures of potato starch with β-glucan extracted from different varieties of barley
Estimated glycemic index
Based on the starch digestibility, the estimated glycemic index (GI) was calculated and shown in Tables 4 and 5. The estimated GI of raw and heated barley flour belonged to low-GI food and medium-GI food as 43.14–46.06 and 55.36–61.97, respectively (Table 4). The estimated GI of barley flour in the current study was lower than the estimated GI of barley as shown in Foster-Powell et al. [47]. According to Zhang et al. [48], normal and waxy type cereals had 20–30 and 0–5% amylose content, respectively. Barley was mostly constituted with low amount of amylose and high amount of amylopectin. When amylopectin was not completely hydrolyzed, barley possibly indicated low estimated GI. Although less-hulled barley and beer barley were normal type barley, estimated GI was lower than that of other barley varieties. Starch structure of less-hulled barley and beer barley could be more complicated, like they were mixed with indigestible carbohydrates because these varieties were not completely polished. The estimated GI of barley flour increased when heated. The barley flour was gelatinized by heating and formed swelling amylopectin and amylose. The greater swelling in the barley flour starch was the faster starch digestibility was [48].
Table 4.
Estimated glycemic index (GI) for different barley flour
| Barley flour | Estimated GI | |
|---|---|---|
| Non-heated | Heated | |
| Less-hulled | 44.56 ± 0.14c1,2 | 57.26 ± 0.29d |
| Beer | 43.14 ± 0.41d | 55.40 ± 1.13e |
| Black | 45.75 ± 1.11ab | 55.36 ± 1.36f |
| Waxy-naked | 45.05 ± 1.22bc | 58.66 ± 1.22c |
| Naked | 46.06 ± 0.35a | 59.85 ± 1.26b |
| Blue | 44.99 ± 0.35c | 61.97 ± 0.78a |
1Each value is mean ± standard deviation
2Means with the different letter in a column indicate significant difference (p < 0.05) by Duncan’s multiple range test
Table 5.
Estimated glycemic index (GI) for the mixture of potato starch and β-glucan extracted from different varieties of barley
| Mixture of potato starch and β-glucan extracted from barley | Estimated GI |
|---|---|
| Less-hulled | 62.14 ± 0.35a1,2 |
| Beer | 59.71 ± 1.44b |
| Black | 62.68 ± 1.62a |
| Waxy-naked | 63.19 ± 0.27a |
| Naked | 62.21 ± 0.80a |
| Blue | 62.47 ± 0.39a |
| Potato starch (non-heated) | 47.98 ± 0.19 |
| Potato starch (heated) | 100.50 ± 1.43 |
| White bread | 94.61 ± 0.25 |
1Each value is mean ± standard deviation
2Means with the different letter in a column indicate significant difference (p < 0.05) by Duncan’s multiple range test
The estimated glycemic index (GI) of the mixtures with potato starch and β-glucan extracted from different varieties of barley was reduced by addition of β-glucan (Table 5). The estimated GI of heated potato starch was the highest as 100.50 and the estimated GI of all mixtures with potato starch and β-glucans were much lower than heated potato starch as 59.71–63.19. These results indicated that β-glucan helped to reduce the estimated GI of the heated potato starch. The GI was first introduced as a mean for identifying carbohydrate-rich foods based on their ability to raise postprandial blood glucose levels [47]. According to Cavallero et al. [49] study, a linear decrease in glycemic index was found for increasing barley β-glucan content when consumed as bread. They indicated that the barley bread contained 2.0, 2.8, and 5.7% β-glucan decreased glycemic index to 85.42 ± 13.72, 74.46 ± 15.48, 69.67 ± 7.19, respectively. The β-glucan with high molecular weight formed highly viscous solution and showed low GI value [19]. The viscous property of the mixtures with potato starch and β-glucan in the current study helped to retard the susceptibility of enzyme to digest starch. This study demonstrated that starch digestibility was affected by heating, starch structure, and presence of β-glucan and especially dietary fiber including β-glucan from barley could slow starch digestion rate indicating the low GI value.
In summary, the physicochemical and in vitro physiological properties of six different varieties of barley grown in Jeju were determined. The amount of β-glucan in hull-less barley (black, waxy-naked, naked, and blue barley) flours was high as 5.91–6.75%. Viscosity of barley flour suspensions was increased with β-glucan contents and heating treatment. From the waxy-naked barley, β-glucan was highly extracted with the yield of 95.46%. Although resolubility of β-glucan extracts was similar to each other, the ratio of β(1 → 4) to β(1 → 3) linkages was statistically different. In vitro bile-acid binding was mostly influenced by β-glucan purification. In vitro starch hydrolysis of barley flour and the mixtures of potato starch and β-glucan extracts were influenced by β-glucan concentration and heat treatment. Estimated GI was increased by starch gelatinization and decreased by addition of β-glucan extracts. These results indicated that β-glucan was the main factor to influence in vitro bile acid binding and in vitro starch digestibility.
Acknowledgements
This research was supported by the 2016 scientific promotion program funded by Jeju National University.
References
- 1.Newman CW, Newman RK. A brief history of barley foods. Cereal Foods World. 2006;51:4–7. [Google Scholar]
- 2.Baik BK, Steven EU. Barley for food: Characteristics, improvement, and renewed interest. J. Cereal Sci. 2008;48:233–242. doi: 10.1016/j.jcs.2008.02.002. [DOI] [Google Scholar]
- 3.Izydorczyk MS, Storsley J, Labossiere D, MacGregor AW, Rossnagel BG. Variation total and soluvle β-glucan content in hulless barely: effects of thermal, physical, and enzymic treatments. J. Agric. Food Chem. 2000;48:982–989. doi: 10.1021/jf991102f. [DOI] [PubMed] [Google Scholar]
- 4.Quinde Z, Ulllrich S, Baik BK. Genotypic variation in colour and discolouration potential of barley-based food products. Cereal Chem. 2004;81:752–758. doi: 10.1094/CCHEM.2004.81.6.752. [DOI] [Google Scholar]
- 5.Izydorczyk MS, Lagasse SL, Hatcher DW, Dexter JE, Rossnagel BG. The enrichment of Asian noodles with fibre-rich fractions derived from roller milling of hull-less barley. J. Sci. Food Agr. 2005;85:2094–2104. doi: 10.1002/jsfa.2242. [DOI] [Google Scholar]
- 6.Wood PJ, Siddiqui IR, Paton D. Extraction of high viscosity gums from oat. Cereal Chem. 1978;55:1038–1049. [Google Scholar]
- 7.Charles SB, Louise JC. The potential use of cereal (1 → 3, 1 → 4)-β-D-glucans as functional food ingredients. J. Cereal Sci. 2005;42:1–13. doi: 10.1016/j.jcs.2005.01.002. [DOI] [Google Scholar]
- 8.Izydorczyk MS, Dexter JE. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products. Food Res. Int. 2008;41:850–868. doi: 10.1016/j.foodres.2008.04.001. [DOI] [Google Scholar]
- 9.Stone BA, Clarke AE. (1 → 3, 1 → 4)-β-Glucan in higher plants. pp. 431-489. In: Chemistry and Biology of (1 → 3)-β-Glucans. Stone BA, Clarke AE (eds). La Trobe University Press, Victoria, Australia (1992)
- 10.Tudorica CM, Kuri V, Brennan CS. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J. Agri. Food Chem. 2002;50:347–356. doi: 10.1021/jf0106953. [DOI] [PubMed] [Google Scholar]
- 11.Jiang G, Vasanrhan T. MALDI-MS and HPLC quantification of oligosaccharides of lichenase-hydrolysed water-soluble β-glucans from ten barley varieties. J. Agric. Food Chem. 2000;48:3305–3310. doi: 10.1021/jf0001278. [DOI] [PubMed] [Google Scholar]
- 12.Wood PJ. Relationship between solution properties of cereal β-glucans and physiological effect – a review. Trend Food Sci. Tech. 2004;15:320–646. doi: 10.1016/j.tifs.2003.03.001. [DOI] [Google Scholar]
- 13.Kalra S, Jood S. Effect of dietary barley β-glucan on cholesterol and lipoprotein fractions in rats. J. Cereal Sci. 2000;31:141–145. doi: 10.1006/jcrs.1999.0290. [DOI] [Google Scholar]
- 14.Butt MS, Tahir-Nadeem M, Khan MKI, Shabir R, Butt MS. Oat: unique among the cereals. Eur. J. Nutr. 2008;47:68–79. doi: 10.1007/s00394-008-0698-7. [DOI] [PubMed] [Google Scholar]
- 15.Lazaridou A, Biliaderis CG. Molecular aspects of cereal β-glucan functionality: physical properties, technological applications and physiological effects. J. Cereal Sci. 2007;46:101–118. doi: 10.1016/j.jcs.2007.05.003. [DOI] [Google Scholar]
- 16.Frank J, Sundberg B, Kamal-Eldin A, Vessby B, Aman P. Yeast-leavened oat breads with high or low molecular weight β-glucan do not differ in their effects on blood concentrations of lipids, insulin, or glucose in humans. J. Nutr. 2004;134:1384–1388. doi: 10.1093/jn/134.6.1384. [DOI] [PubMed] [Google Scholar]
- 17.Kahlon TS, Woodruff CL. In vitro binding of bile acids by rice bran, oat bran, barley and β-glucan enriched barley. Cereal Chem. 2003;80:260–263. doi: 10.1094/CCHEM.2003.80.3.260. [DOI] [Google Scholar]
- 18.Kahlon TS, Smith GE. In vitro binding of bile acids by bananas, peaches, pineapple, grapes, pears, apricots and nectarines. Food Chem. 2007;101:1046–1051. doi: 10.1016/j.foodchem.2006.02.059. [DOI] [Google Scholar]
- 19.Kim HJ, White PJ. Impact of the molecular weight, viscosity, and solubility of β-glucan on in vitro oat starch digestibility. J. Agric. Food Chem. 2013;61:3270–3277. doi: 10.1021/jf305348j. [DOI] [PubMed] [Google Scholar]
- 20.Bjorck I, Lijeverg H, Ostman E. Low glycemic-index foods. Br. J. Nutr. 2000;83:149–155. doi: 10.1017/S0007114500001094. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, White PJ. In vitro digestion rate and estimated glycemic index of oat flours from typical and high β-glucan oat lines. J. Agric. Food Chem. 2012;60:5237–5242. doi: 10.1021/jf300429u. [DOI] [PubMed] [Google Scholar]
- 22.AOAC. Official methods of analysis of AOAC Intl. 16th ed. Method 991.43. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
- 23.AACC International. Approved Method of the AACC, 10th ed. Method 21-23.01, 76.13. Association of Cereal Chemists, St. Paul, MN, USA (2000)
- 24.Jeong HS, Kang TS, Park HJ, Jung IS. Lee HY Characteristics of viscosity and components of soluble extract in oats. Food Eng. Prog. 2004;8:40–46. [Google Scholar]
- 25.Kim HJ, White PJ. In vitro bile-acid binding and fermentation of high, medium, and low molecular weight β-glucan. J. Agric. Food Chem. 2010;58:628–634. doi: 10.1021/jf902508t. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Jang GY, Kim HY, Woo KS, Hwang IG, Kim KJ, Lee MJ, Kim TJ, Lee JS, Jeong HS. Physicochemical properties of barley β-glucan with different heating temperatures. Korean J. Soc. Food Sci. Nutr. 2012;41:1764–1770. doi: 10.3746/jkfn.2012.41.12.1764. [DOI] [Google Scholar]
- 27.Woodward JR, Fincher GB, Stone BA. Water-soluble (1 → 3), (1 → 4)-β-D-glucans from barley (Hordeum vulgare) endosperm. II. Fine structure. Carbohydr. Polym. 1980;3:207–225. doi: 10.1016/0144-8617(83)90019-X. [DOI] [Google Scholar]
- 28.Kim HJ, White PJ. Interactional effects of β-glucan, starch, and protein in heated oat slurries on viscosity and in vitro bile acid binding. J. Agric. Food Chem. 2012;60:6217–6222. doi: 10.1021/jf300786f. [DOI] [PubMed] [Google Scholar]
- 29.Goni I, Garcia–Alons A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. (N.Y) 17: 427–437 (1997)
- 30.Lee WJ. Changes in dietary fiber content of barley during pealing and cooking. Korean J. Food Sci. Technol. 1992;24:180–182. [Google Scholar]
- 31.Lee YT. β-Glucans in barley and oat their change in solubility by processing. Agric. Chem. Biotechnol. 1996;39:482–487. [Google Scholar]
- 32.Skendi A, Billiaderis CG, Lazaridou A, Izydorczyk MS. Structure and rheological properties of water soluble β-glucans from oat sultivars of Avena sativa and Avena bysantina. J. Cereal Sci. 2003;38:15–31. doi: 10.1016/S0733-5210(02)00137-6. [DOI] [Google Scholar]
- 33.Guleria P, Kumari S, Dangi N. β-glucan: health benefits and role in food industry-a review. J. ERS. Tech&Eng. 2015;8:255–263. [Google Scholar]
- 34.Wood PJ. Relationship between solution properties of cereal β-glucans and physiological effect – a review. Trend in Food Sci&Tech. 2004;15:320–646. [Google Scholar]
- 35.Brandt LM, Jeltema MA, Zabik ME, Jeltema BD. Effects of cooking in solutions of varying pH on the dietary fiber components of vegetables. J. Food Sci. 1984;49:133–141. doi: 10.1111/j.1365-2621.1984.tb13237.x. [DOI] [Google Scholar]
- 36.Izydorczyk MS, Jacobs M, Dexter JE. Distribution and structural variation of non-starch polysaccharides in milling fractions of hull-less barely. Cereal Chem. 2003;80:645–653. doi: 10.1094/CCHEM.2003.80.6.645. [DOI] [Google Scholar]
- 37.Brennan CS, Cleary LJ. The potential use of cereal (1 → 3, 1 → 4)-β-D-glucans as functional food ingredients. J. Cereal Sci. 2005;42:1–13. doi: 10.1016/j.jcs.2005.01.002. [DOI] [Google Scholar]
- 38.McCleary BV. Development of an integrated total dietary fiber method consistent with the Codex Alimentarius definition. Cereal Foods World. 2010;55:24–28. [Google Scholar]
- 39.Park HJ, Kang TS, Lee HB, Kim KY, Jang KI, Noh YH, Jeong HS. Purification of oat β-glucan byα-amylase treatment and characterization of its physiochemical properties. Korean J. Food Sci. Technol. 2005;5:776–782. [Google Scholar]
- 40.Choi HD, Seog HM, Choi IW, Park YK, Lee CR, Shin KS. Molecular structure of β-glucans isolated from non-waxy and waxy barley. Korean J. Food Sci. Biotechnol. 2004;13:744–748. [Google Scholar]
- 41.Kim HJ, White PJ. Optimizing the molecular weight of oat β-glucan for in vitro bile acid binding and fermentation. J. Agric. Food Chem. 2011;59:10322–10328. doi: 10.1021/jf202226u. [DOI] [PubMed] [Google Scholar]
- 42.Yao N, White PJ, Jannink JL, Alavi S. Impact of dry solids and bile acid concentrations on bile acid binding capacity of extruded oat cereals. J. Agric. Food Chem. 2008;56:8672–8679. doi: 10.1021/jf802284h. [DOI] [PubMed] [Google Scholar]
- 43.Siebenhandl S, Grausgruber H, Pellegrini N, Rio DD, Fogliano V, Pernice R, Berghofer E. Phytochemical profile of main antioxidant in different fraction of purple and blue wheat, and black barley. J. Agric. Food Chem. 2007;55:8541–8547. doi: 10.1021/jf072021j. [DOI] [PubMed] [Google Scholar]
- 44.Zhou M, Robards K, Glennie-Holmes M, Helliwell S. Structure and pasting properties of oat starch. Cereal Chem. 1998;75:273–281. doi: 10.1094/CCHEM.1998.75.3.273. [DOI] [Google Scholar]
- 45.Zheng GH, Sosulski FW. Determination of water separation from cooked starch and flour pastes after refrigeration and freeze-thaw. J. Food Sci. 1998;63:134–139. doi: 10.1111/j.1365-2621.1998.tb15693.x. [DOI] [Google Scholar]
- 46.Phillip DR, Stone BA. Water-soluble (1 → 3), (1 → 4)-β-glucans from barley (Hordeum vulgare) endosperm. IV. Comparison of 40 C and 65 C soluble fractions. Carbohydr. Polym. 1988;8:85–97. doi: 10.1016/0144-8617(88)90013-6. [DOI] [Google Scholar]
- 47.Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am. J. Cli. Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Q, Abe T, Takahachi T, Sarahara T. Variations in in vitro starch digestion of glutinous rice flour. J. Agric. Food Chem. 1996;44:2672–2674. doi: 10.1021/jf9508110. [DOI] [Google Scholar]
- 49.Cavallero A, Empilli S, Brighenti F, Stanca AM. High (1 → 3,1 → 4)-β-glucan barley fractions in bread making and their effects on human glycemic response. J. Cereal Sci. 2002;36:59–66. doi: 10.1006/jcrs.2002.0454. [DOI] [Google Scholar]