Abstract
The effects of ultrasound-assisted extraction (UAE) variables—namely extraction temperature (40–60 °C), ultrasonic power (50–150 W), and sonication time (40–60 min)—on the extractive value (EV) of bioactive phenolics from Malva sylvestris leaves were investigated and optimized using Response surface methodology. The effects of extraction solvents (ethanol, ethyl acetate, and n-hexane) on EV, free radical scavenging activity (FRSA), total phenolic content (TPC), and major bioactive phenolics were studied using agitated bed extraction (ABE), and the results were compared with the UAE findings. Under the optimal UAE conditions (48 °C, 110.00 W, and 48.77 min) the experimental EV was 279.89 ± 0.21 mg/g with 71.12 ± 0.15% DPPHsc, 73.35 ± 0.11% ABTSsc, and a TPC of 152.25 ± 0.14 mg GAE/g. Ethanolic ABE results in higher EV (320.16 ± 0.25 mg g−1) compared to UAE, while the FRSA and TPC values were reduced. HPLC analysis revealed that the concentration of bioactive phenolics increased significantly (p < 0.05) under the optimal UAE conditions.
Keywords: Malva sylvestris, Ultrasound-assisted extraction, Agitated bed extraction, Bioactive phenolic compound, HPLC
Introduction
In biological systems, the presence of natural antioxidants along with endogenous defenses (e.g., enzymes, proteins, and vitamins) may help prevent or slow the oxidative stress induced by free radicals. Oxidative damage may contribute to the development of age-related and degenerative diseases [1]. The protective effects of beneficial compounds for human health have been attributed to their antioxidant activity [1, 2]. The desire for a healthier diet allied with the increasing consumer demands to replace synthetic antioxidants such as butylated hydroxyl toluene (BHT), butylated hydroxylanisole (BHA), tertiary butyl hydroquinone (TBHQ), and gallates in food has pushed the food industry to examine new sources of natural antioxidants and develop efficient extraction techniques [3].
Ultrasound refers to sonic waves with frequencies higher than that of sound audible to the human ear. Ultrasonic waves for application in food processing can be divided into two categories based on the difference in sound intensity and frequency. High-frequency ultrasound, which operates at frequencies of 2–20 MHz with sound intensities in the range of 0.1–1 W/cm2, is used in food quality analysis, medical imaging, and non-destructive inspection. Power ultrasound, known as high-intensity ultrasound, operates at lower frequencies, typically 20–100 kHz, with a sound intensity ranging from 10 to 1000 W/cm2 [4]. The high energy level available in power ultrasound makes it suitable for use in the food industry to enhance processes such as oil extraction [5], bioactive-compound extraction [6], surface decontamination [7], microbial inactivation [8], enzyme inactivation [9], and starch–protein separation [10]. The use of power ultrasound is also called sonication, and most applications include a liquid medium [11]. High-intensity ultrasound is used as an inexpensive, reproducible, simple, and efficient alternative method of industrial relevance that improves the extraction process of bioactive compounds in food [12]. All the mechanical effects involved in ultrasonic process can accelerate the internal diffusion of bioactive-compounds result in the improved mass transfer of targeted compounds [13, 14]. Ultrasound may be used for dried substrates to facilitate swelling and hydration and cause an enlargement of the pores of the cell wall [15]; additional benefits result from the disruption of the biological cell walls during the ultrasonic cavitation to facilitate the release of contents [16]. Ultrasound has been recognized for potential industrial applications in the phyto-pharmaceutical extraction industry for a wide range of herbal extracts. Chemat et al. [17] published a very helpful review paper on ultrasound-assisted extraction (UAE) of food and natural products. They described the UAE mechanisms, techniques, combinations, protocols, and applications.
Malva sylvestris L. (Malvaceae), usually known as common mallow, is native to Europe, Asia, and North Africa. It is an annual plant with purple flowers and shallowly lobed leaves. M. sylvestris is commonly used as a vegetable and medicinal plant in Iran, where it is called Panirak. Some parts of this plant such as the roots, flowers, seeds, and leaves have been employed in traditional medicine owing to the plant’s therapeutic relevance. Nowadays, the consumption of M. sylvestris is widespread because new research has revealed important therapeutic properties of this plant [18]. The leaves of this plant have potent anti-inflammatory, antioxidant, anticomplementary, anticancer, and skin tissue integrity activity [18]. M. sylvestris flowers are consumed as a remedy for eczema, bronchitis, cut wound, dermal infected wounds, inflammations, and digestive problems [19]. The total cholesterol and triglycerides of plasma can be decreased by using M. sylvestris anthocyanins [18]. Young M. sylvestris leaves are used raw in salads and soups and as boiled vegetables [20].
Based on our knowledge, there is no available information on UAE of valuable bioactive compounds from M. sylvestris leaves. The effect of operating UAE variables such as extraction temperature, ultrasonic power, and sonication time on the extractive value (EV) of bioactive compounds was evaluated and optimized using a response surface methodology (RSM) employing a three-variable, three-level Box-Behnken design (BBD). The effect of extraction solvent including ethanol, ethyl acetate, and n-hexane on EV, free radical scavenging activity (FRSA), total phenolic content (TPC), and composition of phenolic compounds was investigated using the agitated bed extraction (ABE) technique. Furthermore, the effect of UAE on the extraction efficiency in terms of EV, FRSA, TPC, and composition of phenolic compounds was studied and compared with that obtained using the ABE technique.
Materials and methods
Materials
The M. sylvestris plants were harvested from a cultivated area in Zanjan, Iran. Leaves were separated and washed under tap water. They were dried in an oven with air circulation (K.J25, Pars Azma, Tehran, Iran) at 40 °C for 24 h. Before extraction, dried leaves were ground finely using a blade mixer (Cuisinart Blender Blade, Ginsong, Zhejiang, China) for 10 s to produce a powder that can pass through an 18-mesh (1.00 mm) stainless steel sieve. Methanol (chromatography grade), ethanol (analytical grade), ethyl acetate (analytical grade), and n-hexane (analytical grade) were obtained from Merck, Darmstadt, Germany. 2-2′Azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS·+), 1,1-diphenyl-2- picrylhydrazyl (DPPH·), trifluoroacetic acid, potassium persulphate, and Folin–Ciocalteu reagent (FCR) were purchased from Sigma-Aldrich Chemie GmbH, Kappelweg, Schnelldorf, Germany. All phenolic compound standards including gallic acid, genistein, quercetin, myricetin, apigenin, and kaempferol were purchased from Sigma-Aldrich Chemie GmbH, Kappelweg, Schnelldorf, Germany.
Agitated bed extraction
ABE was performed at 40 °C by placing M. sylvestris leaves into 150-mL Erlenmeyer flasks containing different extraction solvents (ethanol, ethyl acetate, and n-hexane) using a sample:solvent ratio of 1:20 (w/v). Extractions were conducted in an orbital shaking incubator (Sher600-1, Noorsanat, Alborz, Iran) with agitation speed of 150 rpm for 5 h. The levels of parameters were selected based on our preliminary experiments.
Ultrasound-assisted extraction
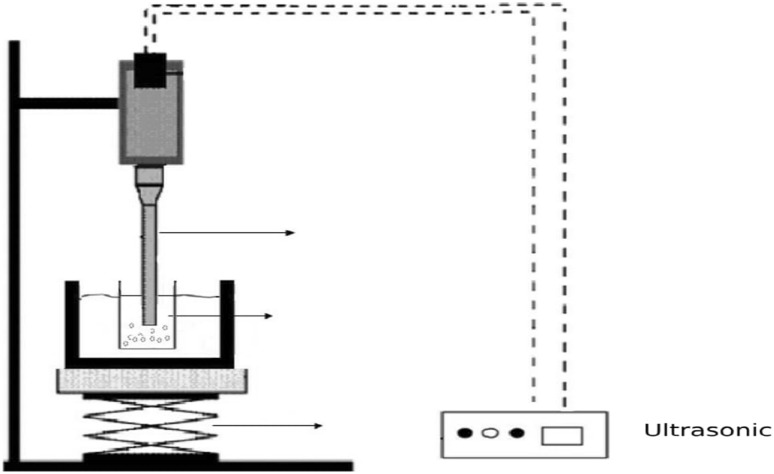
The experimental setup used for UAE is depicted schematically in Fig. 1. M. sylvestris leaves were added to 150-mL Erlenmeyer flasks and then mixed with appropriate solvent selected from the ABE study to give a feed:solvent ratio of 1:20 (w/v). The extraction was performed using a 200-W ultrasound equipment (UP200H/UP200S Hielscher, Germany) with a titanium ultrasonic probe (3-mm diameter). The probe system delivers power ultrasonic energy directly to the leaves being treated. During extraction, the temperature was controlled at the desired levels (40, 50, and 60 °C) within ± 1 °C. Ultrasound irradiations were performed for 40, 50 and 60 min. The applied ultrasonic power levels were 50, 100, and 150 W. The extraction process was optimized by using RSM to maximize the EV of bioactive compounds. The FRSA, TPC, and major phenolic compound of the extract obtained under optimal conditions were determined and the results were compared with those obtained using the ABE technique.
Fig. 1.
Schematic representation of the experimental setup for UAE
Determination of extractive value
After extraction, the solvent was removed from the extracts via evaporation under vacuum using a rotary evaporator (R-250, Flawil, Switzerland) at 40 °C. Then, the extracts were kept in an oven at 40 °C for 1 h before transferring them into the desiccator to determine the final constant weight. The EV was calculated using the following equation:
| 1 |
where m e is the mass of crude extract separated from the sample (g) and m s is the mass of extracted sample (g). Then, the extracts were kept at −18 °C in the dark for further analysis.
Free radical scavenging activity assays
In this study, the FRSA of M. sylvestris leaf extracts was expressed as a percentage of that of 2,2′-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS·+) and 1,1-diphenyl-2-picrylhydrazyl (DPPH·) (%ABTSsc and %DPPHsc, respectively).
DPPH· radical scavenging activity
The DPPH· radical scavenging method involves neutralization of free radicals of DPPH· by the antioxidant compounds. The procedure was same as that described by Bimakr et al. [21] with minor modification. In short, 2 mL of sample (0.1 mg/mL) (dissolved in ethanol) was mixed with 2 mL of an ethanolic solution of DPPH· (0.1 mM) and the sampleʼ s absorbance was measured at 517 nm for 60 min with 10-min intervals using a UV–Vis spectrophotometer (Specord 250, Jena, Germany). Ethanolic DPPH· solution was used as a blank sample. The inhibition percent of scavenged DPPH· (%DPPHsc) was calculated as 100 × (Ab − As)/Ab, where Ab is the absorbance of the blank and As is the absorbance of the sample.
ABTS·+ radical scavenging activity
The ABTS·+ radical scavenging activity of extracts was determined by a procedure reported by Bimakr et al. [21]. The ABTS· + solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium persulphate in 20 mL of distilled water. The solution was kept in the dark at room temperature for 16 h. The ABTS·+ solution was diluted with 80% (v/v) ethanol to obtain an absorbance between 0.8 and 0.9 at 734 nm. 3.9 mL of ABTS·+ solution was added to 2 mL of the sample (0.1 mg/mL). The absorbance of the mixture was recorded at 734 nm for 10 min at 2-min intervals using a UV–Vis spectrophotometer (Specord 250, Jena, Germany). Ethanol was used as a blank. The inhibition percent of scavenged ABTS·+ (%ABTSsc) was calculated as 100 × (Ab − As)/Ab, where Ab is the absorbance of the blank and As is the absorbance of the sample.
Total phenolic content
The TPC of extracts was estimated using the Folin–Ciocalteu method, based on a colorimetric oxidation–reduction reaction of phenols [22]. The procedure was same as that described by Bimakr et al. [21] with slight modification. In short, 1 mL of deionized water was mixed with 7 mg of a test sample. Then, 1 mL of Folin–Ciocalteu reagent (freshly diluted 10 times with distilled water) was added to 2 mL of the test sample. After 5 min, 7.5 mL of aqueous carbonate sodium (Na2CO3, 60 mg/mL) solution was added and the mixture was kept for 30 min at room temperature. The color change of samples was determined at 765 nm (Specord 250, Jena, Germany). A standard calibration curve was prepared using different concentrations of gallic acid in ethanol (50–500 ppm). The TPC values were calculated on the basis of this calibration curve and are expressed as milligrams of gallic acid equivalents per g of extract.
High-performance liquid chromatography analysis
The determination of the major phenolic compounds in M. sylvestris leaf extracts was performed by using a high-performance liquid chromatography (HPLC) system comprising a Varian 9012 HPLC pump (CA, USA), a six-port Cheminert HPLC valve from Valco (Houston, USA) with a 20-µL sample loop, and a Varian 9050 UV–Vis detector. Chromatographic data were recorded and analyzed using Chromana software (version 3.6.4). Efficient chromatographic separation was obtained by using an Eclipse RP-C18 column (25 cm × 4.6 mm × 5 μm, Supelco, USA) with solvent A (trifluoroacetic acid, 2.5 pH in deionized water) and solvent B (methanol, HPLC grade). All solutions were filtered through a 0.45-µm filter before injection. The major bioactive phenolic compounds were identified by matching their retention time against those of available standard compounds including gallic acid, genistein, quercetin, myricetin, apigenin, and kaempferol. The bioactive phenolic compounds were quantified using regression equations from their respective standard curves. The limits of detection (LOD) and limits of quantification (LOQ) were established at signal-to-noise ratios of 3 and 10, respectively [23].
Experimental design and statistical analysis
The results obtained using the ABE technique were statistically analyzed via analysis of variance (ANOVA), and mean differences between EV were compared using Tukey test at a confidence level of 95% using Design-Expert software (Trial Version 7.0.3, Stat-Ease Inc., Minneapolis, MN). UAE optimization was conducted by using RSM to obtain the maximum EV. A BBD with three independent variables was used to determine the response pattern and then to establish a model. The three independent variables used in this study were extraction temperature (X1), ultrasonic power (X2), and sonication time (X3), with three levels for each variable, while the dependent variable was the EV of bioactive compounds. This generated 17 treatments with 5 replications at the center point (Table 1). The effect of unexplained variability induced by extraneous factors on the observed response was minimized by randomizing the order of experiments [24]. A second-order polynomial model was used to predict the EV as a function of the abovementioned independent variables as follows:
| 2 |
where Y is the estimated response; b0, bj, bjj, and bij are the regression coefficients for intercept, linearity, square, and interaction, respectively; and Xi and Xj are the independent coded variables. The significant terms (p < 0.05) in the model were found via ANOVA based on the F-ratio and p value. The model adequacies were determined using model analysis, the lack-of-fit test, the coefficient of determination, and the adjusted R2 [25]. In addition, the quality of the fit between the experimental and predicted data was determined according to values of the mean relative deviation modulus (E), which can be calculated as follows:
| 3 |
where Vexp and Vpre are the experimental and predicted values, respectively, and n is the number of experimental data. A model is considered acceptable if the E value is less than 10% [26]. The optimum level of UAE independent variables aiming to maximize the EV value was achieved using graphical and numerical optimization procedures. The experimental design matrix, data analysis, regression coefficients, generation of response surface plots, and optimization procedure were also created using Design-Expert statistical software (Trial Version 7.0.3, Stat-Ease Inc., Minneapolis, MN).
Table 1.
BBD and response for the EV of bioactive compounds from M. sylvestris leaves
| Run | Coded levels | Response | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | EV (mg/g) | |||
| Extraction temperature (°C) | Ultrasonic power (W) | Sonication time (min) | Ye | Yp | Ye − Yp | |
| 1 | 50.00 (0) | 100.00 (0) | 50.00 (0) | 280.95 | 280.89 | 0.05 |
| 2 | 60.00 (+1) | 100.00 (0) | 60.00 (+1) | 278.20 | 278.24 | −0.03 |
| 3 | 50.00 (0) | 100.00 (0) | 50.00 (0) | 280.92 | 280.89 | 0.02 |
| 4 | 60.00 (+1) | 150.00 (+1) | 50.00 (0) | 275.80 | 275.92 | −0.12 |
| 5 | 50.00 (0) | 50.00 (−1) | 40.00 (−1) | 275.23 | 275.39 | −0.16 |
| 6 | 50.00 (0) | 100.00 (0) | 50.00 (0) | 281.00 | 280.89 | 0.11 |
| 7 | 50.00 (0) | 150.00 (+1) | 60.00 (+1) | 278.50 | 278.34 | 0.16 |
| 8 | 50.00 (0) | 50.00 (−1) | 60.00 (+1) | 277.60 | 277.77 | −0.17 |
| 9 | 60.00 (+1) | 50.00 (−1) | 50.00 (0) | 275.04 | 274.83 | 0.21 |
| 10 | 50.00 (0) | 150.00 (+1) | 40.00 (−1) | 279.00 | 278.83 | 0.17 |
| 11 | 50.00 (0) | 100.00 (0) | 50.00 (0) | 280.90 | 280.89 | 0.01 |
| 12 | 60.00 (+1) | 100.00 (0) | 40.00 (−1) | 276.10 | 276.15 | −0.05 |
| 13 | 40.00 (−1) | 50.00 (−1) | 50.00 (0) | 275.40 | 275.28 | 0.12 |
| 14 | 40.00 (−1) | 100.00 (0) | 40.00 (−1) | 278.70 | 278.66 | 0.03 |
| 15 | 40.00 (−1) | 150.00 (+1) | 50.00 (0) | 278.00 | 278.21 | −0.21 |
| 16 | 40.00 (−1) | 100.00 (0) | 60.00 (+1) | 278.50 | 278.45 | 0.04 |
| 17 | 50.00 (0) | 100.00 (0) | 50.00 (0) | 280.70 | 280.89 | −0.19 |
Y e experimental values, Y p predicted values, Y e −Y p residual values
Results and discussion
Effect of different solvents on the EV of bioactive compounds using agitated bed extraction
In this study, the effects of different solvents on the EV of bioactive compounds from M. sylvestris leaves using ABE have been studied, and the results are shown in Table 2. The solubility of different natural products varies with different solvents, i.e., a polar solute is soluble in polar solvents such as ethanol, whereas non-polar solutes dissolve in non-polar solvents such as n-hexane. The obtained EV value was higher in polar solvents such as ethanol (320.16 ± 0.25 mg/g) and lower in the case of a non-polar solvent such as n-hexane (27.10 ± 0.12 mg/g), which exhibited the lowest value. As shown in Table 2, the FRSA and TPC of M. sylvestris leaf extracts increased with increasing polarity of the extraction solvent. There was a high correlation (0.98) between %DPPHsc and %ABTSsc, which reflects the reliability of the FRSA results. A strong positive linear correlation between TPC and %ABTSsc (0.90) and DPPHsc % (0.95) was also observed. The presence of phenolic compounds in plant extracts contributes greatly to their antioxidant activity potential. Oroian and Escriche [1] stated that phenolic compounds are responsible for the antioxidant activities of edible and non-edible plant products. Furthermore, the correlation between phenolic compounds and antioxidant activity has been described in various previous studies [27–29]. These findings reflected the presence of phenolic compounds in M. sylvestris leaf extracts obtained using ABE technique. The maximum extent of EV, FRSA, and TPC from M. sylvestris leaves was obtained using ethanol as the extraction solvent; hence, it was determined as the preferable extraction solvent.
Table 2.
Results for EV, FRSA (%DPPHsc and %ABTSsc), and TPC of M. sylvestris leaf extracts obtained via different extraction techniques
| Extraction method | Extraction solvent | EV (mg/g) | %DPPHsc | %ABTSsc | TPC (mg GAE/g) |
|---|---|---|---|---|---|
| ABEa | n-Hexane | 27.10 ± 0.12 | 11.12 ± 0.15 | 8.88 ± 0.15 | – |
| Ethyl acetate | 224.14 ± 0.13 | 34.75 ± 0.18 | 26.84 ± 0.19 | 85.24 ± 0.15 | |
| Ethanol | 320.16 ± 0.25 | 64.58 ± 0.17 | 61.42 ± 0.11 | 128.88 ± 0.08 | |
| UAEb | Ethanol | 279.89 ± 0.21 | 71.12 ± 0.15 | 73.35 ± 0.11 | 152.25 ± 0.14 |
| BHT | – | 94.68 ± 0.21 | 93.43 ± 0.6 | – | |
| Catechin | – | 97.54 ± 0.7 | 98.10 ± 0.8 | – |
aAgitated bed extraction
bOptimal conditions of ultrasound-assisted extraction (48 °C extraction temperature, 110-W ultrasonic power, and 48.77-min sonication time)
Effects of UAE variables on the EV of bioactive compounds
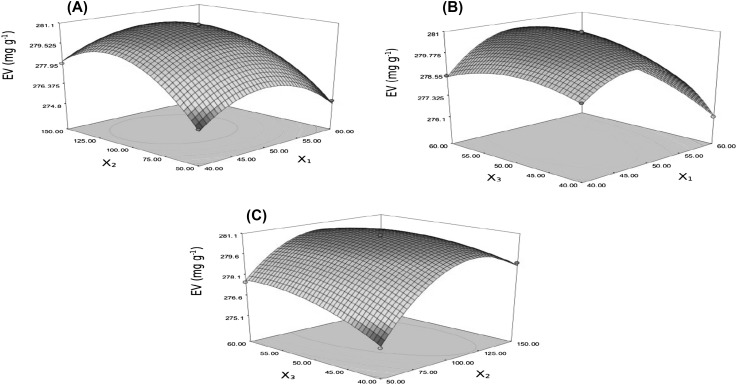
To express the effect of independent variables on EV within the experimental region under investigation, generating response surface plots are highly recommended. In this study, both multiple graphical and numerical optimization procedures were applied to determine the optimal level of the independent variables to extract valuable bioactive compounds from the M. sylvestris leaves. Three-dimensional (3-D) surface plots were constructed according to Eq. (2) (Fig. 2(A)–(C)). The 3-D plots were drawn by maintaining one variable constant at the center point while varying the other two variables within the experimental range to show the response of variables related to two continuous design variables. As can be seen in Fig. 2(A), (C), EV increased with increasing ultrasonic power up to a certain threshold (around 120 W). From a botanical standpoint, vegetal tissue comprises cells surrounded by membranes and cell walls. The vegetal cell structure was bombed by supersonic jets from bubble collapse, and micro-cavitation bubbles caused capillaries to improve the extraction efficiency. The extraction process improved due to application of large ultrasonic power [30, 31]. Applying large ultrasonic power resulted in generation and collapse of more bubbles. Since the collapse of bubbles occurred over a very short duration and the pressure and temperature were very high inside the bubbles, a high-speed jet and violent shock wave were generated, which could enhance the mass transfer rate [32]. In this study, EV reached a maximum value of 280.95 ± 0.10 mg/g when ultrasonic power was increased to a certain threshold, with no further improvement thereafter (Fig. 2(A), (C)). This phenomenon might be attributed to the presence of hard cell walls, which are not very permeable [30, 33]. Similar results were obtained by Zhang et al. [33] and Sivakumar et al. [34] in the UAE of oil from flaxseed and tannin from myrobalan, respectively.
Fig. 2.
Response surface plots (A–C) showing the effects of different UAE parameters (X1: extraction temperature, °C; X2: ultrasonic power, W; X3: sonication time, min) on EV (mg/g)
Figure 2(B), (C) shows the influence of sonication time on the EV of bioactive compounds from M. sylvestris leaves. EV increased rapidly with sonication time for the first 50 min. Ultrasonic waves could result in cell-wall disruptions, which accelerate the release of intracellular material into the solvent. The extraction mechanism of the UAE process occurs mainly in two stages: [1] dissolution of soluble components on surfaces of the leaf matrix, known as washing, and [2] mass transfer of the solute from the leaf matrix into the solvent due to diffusion and osmotic processes, known as slow extraction [35]. Based on the results, EV had no significant (p > 0.05) increment when the sonication time was increased from 50 to 60 min. This phenomenon may have occurred because most of the extracts had already been extracted during the first 50 min of sonication.
The EV of bioactive compounds rose as extraction temperature increased from 40 to nearly 50 °C (Fig. 2(A), (B)). The variation of EV with temperature under UAE may be attributed to a combination of the cavitation and thermal effects. From the thermal effect point of view, increasing temperature had a positive effect on the extraction yield due to softening and swelling of materials, which increase solute solubility and diffusivity. From the cavitation effect point of view, increasing temperature had a negative effect because cavitation intensity decreases with increasing temperature [15]. In the present study, when extraction temperature crosses a certain threshold, EV starts decreasing (Fig. 1(A), (B)). As mentioned earlier, the optimal conditions for UAE were identified as an extraction temperature of 48 °C, ultrasonic power of 110 W, and sonication time of 48.77 min. The extract obtained under the optimal UAE conditions exhibited high FRSA (71.12 ± 0.15%DPPHsc and 73.35 ± 0.11%ABTSsc). Furthermore, this extract was a potential source of valuable bioactive phenolic compounds (152.25 ± 0.14 mg GAE/g).
Optimization of UAE of bioactive compounds
Model fitting
Since different variables can influence the UAE performance of valuable bioactive compounds from M. sylvestris leaves, optimizing the operating conditions is essential to achieve a successful process. In this study, ultrasonic power, extraction temperature, and sonication time were considered to be the most important variables. The UAE conditions of bioactive compounds obtained from M. sylvestris leaves were optimized using different independent variable combinations according to the BBD. The experiment design and corresponding response data are shown in Table 1. A mathematical model representing EV as a function of the independent variables considered herein within the region under investigation is expressed by the following equation:
| 4 |
where Y is the EV, and X1, X2, and X3 are the coded variables for extraction temperature, ultrasonic power, and sonication time, respectively. The model adequacy was determined using model analysis, the lack-of-fit test (0.064 > 0.05), coefficient of determination (R2 = 0.996), adjusted R2 (0.991), and E-value (0.053%) (Table 3). The p-value of the model was less than 0.05, indicating that the model is statistically significant. Meanwhile, the lack of fit of the model was insignificant (p > 0.05), suggesting adequacy of the model in predicting EV over the studied range of independent variables (Table 3). A high regression coefficient (R2) value indicates a sufficient relevance of the dependent variable in the model [24]. In the current study, the high value of R2 (0.996) showed that the true behaviour of the system was very well defined by the regression model. In addition, the suitability of the model was supported by the closeness of the adjusted R2 (0.991) to unity, presenting a high degree of correlation between the experimental and predicted values [21]. The small E-value (0.053%) indicated that the model obtained was acceptable.
Table 3.
Regression coefficients and significant probability (P values and F-ratio) of the effects of the independent variables of UAE on the EV of bioactive compounds from M. sylvestris leaves
| EV (mg g−1) | β | F-ratio | P value | |
|---|---|---|---|---|
| Cons. | 280.89 | 204.99 | < 0.0001* | |
| X1 | − 0.68 | 92.57 | < 0.0001* | |
| X2 | 1.00 | 200.22 | < 0.0001* | |
| X3 | 0.47 | 44.13 | 0.003* | |
| X21 | − 2.27 | 371.16 | < 0.0001* | |
| X22 | 2.56 | 539.31 | < 0.0001* | |
| X23 | − 0.75 | 687.20 | 0.0001* | |
| X1X2 | − 0.46 | 21.03 | 0.002* | |
| X1X3 | 0.57 | 32.85 | 0.000* | |
| X2X3 | − 0.72 | 51.15 | 0.000* | |
| R-squared (R2) | 0.996 | |||
| Pred R2 | 0.949 | |||
| Lack of fit | 0.064 | |||
| Adj R2 | 0.991 | |||
| E (%) | 0.053 | |||
Cons constant, X 1 extraction temperature, X 2 ultrasonic power, X 3 sonication time; X 21, X 22, and X 23 the quadratic effects of extraction temperature, ultrasonic power, and sonication time, respectively, X 1X2, X 1X3 and X 2X3 the interaction effect of extraction temperature, ultrasonic power, and sonication time, respectively, β regression coefficient
*p-values < 0.05 are considered significant
Verification of the final reduced model
The optimal conditions of UAE aimed to maximize EV were found to be as follows: extraction temperature of 48 °C, ultrasonic power of 110 W, and sonication time of 48.77 min. Under these conditions, the obtained experimental EV was 279.89 ± 0.21 mg/g with no significant difference (p > 0.05) from the predicted EV (281 mg/g). The adequacy of the final reduced model was verified using residual values. As presented in Table 1, the experimental data were compared with the predicted values obtained using Eq. (4). A close agreement and no significant difference (p > 0.05) were found between the obtained experimental and predicted values.
Identification and quantification of major bioactive phenolic compounds
Different bioactive phenolic compounds including gallic acid, genistein, quercetin, apigenin, myricetin, and kaempferol were detected and quantified after evaluating the retention times and ultraviolet spectra of bioactive phenolic compounds. The linear relationships between peak areas and concentrations, test ranges, LOD and LOQ values of standard phenolic compounds are shown in Table 4. The contents of major bioactive phenolic compounds of M. sylvestris leaf extracts obtained via different extraction methods are listed in Table 5. The main phenolic compound was quercetin, which exhibited a value of 206.21 ± 0.10 mg/gunder the optimal conditions of UAE, followed by apigenin, genistein, gallic acid, myricetin, and kaempferol. The contents of the bioactive phenolic compounds of M. sylvestris leaves under the minimum level (40 °C extraction temperature, 50-W ultrasonic power, and 40-min sonication time) and the maximum level (60 °C extraction temperature, 150-W ultrasonic power, and 60-min sonication time) of each studied variable are compared with those obtained under the optimum level. The results show that the highest value of bioactive phenolic compounds could be obtained under the optimized conditions of UAE. These findings highlight the importance of process optimization. Lower concentration of bioactive phenolic compounds was determined in ABE extracts compared with those obtained via UAE (Table 5). According to the results obtained using the ABE process, ethanol ensures high bioactive-phenolic-compound recovery and provides the extracts rich in phenolic compounds, while less-polar solvents such as n-hexane afford lower recoveries of bioactive phenolic compounds. Therefore, UAE can a suitable method to isolate bioactive phenolic compounds from M. sylvestris leaves.
Table 4.
Linear relationships between peak areas and concentrations and LOD and LOQ of phenolic compounds
| Phenolic compound | Linearity range (μg/mL) | Correlation coefficient (r2) | LODa (μg/mL) | LOQb (μg/mL) |
|---|---|---|---|---|
| Gallic acid | 0.36–680 | 0.9990 | 0.11 | 0.36 |
| Genistein | 0.09–600 | 0.9889 | 0.03 | 0.10 |
| Quercetin | 0.05–520 | 0.9987 | 0.02 | 0.06 |
| Apigenin | 0.20–500 | 0.9883 | 0.07 | 0.23 |
| Myricetin | 0.13–400 | 0.9995 | 0.04 | 0.13 |
| Kaempferol | 0.30–380 | 0.9998 | 0.09 | 0.30 |
aLimit of detection
bLimit of quantification
Table 5.
Identification and quantification of the major bioactive phenolic compounds of M. sylvestris leaf extracts obtained via different extraction methods
| Extraction method | Extraction solvent | Phenolic compound (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| Gallic acid | Genistein | Quercetin | Apigenin | Myricetin | Kaempferol | ||
| ABEa | n-Hexane | 53.35 ± 0.11 | – | 84.42 ± 0.06 | – | 78.18 ± 0.08 | – |
| Ethyl acetate | 88.25 ± 0.10 | 85.36 ± 0.12 | 118.65 ± 0.09 | 91.21 ± 0.10 | 98.00 ± 0.15 | 80.32 ± 0.05 | |
| Ethanol | 114.32 ± 0.06 | 112.40 ± .010 | 150.36 ± 0.05 | 131.12 ± 0.013 | 118.2 ± 0.01 | 115.54 ± 0.08 | |
| UAEb | Type 1c | 124.14 ± 0.18 | 115.87 ± 0.15 | 175.74 ± 0.17 | 145.78 ± 0.10 | 124.10 ± 0.18 | – |
| Type 2d | 163.42 ± 0.12 | 168.12 ± 0.14 | 206.21 ± 0.10 | 180.25 ± 0.05 | 159.12 ± 0.12 | 121.51 ± 0.10 | |
| Type 3e | 146.54 ± 0.12 | 153.24 ± 0.14 | 183.93 ± 0.11 | 173.25 ± 0.10 | 131.20 ± 0.14 | 104.27 ± 0.15 | |
aAgitated bed extraction
bUltrasound-assisted extraction
c40 °C extraction temperature, 50-W ultrasonic power, and 40-min sonication time
d48 °C extraction temperature, 110-W ultrasonic power, and 48.77-min sonication time
e60 °C extraction temperature, 150-W ultrasonic power, and 60-min sonication time
Comparison between different extraction methods
In general, obtaining extracts with high antioxidant activity from low-cost raw materials by applying environmentally friendly techniques is of great interest to researchers. The use of different extraction methods might afford different efficiencies and yield. The primary aim of the extraction process is to determine the preferable process conditions, which enables obtaining the highest extraction yield of bioactive compounds. In ABE, agitation resulted in an enhanced EV, as the maximum bioactive-compound EV was obtained by applying ethanolic ABE (320.16 ± 0.25 mg/g). During UAE, the release of bioactive compounds from vegetal cell could be achieved due to the cavitation phenomenon, which causes localized stirring. The simultaneous occurrence of this stirring effect and repeated washing of the valuable bioactive compounds with solvent could greatly enhance the extraction efficiency [11]. From the results obtained herein, it can be observed that about 82% of EV obtained via ABE could be achieved by applying UAE, which reveals the superiority of UAE over ABE. The FRSA and TPC analyses results revealed that UAE afforded extracts with relatively higher FRSA and TPC values compared with ABE in much shorter time (48.77 min). As shown by the HPLC analysis (Table 5), the phenolic-compound compositions of the extracts obtained via UAE and ABE of M. sylvestris leaves were different. The highest levels of major bioactive phenolic compounds were found in the extracts obtained under the optimal conditions of UAE. In another study, Bimakr et al. [21] studied the characterization of valuable compounds from winter melon (Benincasa hispida (Thunb.) Cogn.) seeds using different extraction methods including UAE, supercritical carbon dioxide extraction combined with the pressure swing technique (SCE-PST), and the conventional method. They reported that UAE was the fastest extraction method (~36 min) compared with SCE-PST (~50 min), and the conventional method (~360 min). They pointed out that the extraction efficiency of UAE in terms of crude extraction yield was lower than those of the other techniques. However, the quality of the extract obtained via UAE in terms of antioxidant activity was significantly higher (p < 0.05) than that obtained via conventional method.
In general, it was found that ethanolic ABE afforded higher EV values (320.16 ± 0.25 mg/g) than optimized UAE (279.89 ± 021 mg/g). However, the quality of UAE extracts in terms of FRSA, TPC, and concentration of bioactive phenolic compounds was significantly (p < 0.05) better than those obtained via ABE. Applying optimized UAE conditions the extraction time (48.77 min) was shortened compared with that needed in ABE technique (5 h). Since the highest concentrations of valuable bioactive phenolic compounds were obtained in UAE extracts, it can be said that UAE helps in reducing the thermal degradation of sensitive valuable bioactive compounds from M. sylvestris leaves. Therefore, ultrasound irradiation can improve the process of extracting bioactive compounds from M. sylvestris leaves. The results also show that M. sylvestris leaves are a potential source of valuable bioactive compounds, which makes it beneficial for human health because it prevents or reduces oxidative damage.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Oroian M, Escriche I. Antioxidants: characterization, natural sources, extraction and analysis. Food Res. Int. 2015;74:10–36. doi: 10.1016/j.foodres.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2013;6:806–817. doi: 10.1007/s11947-012-0877-7. [DOI] [Google Scholar]
- 3.Martinez JL. Supercritical fluid extraction of nutraceuticals and bioactive compounds. New York: United States of America, CRC Press; 2008. [Google Scholar]
- 4.Feng H, Yang W. Power ultrasound. In: Handbook of food science, technology, and engineering, edited by Hui, Y.H. New York, CRC Press. (2005)
- 5.Sharma A, Gupta MN. Oil extraction from almond, apricot and rice by three-phase partitioning after ultrasonication. Eur. J. Lipid Sci. Tech. 2004;106:183–186. doi: 10.1002/ejlt.200300897. [DOI] [Google Scholar]
- 6.Wang L, Weller CL. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006;17:300–312. doi: 10.1016/j.tifs.2005.12.004. [DOI] [Google Scholar]
- 7.Zhou B, Feng H, Lou Y. Ultrasound enhanced sanitizer efficacy in reduction of Escherichia coli O157:H7 population on spinach leaves. J. Food Sci. 2009;74:308–313. doi: 10.1111/j.1750-3841.2009.01247.x. [DOI] [PubMed] [Google Scholar]
- 8.Baumann A, Martin SE, Feng H. Removal of Listeria monocytogenes biofilms from stainless steel using ultrasound and ozone. J. Food Prot. 2009;72:1306–1309. doi: 10.4315/0362-028X-72.6.1306. [DOI] [PubMed] [Google Scholar]
- 9.Raviyan P, Zhang Z, Feng H. Ultrasonication for tomato pectin methyl esterase inactivation: effect of cavitation intensity and temperature on inactivation. J. Food Eng. 2005;70:189–196. doi: 10.1016/j.jfoodeng.2004.09.028. [DOI] [Google Scholar]
- 10.Zhang Z, Feng H, Niu Y, Eckhoff SR. Starch recovery from degermed corn flour and hominy feed using power ultrasound. Cereal Chem. 2005;82:447–449. doi: 10.1094/CC-82-0447. [DOI] [Google Scholar]
- 11.Zhang HQ, Barbosa-Canovas GV, Balasubramaniam VM, Dunne CP, Farkas DF, Yuan JT. Nonthermal processing technologies for food. United Kingdom: Wiley, IFT press; 2011. [Google Scholar]
- 12.Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A. Optimization of conventional and ultrasound-assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT Food Sci. Technol. 2015;64:1114–1122. doi: 10.1016/j.lwt.2015.07.024. [DOI] [Google Scholar]
- 13.Jian-Bing J, Xiang-Hong L, Mei-Qiang C, Zhi-Chao X. Improvement of leaching process of Geniposide with ultrasound. Ultrason. Sonochem. 2006;13:455–462. doi: 10.1016/j.ultsonch.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Rostagno MA, Palma M, Barroso CG. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A. 2003;1012:119–128. doi: 10.1016/S0021-9673(03)01184-1. [DOI] [PubMed] [Google Scholar]
- 15.Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001;8:303–313. doi: 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- 16.Cristina Soria A, Villamiel M. Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci. Technol. 2010;21:323–331. doi: 10.1016/j.tifs.2010.04.003. [DOI] [Google Scholar]
- 17.Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Samavatia V, Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its antioxidant activity. Int. J. Biol. Macromolec. 2013;60:427–436. doi: 10.1016/j.ijbiomac.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 19.Pirbalouti AG, Yousefi M, Heshmetollah N, Karimi I, Koohpayeh A. Bioactivity of Malva Sylvestris L., a Medicinal Plant from Iran. E. J. Bio. 5: 62–66 (2009)
- 20.Wang ZY. Impact of anthocyanin from Malva sylvestris on plasma lipids and free radical. J For Res. 2005;16:228–232. doi: 10.1007/BF02856821. [DOI] [Google Scholar]
- 21.Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Characterization of valuable compounds from winter melon (Benincasa hispida (Thunb.) Cogn.) seeds using supercritical carbon dioxide extraction combined with pressure swing technique. Food Bioprocess Tech. 9: 396–406 (2016)
- 22.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 23.Sehati N, Dalali N, Soltanpour S, Seyed Dorraji MS. Application of hollow fiber membrane mediated with titanium nanowire/reduced grapheme oxide nanocomposite in preconcentration of clotrimazole and tylosin. J. Chromatogr. A. 2015;1420:46–53. doi: 10.1016/j.chroma.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 24.Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- 25.Weng WL, Liu YC, Lin CW. Studies on the optimum models of the dairy product Kou Woan Lao using response surface methodology. Asian-Australasian J. Anim. Sci. 2001;14:1470–1476. doi: 10.5713/ajas.2001.1470. [DOI] [Google Scholar]
- 26.Deng Y, Zhao Y. Effect of pulsed vacuum and ultrasound osmo-pretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji) LWT Food Sci. Technol. 2008;41:1575–1585. doi: 10.1016/j.lwt.2007.10.018. [DOI] [Google Scholar]
- 27.He L, Xu H, Liu X, He W, Yuan F, Hou Z. Identification of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant capacities by HPLC-ABTS+ assay. Food Res. Int. 2011;44:1161–1167. doi: 10.1016/j.foodres.2010.05.023. [DOI] [Google Scholar]
- 28.Anesini C, Ferraro GE, Filip R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J. Agric. Food Chem. 2008;56:9225–9229. doi: 10.1021/jf8022782. [DOI] [PubMed] [Google Scholar]
- 29.Gan CY, Latiff AA. Optimization of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011;124:1277–1283. doi: 10.1016/j.foodchem.2010.07.074. [DOI] [Google Scholar]
- 30.Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules. 2012;17:11748–11762. doi: 10.3390/molecules171011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caresa MG, Vargas Y, Gaete L, Sainz J, Alarcon J. Ultrasonically assisted extraction of bioactive principles from Quillaja Saponaria Molina. Phys. Procedia. 2010;3:169–178. doi: 10.1016/j.phpro.2010.01.024. [DOI] [Google Scholar]
- 32.Bimakr M, Russly AR, Farah ST, Noranizan MA, Zaidul ISM, Ali G. Ultrasound-assisted extraction of valuable compounds from winter melon (Benincasa hispida) seeds. Int. Food Res. J. 2013;20:331–338. [Google Scholar]
- 33.Zhang ZS, Wang LJ, Li D, Jiao SS, Chen ZXD, Mao H. Ultrasound-assisted extraction of oil from flaxseed. Sep. Purif. Technol. 2008;62:192–198. doi: 10.1016/j.seppur.2008.01.014. [DOI] [Google Scholar]
- 34.Sivakumar V, Ravi Verma V, Rao PG, Swaminathan G. Studies on the use of power ultrasound in solid-liquid myrobalan extraction process. J. Clean. Prod. 2007;15:1813–1818. doi: 10.1016/j.jclepro.2006.06.006. [DOI] [Google Scholar]
- 35.Shekhar UK, Tiwari BK, Smyth TJ, Donnell CP. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed using response surface methodology. Ultrason. Sonochem. 2015;23:308–316. doi: 10.1016/j.ultsonch.2014.10.007. [DOI] [PubMed] [Google Scholar]