Abstract
To enhance the biological activities of sprout soybean, beans were treated with steaming (SS), germinating (GS), or roasting (RS) prior to fermentation with Irpex lacteus mycelia for 20 days. The total phenolic, flavonoid, isoflavone, and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of each fermented bean preparation were examined every 5 days for 20 days. The total phenolic content of SS, GS, and RS preparations was 9.61, 10.23, and 10.46 mg/g, respectively, after 15 days of fermentation. These concentrations were approximately 4–5 folds higher compared to initial levels. The total flavonoid content was 8–9 folds higher than initial levels. The isoflavone content was highest in the RS sample (6.84 mg/g). The DPPH radical scavenging activity of beans fermented with I. lacteus mycelia was increased 2–8 folds after 20 days of fermentation. These results indicate that antioxidant activity components were increased by fermentation of I. lacteus mycelia irrespective of soybean treatments.
Keywords: Irpex lacteus mycelia, Sprout soybean, Germinating, Roasting, Isoflavone
Introduction
Soybeans (Glycine max L.) contain bioactive components such as high-quality proteins, fats, fibers, oligosaccharides, linolenic acid, saponin, lecithin, and isoflavones [1]. These bioactive components can prevent stroke and heart disease as well as various life style diseases [2–4]. Isoflavones are a main bioactive component of soybeans and have similar effects to estrogen which is effective in preventing osteoporosis and relieving menopausal symptoms. Soybeans have also been reported to help prevent prostate cancer in men as well as breast and uterine cancer in women [5].
Sprout soybean, a microcarpa [6], contains more isoflavones than other beans [7, 8]. Isoflavones exist in two forms, glycosides and aglycones. The absorption rates and bioactivity of isoflavones are higher in aglycones than glycosides. Therefore, it is important to convert isoflavones to forms with higher bioavailability [9]. Accordingly, many studies have aimed to convert isoflavones to their aglycone forms and to increase bioactive isoflavone content [10, 11]. The approaches used in these studies have included fermenting soybeans with Cheonggukjang or lactic acid bacteria after germinating or roasting [12, 13]. More recently, microorganism fermentation, heating, enzymatic reactions, salting, and germination processes have been applied to soybeans to increase their aglycone content [14, 15].
Mushroom mycelia have been studied for their potential food applications because they are less toxic and contain more bioactive components than their fruiting bodies [16]. For example, mushroom basidiomycetes are used to generate different fermented food such as bread, cheese, and alcoholic drinks [17, 18]. Moreover, liquid incubation using mushroom mycelia is actively studied as a method for producing pharmaceutical active components [19, 20]. In another approach, applications of fluid mushroom basidiomycetes on solid cultures of plants such as ginseng, Siberian ginseng, and rice were also studied to improve their effectiveness of these cultures [21, 22]. Irpex lacteus, a white-rot fungus [23] that usually grows on oak trees, has an extensive biological degradation capacity. Thus, this fungus can be used in environmental purification applications such as dye decolorization, breakdown of explosives, and decomposition of bisphenol A, endocrine disrupters and industrial waste [24]. I. lacteus has also been reported to show antibacterial and antifungal activity [23] and to contain nematicidal chemicals such as 5-(pentyl)-2-furalaldehyde, 5-(4-pentyl)-2-furalaldehyde and methyl-3-ρ-anisoloxypropionide, as well as other enzymes of interest such as carboxymethylcellulase, chitinase, cellulase, semicellulase, and acidic protease [24]. Kobayashi et al. [25] isolated a proteinase from I. lacteus and used it as a rennet substitute to produce Gouda cheese [26].
In this study, to enhance the antioxidant activities of sprout soybean, soybeans were first pretreated by steaming, germinating, or roasting and then fermented with I. lacteus mycelia. Finally, their bioactive content was analyzed.
Materials and method
Sample collection and preparation
Sprout soybeans, which were harvested near Jeonju, Korea, 2014, were provided by the Ongoul-farm Cooperative (Jeonju, Korea). Mycelia of the I. lacteus KCTC 26201 strain were transfer-cultured successively on potato dextrose agar (PDA, Difco, Sparks, MD, USA) at 25 °C for 5 days and stored in a refrigerator at 4 °C for further use. Sprout soybeans were dried in a heater controlled at 50 °C for 48 h, ground to powder with a grinder (HR 2860, Philips Type, Hong Kong, China), and stored at 4 °C.
Analysis of proximate sprout soybean components
Proximate sprout soybean components were analyzed by AOAC [27]. Moisture content was measured via the high pressure-heat drying method, ash content via the dry ashing method using a Maffle’s furnace at 550 °C, crude protein via the Kjeldahl nitrogen determination method, and crude fat via Soxhlet method using diethyl ether.
Preparation of starter cultures
Soybean broth (5% = 5 g in 100 mL distilled water) was sterilized at 121 °C for 15 min. Activated mushroom mycelia were inoculated on PDA (potato dextrose agar) five or six times with a cork borer (∮5 mm) and cultured at 25 °C with shaking at 120 rpm for 5 days. To culture liquid spawn, 10 mL of the culture fluid was inoculated onto soybean liquid medium and cultured at 25 °C with shaking at 120 rpm for 5 days.
Preparation of steaming beans, germinated beans, and roasted beans
Sprout soybeans were immersed in water for 10 h after washing with tap water. Excess water was drained and removed with a sifter for 1 h (moisture content 54.27–57.50%). The moist beans were steamed after sterilizing in an autoclave at 121 °C for 15 min [28]. To prepare germinated sprout soybeans, the beans were washed with tap water and watering every 3 h (moisture content, 61.28–61.79%). The germinated beans were used for media after sterilizing [10]. To prepare roasted sprout soybeans, the beans were roasted on an Electric Kettle (Dahwa Precision, Seongnam, Korea) at 120 °C for 30 min (moisture content, 4.10–4.25%) after washing, immersing, and removal of excess water. Roasted beans were used for media after sterilizing [11, 12].
Cultivation of mushroom mycelia
To culture mushroom mycelia, an aliquot of liquid spawn (10%, v/w) was inoculated onto the steamed, germinated, or roasted beans. Next, the mushroom mycelia cultured on each bean type were sampled, freeze-dried, pulverized with a grinder (HR 2860, Philips Type, Hong Kong, China), and passed through a sieve with 425-μm mesh (Laboratory Test Sieve, Endecotts Ltd., London, UK). The resultant samples were used in subsequent assays.
Preparation of extracts
The mushroom mycelia powder (1 g) was resuspended in 20 mL of methanol (50%) and extracted in a shaking incubator (37 °C, 150 rpm) for 5 h [28]. The extract was analyzed for total phenolic content, total flavonoid content, isoflavone content, and DPPH radical scavenging ability.
Qualification and quantification of phenols, flavonoids, and isoflavones
Total phenolic compounds were measured using the modified Folin–Ciocalteu method. The absorbance of gallic acid, the assay standard, was measured at 765 nm using a spectrometer (UV-1601, Shimadzu, Kyoto, Japan) [29]. Total flavonoid contents were measured by the method described by the Korea Health Supplements Association [30]. Briefly, the absorbance of the liquid layer was measured at 415 nm using a spectrometer (UV-1601). Total flavonoid content (mg/g) was analyzed based on the quercetin calibration curve. Isoflavone content was analyzed by high performance liquid chromatography (Waters 2695, alliance, Milford, MA, USA) with a YMC-Triart C18 column (4.6 × 250 mm, 5 μm, Kyoto, Japan) [31]. For quantitative analysis, the absorbance of each sample at 254 nm was measured by a photodiode array detector (Waters™ 996, Milford, MA, USA).
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging ability
DPPH radical scavenging ability was measured by the modified Blois method [32]. Briefly, 0.5 mL of extract was added to 0.2 mM DPPH (2 mL) and mixed by vortexing, after which the absorbance of the solution was measured at 517 nm. The DPPH radical scavenging ability was calculated according to the equation below. All experiments were conducted three times; data are presented as average. The radical scavenging ability of vitamin C (0.1 mM) was measured as a control group.
Statistical analysis procedure
All of the results were analyzed statistically by ANOVA, Statistical analyses were performed using SPSS (Statistical Package for Social Sciences, ver 12.0. SPSS Inc., Chicago, IL, USA). Significant differences were determined by Duncan’s multiple range test (P < 0.05).
Results and discussion
Proximate components of sprout soybeans
Sprout soybeans contained moisture (10.16–14.09%), ash (4.5–5.3%), crude protein (35.3–43.1%), crude fat (13.5–16.5%), and carbohydrates (15.5–20.2%). The average weight of 100 sprout soybean grains used for this study was 6 g, classifying the soybeans as extreme-microcarpa.
Total phenolic and flavonoid content
Polyphenolic compounds, which are secondary metabolites from plants, have been reported to have varied physiological activity in the human body. Therefore, efforts have been made to extract antioxidants from plants [33]. Mushroom mycelia (10%) were inoculated onto processed beans (steamed, germinated, or roasted) and cultures, after which the total phenolic and flavonoids content of the beans was analyzed at 5-day intervals for 20 days (Figs. 1, 2).
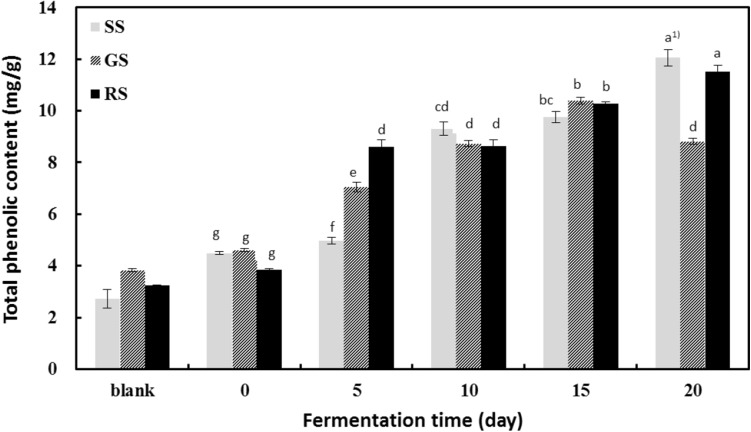
Fig. 1.
Changes in total phenolic content of sprout soybeans after fermentation with Irpex lacteus mycelia. Data are expressed as mean ± SD of triplicate experiments. SS steamed sprout soybeans, GS germinated sprout soybeans, RS roasted sprout soybeans. Mean values with different superscripts are significantly different at P < 0.05 by Duncan’s multiple range test
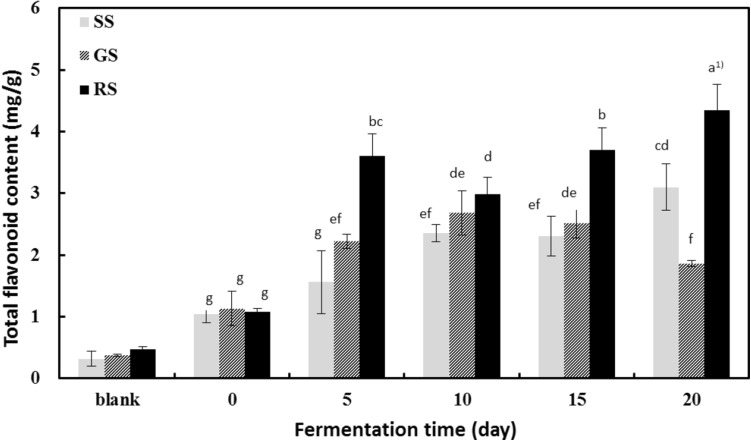
Fig. 2.
Changes in total flavonoid content of sprout soybeans after fermentation with Irpex lacteus mycelia. Data are expressed as mean ± SD of triplicate experiments. SS steamed sprout soybeans, GS germinated sprout soybeans, RS roasted sprout soybeans. Mean values with different superscripts are significantly different at P < 0.05 by Duncan’s multiple range test
Nonprocessed sprout soybeans comprised approximately 3 mg/g phenolic compounds; this content was not significantly different from the content of any processed bean type after freeze-drying (P < 0.05). After 15 days of fermentation, however, the total phenolic content increased from 2.71 to 9.61 mg/g in the steamed beans, 3.83 to 10.23 mg/g in the germinated beans, and 3.24 to 10.46 mg/g in the roasted beans. Thus, all processed beans showed approximately 4–5 times higher total phenolic content than their unprocessed counterparts. The phenolic content of steamed beans continued to increase until 20 days of fermentation. The phenolic content of steamed and roasted beans increased gradually until 15 days, after which the phenolic content of steamed beans decreased. These results are consistent with those of Joung et al. [21], who investigated the antioxidant activity of ginseng cultured with various types of mushroom mycelia (e.g., Phellinus linteus, Ganoderma lucidum and Hericium erinaceum). Joung et al. [21] showed that the phenolic content in ginseng cultured with mushroom mycelia were higher than those in raw ginseng. Our results are also similar to those of Shon [28]. In the study by Shon, beans were fermented with Tricholoma matsutake (pine mushroom) mycelia and examined for changes in bioactive content. Shon found that the bioactive activity was greatest after 9 days of fermentation; moreover, the bioactive content differed depending on the type of mycelia and culture medium. One explanation for this difference is that new phenolic compounds are produced due to the degradation of macromolecular compounds into low molecular weight phenolic compounds during fermentation; different mushrooms may possess distinct enzymes that lead to varying types of enzymatic hydrolysis [33].
The total flavonoid content, as determined based on quercetin quantitation, ranged from 0.32 to 0.47 mg/g, depending on the processing method. However, the flavonoid content of beans processed by different methods was not significantly different (P < 0.05). In contrast, after 15 days of fermentation, the total flavonoid content increased from 0.32 to 2.53 mg/g in the steamed beans, 0.38 to 2.64 mg/g in the germinated beans, and 0.47 to 3.41 mg/g in the roasted beans. Thus, the postfermentation flavonoid content was approximately 8–9 times higher than the prefermentation counterparts. Beans fermented with mushroom mycelia also showed a tendency toward increased total flavonoid content after 20 days of fermentation. Specifically, the flavonoid content increased from 1.04 to 3.10 mg/g in the steamed beans, 1.13 to 1.86 mg/g in the germinated beans, and 1.08 to 4.34 mg/g in the roasted beans compared with the control beans. Each of these values increased approximately 2- to 4-fold after fermentation. Thus, the data indicate that the total flavonoid content in all three types of processed bean increased after fermentation, consistent with the results obtained for the total phenolic content. These increases are presumably due to the enzymatic activity induced by mushroom mycelia and heat treatment.
Change of total isoflavones in beans fermented with mushroom mycelia
Table 1 shows the changes in isoflavones, including glycoside forms (daidzin, glycitin, and genistin) and aglycone forms (daidzein, glycitein, and genistein), according to the processing method for beans that were fermented with I. lacteus mycelia (10%) for 20 days. The total isoflavone content (dry base) were 2.37 mg/g in steamed beans, 4.91 mg/g in germinated beans, and 6.85 mg/g in roasted beans, the latter of which was the highest. These values are higher than those described by Moon et al. [7], who reported 276.09 mg/100 g of isoflavones in sprout soybeans. This difference might be due to the different varieties of sprout soybeans used and/or the distinct growing conditions [7, 8].
Table 1.
Changes in isoflavone content of sprout soybeans after fermentation with Irpex lacteus mycelia
| Samples | Fermentation days | Isoflavone contents (mg/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Glycoside type | Aglycone type | Total aglycone | Total isoflavones | ||||||
| Daidzin | Glycitin | Genistin | Daidzein | Glycitein | Genistein | ||||
| SS | 1.62 ± 0.22 | 0.43 ± 0.07 | 1.59 ± 0.21 | 0.45 ± 0.05 | ND | 0.43 ± 0.06 | 0.89i | 4.52d | |
| 0 | 2.61 ± 0.01 | 0.61 ± 0.00 | 2.57 ± 0.02 | 0.86 ± 0.01 | ND | 0.67 ± 0.02 | 1.53h | 7.32a | |
| 5 | ND | ND | 0.09 ± 0.08 | 1.69 ± 0.35 | 0.39 ± 0.02 | 1.53 ± 0.28 | 3.61d | 3.69e | |
| 10 | ND | ND | ND | 2.37 ± 0.03 | 0.44 ± 0.01 | 2.65 ± 0.04 | 5.46a | 5.46c | |
| 15 | ND | ND | ND | 1.40 ± 0.04 | 0.41 ± 0.02 | 1.71 ± 0.03 | 3.52d | 3.52e | |
| 20 | ND | ND | ND | 1.47 ± 0.06 | 0.41 ± 0.02 | 1.18 ± 0.06 | 3.06e | 3.06f | |
| GS | 1.47 ± 0.06 | 0.44 ± 0.05 | 1.53 ± 0.03 | 0.63 ± 0.02 | 0.09 ± 0.02 | 0.75 ± 0.02 | 1.47h | 4.91d | |
| 0 | 2.48 ± 0.01 | 0.64 ± 0.00 | 2.43 ± 0.01 | 0.83 ± 0.00 | ND | 0.63 ± 0.01 | 1.47h | 7.02ab | |
| 5 | ND | ND | 0.08 0.01 | 2.21 ± 0.01 | 0.45 ± 0.01 | 2.58 ± 0.02 | 5.23a | 5.31c | |
| 10 | ND | ND | ND | 2.33 ± 0.09 | 0.43 ± 0.01 | 2.68 ± 0.07 | 5.45a | 5.45c | |
| 15 | ND | ND | ND | 2.34 ± 0.02 | 0.41 ± 0.02 | 2.67 ± 0.00 | 5.41a | 5.41c | |
| 20 | ND | ND | ND | 2.28 ± 0.03 | 0.42 ± 0.01 | 2.69 ± 0.02 | 5.39a | 5.39c | |
| RS | 2.11 ± 0.03 | 0.37 ± 0.01 | 2.30 ± 0.05 | 1.43 ± 0.00 | ND | 0.64 ± 0.01 | 2.06g | 6.84b | |
| 0 | 2.01 ± 0.00 | 0.36 ± 0.01 | 2.14 ± 0.01 | 1.54 ± 0.02 | 0.13 ± 0.01 | 0.70 ± 0.01 | 2.37f | 6.88b | |
| 5 | 0.15 ± 0.01 | ND | 0.34 ± 0.02 | 1.99 ± 0.07 | 0.33 ± 0.03 | 2.05 ± 0.03 | 4.36c | 4.85d | |
| 10 | ND | ND | ND | 2.31 ± 0.04 | 0.44 ± 0.01 | 2.69 ± 0.07 | 5.44a | 5.44c | |
| 15 | ND | ND | ND | 2.30 ± 0.02 | 0.40 ± 0.01 | 2.72 ± 0.06 | 5.43a | 5.43c | |
| 20 | ND | ND | ND | 2.26 ± 0.04 | 0.39 ± 0.01 | 2.07 ± 0.02 | 4.73b | 4.73d | |
Data are expressed as mean ± SD of triplicate experiments
SS steaming sprout soybean, GS germinating sprout soybean, RS roasting sprout soybean, ND not detected
Mean values with different superscripts in the same column are significantly different at P < 0.05 by Duncan’s multiple range test
The total isoflavone content of all processed sprout soybeans decreased during fermentation. Moreover, the results presented here indicate that the isoflavones were converted from glycoside forms into aglycone forms. In particular, glycoside forms were not detected after 10 days of fermentation. The total isoflavone content decreased from 5.99 to 4.08 mg/g in the steamed beans, 7.02 to 5.39 mg/g in the germinated beans, and 6.92 to 4.85 mg/g in the roasted beans after 20 days of fermentation. Also, the isoflavones in the sprout soybeans changed from glycoside forms into aglycone forms after 10 days of fermentation.
The levels of aglycones rose from 0.89 to 5.46 mg/g in the steamed beans, 1.47 to 5.45 mg/g in the germinated beans, and 2.06 to 5.44 mg/g in the roasted beans after 10 days of fermentation. When the soybean was fermented using mushroom mycelia, the conversion to aglycone form isoflavones was confirmed through fermentation of about 10 days. Thus, the conversion rate rose 2.5–7.0 times after fermentation. Sprout soybean has been reported to be rich in isoflavones [8] compared to other beans, especially after 24 h of germination [10]. Moreover, the isoflavone content in roasted Rhynchosia nulubilis was higher than that in nonroasted beans [11]. This tendency is consistent with the results presented by Jeong et al. [15] that glycoside isoflavones in Cheonggukjang dropped by one-third and aglycone isoflavones increased by around 50-fold after 45 h of fermentation, resulting in approximately half of the total original isoflavone content after fermentation. Shon [28] reported that the total isoflavone content were decreased in steamed and germinated beans after fermentation with Tricholoma matsutake mycelia, however, the isoflavone content was highest on the after 9 days of fermentation.
In all processed sprout soybeans fermented with mushroom mycelia, it took around 10 days for glycoside isoflavones to be converted to bioactive aglycone isoflavones. Moreover, on the after 10 days of fermentation, all processed sprout soybeans had 5.45–5.46 mg/g of aglycone isoflavones (Table 1, Fig. 3). Accordingly, these data indicate that high aglycone isoflavone yields can be obtained from fermented beans when sprout soybeans (already rich in isoflavones) are heat-treated and fermented with mushroom mycelia.
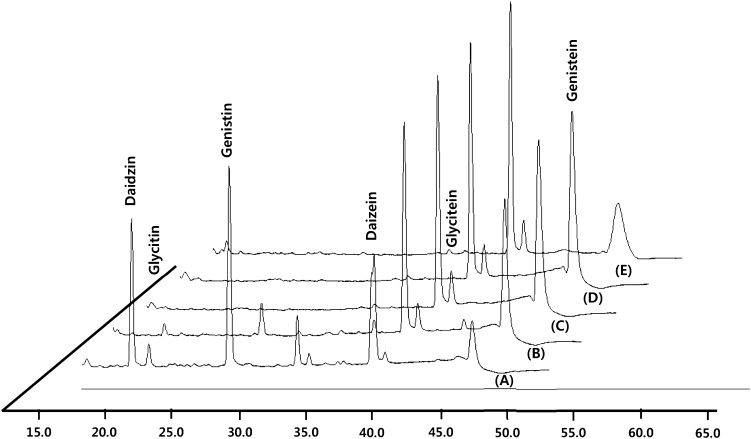
Fig. 3.
HPLC chromatogram changes of roasted sprout soybeans fermented with Irpex lacteus mycelia for 20 days. (A) Chromatogram after 0 day fermentation. (B) Chromatogram after 5 days fermentation. (C) Chromatogram after 10 days fermentation. (D) Chromatogram after 15 days fermentation. (E) Chromatogram after 20 days fermentation
DPPH radical scavenging ability
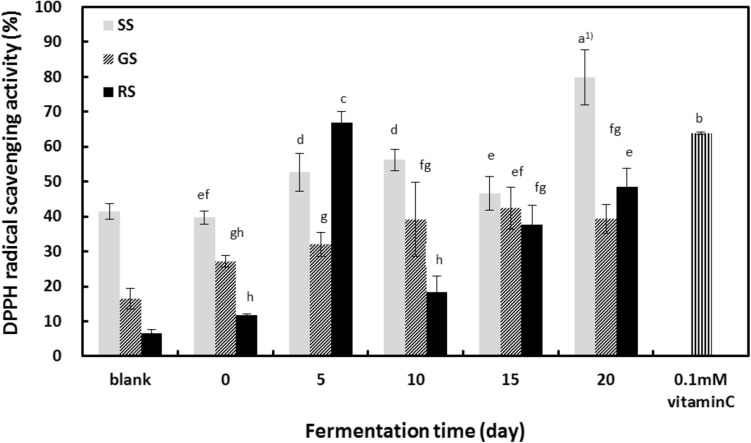
To measure the antioxidant activity in fermented sprout soybeans, the beans underwent different types of processing (steaming, germination, roasting) followed by fermentation with mushroom mycelial fluid (10%) for 20 days. The ability of the soybeans to scavenge DPPH radicals was assessed every five days during this time (Fig. 4). The DPPH radical scavenging ability of all processed sprout soybeans increased after fermentation. Roasted beans showed the highest increase (6.64–53.79%), followed by germinated beans (16.50–45.64%) and steamed beans (41.43–84.66%). Therefore, the DPPH radical scavenging ability of beans increased about 2- to 8-fold after fermentation. This finding is consistent with the results of Cho and Joo [34] and Kim et al. [35], both of whom showed that DPPH radical scavenging ability is correlated with phenolic content. Also, Shon [28] reported that the DPPH radical scavenging ability of germinated beans and steamed beans fermented with Tricholoma matsutake mycelia tended to increase sharply in the early stage of fermentation. Kim et al. [35] studied the effect of temperature on small black soybean antioxidant activity. Kim and colleagues found that beans roasted at 250 °C for 30 min showed the greatest phenolic content and the greatest DPPH radical scavenging ability (around 58% greater than control beans). There was no significant difference in the antioxidant activity of the processing method. However, the data presented here indicate that fermenting steamed, germinated, or roasted sprout soybeans with I. lacteus mycelia greatly increased their DPPH radical scavenging ability. The correlation coefficient between the amount of bioactive compounds and the antioxidant activity is shown in the Table 2. The correlation coefficient between DPPH radical scavenging ability and total phenolic content in all soybean fermented by I. lacteus mycelia was relatively high (0.6190–0.9888), and a relatively low correlation with isoflavone content (0.3673–0.7054). A likely explanation is that heat-treating beans alters their physicochemical properties, resulting in increased levels of different bioactive compounds [9, 35].
Fig. 4.
Changes in DPPH radical scavenging activity of sprout soybeans after fermentation with Irpex lacteus mycelia. Data are expressed as mean ± SD of triplicate experiments. SS steamed sprout soybeans, GS germinated sprout soybeans, RS roasted sprout soybeans. Mean values with different superscripts are significantly different at P < 0.05 by Duncan’s multiple range test
Table 2.
Correlation coefficients between DPPH radical scavenging activity, total phenol content, total flavonoid content, and isoflavone content of the sprout soybean fermented with Irpex lacteus mycelia
| R 2 | SS | GS | RS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total phenol | Total flavonoid | Isoflavone | Total phenol | Total flavonoid | Isoflavone | Total phenol | Total flavonoid | Isoflavone | |
| DPPH radical scavenging activity | 0.6190 | 0.3592 | 0.3940 | 0.9888 | 0.70538 | 0.5680 | 0.7576 | 0.5091 | 0.3673 |
SS steamed sprout soybeans, GS germinated sprout soybeans, RS roasted sprout soybeans
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Kim JA, Jung WS, Chun SC, Yu CY, Ma KH, Gwag JG, Chung IM. A correlation between the level of phenolic compounds and the antioxidant capacity in cooked-with-rice and vegetable soybean (Glycine max L.) varieties. Eur. Food Res. Technol. 2006;224:259–270. doi: 10.1007/s00217-006-0377-y. [DOI] [Google Scholar]
- 2.Malenčić D, Popović M, Miladinović J. Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr.) seeds. Molecules. 2007;12:576–581. doi: 10.3390/12030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeon SH, Lee K, Byoun KE. Studies on changes of isoflavone and nutrients during germination of soybean varieties. Korean J. Human Ecol. 2005;14:485–489. [Google Scholar]
- 4.Kim HY, Kim YS. Biological activities of fermented soybean paste (Doenjang) prepared using germinated soybeans and germinated black soybeans during fermentation. Food Sci. Biotechnol. 2014;23:1533–1540. doi: 10.1007/s10068-014-0209-y. [DOI] [Google Scholar]
- 5.Xu X, Wang HJ, Murphy PA, Hendrich S. Neither background diet nor type of soy food affects short-term isoflavone bioavailability in women. J. Nutr. 2000;130:798–801. doi: 10.1093/jn/130.4.798. [DOI] [PubMed] [Google Scholar]
- 6.Yoon JY. Change of Isoflavones in hypocotyl, cotyledon and seed coat depending on germination period and soybean cultivars. MS Thesis. Konkuk University, Seoul, Korea (2016)
- 7.Moon BK, Jeon KS, Hwang IK. Isoflavone contents in some varieties of soybean and processing conditions. Korean J. Soc. Food Sci. 1996;12:527–534. [Google Scholar]
- 8.Kim YH, Hwang YH, Lee HS. Analysis of isoflavone for 66 varieties of sprout bean and bean sprouts. Korean J. Food Sci. Technol. 2003;35:568–5759. [Google Scholar]
- 9.Jung TD, Shin GH, Kim JM, Choi SI, Lee SJ, Heo IY, Park SJ, Kim HT, Kang BK, Lee OK. Assessment of validation method for bioactive contents of fermented soybean extracts by bioconversion and their antioxidant activities. J. Korean Soc. Food Sci. Nutr. 2016;45:680–689. doi: 10.3746/jkfn.2016.45.5.680. [DOI] [Google Scholar]
- 10.Choi SJ, Choi UK. Changes in isoflavone contents and germination characteristics of germinated soybean under light condition. J. Korean Soc. Food Cult. 2014;29:599–604. doi: 10.7318/KJFC/2014.29.6.599. [DOI] [Google Scholar]
- 11.Lee KH, Kim MJ, Kim AJ. Physicochemical composition and antioxidative activities of Rhynchosia nulubilis according to roasting temperature. J. Korean Soc. Food Sci. Nutr. 2014;43:675–681. doi: 10.3746/jkfn.2014.43.5.675. [DOI] [Google Scholar]
- 12.Cho KM, Hong SY, Math RK, Lee JH, Kambiranda DM, Kim JM, Asraful Islam SM, Yun MG, Cho JJ, Lim WJ, Yun HD. Biotransformation of phenolics (isoflavones, flavanol and phenolic acids) during the fermentation of Cheonggukjang by Bacillus pumilus HY1. Food Chem. 2009;114:413–419. doi: 10.1016/j.foodchem.2008.09.056. [DOI] [Google Scholar]
- 13.Kim B. Production of conjugated linoleic acid-containing of fermented soybean and biological activities by lactic acid fermentation. PD Thesis. Gyeongnam National University of Science and Technology, Jinju, Korea (2015)
- 14.Kim IB, Shin S, Lim BL, Seong GS, Lee YE. Bioconversion of soybean isoflavone by Lactobacillus plantarum and Bifidobacterium longum. Korean J. Food Cookery Sci. 2010;26:214–219. [Google Scholar]
- 15.Jeong PH, Shin DH. Kim YS. Effect of germination and osmopriming treatment on enhancement of isoflavone contents in various soybean cultivars and Cheonggukjang (fermented unsalted soybean paste) J. Food Sci. 2008;73:187–194. doi: 10.1111/j.1750-3841.2008.00897.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim JK, Kim JK, Lim SS. Physiological activities of extract from edible mushrooms. J. Korean Soc. Food Sci. Nutr. 2010;39:1087–1096. doi: 10.3746/jkfn.2010.39.8.1087. [DOI] [Google Scholar]
- 17.Matsui T, Kagemori T, Fukuda S, Ohsugi M, Tabata M. Characteristic of wine produced by mushroom fermentation using Schizophyllum commune NBRC 4929. Mushroom Sci. Biotechnol. 2009;17:107–111. [Google Scholar]
- 18.Matsui T. Development of functional food by mushroom fermentation. Food and Development. 2010;48:11–13. [Google Scholar]
- 19.Kim SM, Kim EJ. Development of resources for functional food and biological activity of Lentinus edoes mycelium. Korean J. Herbol. 2011;26:25–30. [Google Scholar]
- 20.Cho JH, Choi GH, Park IJ, Baek SO, Kim HH, Kim CS. Development of Functional Food Materials from Acanthopanax senticosus-fermented mushroom mycelia. J. Korean Soc. Food Sci. Nutr. 2014;43:411–418. doi: 10.3746/jkfn.2014.43.3.411. [DOI] [Google Scholar]
- 21.Joung EM, Kim HY, Hwang IG, Jeong JH, Yu KW, Lee JS, Jeong HS. Changes of antioxidant activities on cultured ginseng with mushroom mycelia during cultivation. J. Korean Soc. Food Sci. Nutr. 2010;39:1346–1352. doi: 10.3746/jkfn.2010.39.9.1346. [DOI] [Google Scholar]
- 22.Kim HS, You JH, Jo YC, Lee YJ, Park IB, Park JW, Jung MA, Kim YS, Kim SO. Inhibitory effect of Phellinus linteus and rice with Phellinus linteus mycelium on obesity and diabetes. J. Korean Soc. Food Sci. Nutr. 2013;42:1029–1035. doi: 10.3746/jkfn.2013.42.7.1029. [DOI] [Google Scholar]
- 23.Robert R. The fungal pharmacy. The complete guide to medical mushroom & Lichens of North America, North Atlantic Books Berkeley, California, pp. 242–243 (2011)
- 24.Kim YJ, Song HG, Choi HT. Degradation of bisphenol A and removal of its estrogenic activity by two laccase transformants of Irpex lacteus. Korean J. Microbiol. 2008;44:199–202. [Google Scholar]
- 25.Kobayashi H. Substrate specificity of a carboxyl proteinase Iprex lacteus. Agric. Biol. Chem. 1983;47:1921–1923. [Google Scholar]
- 26.Kikuchi E, Kobayashi H, Kusakabe I. Suitability of milk-clotting enzyme from Irpex lacteus for goda cheese manufacture. Jpn. J. Zootech Sci. 1988;59:532–554. [Google Scholar]
- 27.AOAC. Official methods of analysis 18th ed, The association of official analytical Chemists, Washington DC, USA. CH 45. pp. 114–118 (2005)
- 28.Shon KS. Change of phytoestrogen during solid-state fermentation of soybean by mycelia of Tricholoma matsutake. MS Thesis. Gyeongnam National University of Science and Technology, Jinju, Korea (2014)
- 29.Singleton VL, Russi J. Colorimetry of total phenolics with phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- 30.KHSA. Health Functional Food Code. Korea Health Supplements Association. Ministry of Food and Drug safety, Seoul, Korea. pp. 341–343 (2016)
- 31.Kim CS, Lee YS, Kim JS, Hahn YH. High performance liquid chromatographic analysis of isoflavones in soybean foods. Korean J. Food Sci. Technol. 2000;32:25–30. [Google Scholar]
- 32.Blois MS. Antioxidant determination by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 33.Tsujiyama SI. Production of phenolic compounds from plant biomass by in solid-state cultivation. Mushroom Sci. Biotechnol. 2009;17:71–7443. [Google Scholar]
- 34.Cho KM, Joo OS. Enhances antioxidant effect of sweet potato by roasting. Korean J. Food Preserv. 2012;19:735–743. doi: 10.11002/kjfp.2012.19.5.735. [DOI] [Google Scholar]
- 35.Kim HG, Kim GW, Oh H, Yoo SY, Kim YO, Oh MS. Influence of roasting on the antioxidant activity of small black soybean (Glycine max L. Merrill). LWT-Food. Sci. Technol. 2011;44:992–998. [Google Scholar]