Abstract
The current study investigates the oxidative stability of soybean oil packaged with an oxygen scavenging film prepared by pyrogallol coating with concentrations of 5, 10, and 20% at 5, 23, and 60 °C and 95 ± 2% RH respectively. The oil stability was evaluated in terms of peroxide, thiobarbituric acid, and p-anisidine then compared with oil packed without the oxygen scavenging film. The results showed that the LDPE/PG 10 and 20% were efficient in the stabilization of soybean oil, even at high temperature. Peroxide, Thiobarbituric acid, and p-anisidine values, the oil samples packed with LDPE/PG films delayed the oil oxidation. The synergetic effect of LDPE/PG films, which can scavenge oxygen from the packaged product thereby slowing the oxidation of fats, was established in the study. The present study confirmed that active packaging could be introduced as a worthy replacement for direct addition of artificial antioxidants to the soybean oil.
Keywords: Oxygen scavenger, Active packaging, Oxidative stability, Soybean oil, Pyrogallol
Introduction
Oxygen is a key factor in food shelf life since it directly contributes to the oxidation of lipids and fats. On the other hand, oxygen is also responsible for the growth of aerobic microorganisms in food and therefore the loss of food quality [1, 2]. To overcome such critical issues, the oxygen must be removed from packaged foods or reduce the oxygen concentration from the headspace of food product packaging [3]. The existing technologies used to slow down or prevent oxygen-related deterioration in food include: packaging under inert gas, vacuum packaging/skin packaging, deaerating, sparging with an inert gas, modified atmosphere packaging (MAP), heating to expel air, use of chemical additives and preservatives, addition of antioxidants, and use of oxygen scavenger [4].
Food products that contain unsaturated lipids are highly susceptible to oxidation and hence food quality deterioration, such as changes in organoleptic properties, loss of nutritional qualities, and reduced shelf life [5]. These changes in quality are mainly due to hydroperoxides, aldehydes, and ketones, which are the main oxidation products [6]. To inhibit lipid oxidation, several methods have been developed for improving the stability of oils that include genetic modifications, compositional changes via chemical means, and addition of synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylatedhydroxytoluene (BHT) [7]. Nevertheless, in the last two decades, consumers, due to some concern regarding health safety, abstain from using synthetic antioxidants since they are added directly into the food product [8].
The substitute approach is a modified atmosphere packaging in which the gases inside the package are modified (generally using low oxygen and high nitrogen concentration) and combined with a high gas barrier packaging material. Due to the resulting low concentration of oxygen inside the package oxidation and microbial growth is inhibited [9, 10]. Although the MAP technique increases food shelf life, it is less effective due to the problems with oxygen permeability of packaging materials, leakage of air through poor seals and pinholes, outgassing of foodstuffs, and inadequate evacuation and/or gas flushing [11]. Thus there is a need for active packaging materials that can scavenge oxygen from package headspaces and that permeate through the package wall during storage [12]. Over the last few decades, active packaging has been an advanced method in food packaging technology that incorporates an active compound into the packaging material, and thus has the capacity to increase the quality and safety of foods, such as meat and bakery products [13–16]. Oxygen scavenging/absorbing packaging is one type of active packaging in which an oxygen scavenging compound (alone or with active compounds) is incorporated into package materials. The resultant continuous oxygen scavenging would extend the shelf life of a food product without adding further preservatives, including a synthetic antioxidant [17, 18].
The presence of an oxygen scavenger, including iron-based compounds, is popular due to low cost, availability, and effectiveness [19]. However, iron-based oxygen scavengers have several limitations for food packaging use. For example, there is the potential risk of accidental consumption by children and babies, and the fact that packages containing oxygen scavenging sachets cannot be used in microwave ovens; iron-based oxygen scavengers also create issues for metal detectors in food production lines [2]. The use of natural active compounds is a good alternative as they are non-toxic compounds with a lower risk of health concerns related to foodstuff [20]. Vegetables and aromatic plants, which are used as spices, contain significant levels of biological active components with high antioxidant activity [6]. The majority of these components are polyphenols. Polyphenols are plant derived products that are commonly found in fruits and plants. Polyphenols are well-known for their antioxidant properties due to the ability to scavenge free radicals [17]. In addition, many polyphenols have been proved to possess bactericidal effects against both Gram-negative, and Gram-positive bacteria [21, 22]. Pyrogallol is a polyphenol compound. It has been confirmed that some portion of pyrogallol is an active flavonoid constituent and presents a high oxygen absorbing activity since pyrogallol effectively reacts with oxygen [2]. Pyrogallol has also exhibited antioxidant, antibacterial, and antiviral properties [23]. Pyrogallol is also an active ingredient employed in fruit storage to extend the shelf life [24] and is used in the development of active antimicrobial packaging materials [25]. To the best of our knowledge, no prior study has been done regarding oxygen scavenging films utilizing an organic-based scavenger in a food packaging application.
In the present study, different concentrations of pyrogallol as a natural oxygen scavenger were coated onto low-density polyethylene (LDPE) containing sodium carbonate as an alkaline compound. The resulting effect of the films on oxidative stability of soybean oil stability at 5, 23, and 60 °C and 95% RH were evaluated for oxidation paramerts including peroxide value, thiobarbituric acid, p-anisidine Value.
Materials and methods
Materials
A commercially refined soybean oil without any antioxidant was obtained from a local market of Wonju, South Korea. Pyrogallol (99%), sodium carbonate, and ethyl acetate were purchased from Daejung Chemical Co. Ltd (Jeongwang-Dong Siheung-Si Gyeonggi-Do, Korea). Polyurethane was purchased from HICHEM Co., Ltd. (Seoul, Korea). Aromatic polyisocyanate (75%) used as a hardener was purchased from Hanwha Fine Chemical (Yeosu-si, Jeollanam-do, Korea). LDPE resin (XJ700 LDPE) was purchased from LG Chemicals (Yeosu, Korea). All chemicals were of reagent grade and used without additional purification.
Fabrication of oxygen scavenging film
A low-density polyethylene (LDPE) film was modified by blending with sodium carbonate (SC). The modified LDPE films were prepared by using a laboratory scale twin-screw extruder (HEM, HANKOOK E.M. Ltd., Gyeonggi-do, South Korea) with a length/diameter ratio of 40:19. All chemicals were of reagent grade and used without further purification. Before processing, all materials were dried at 80 °C for 24 h to remove moisture. Modified LDPE films with a sodium carbonate loading of 10% (w/w) were prepared. Before extrusion, sodium carbonate and LDPE resin were mixed by shaking in a glass jar. The temperatures of the extruder were set to 170 °C for the header zone, 170 °C for zones 1–6, and 120 °C for the feed zone. The barrel pressure of 5.3 kgf/cm2 was used for melt-compounding and 4.61 kgf/cm2 for the extrusion process.
The prepared modified LDPE films were further coated with pyrogallol. As presented in Table 1, different amounts of pyrogallol were slowly added to a 7 mL ethyl acetate solution followed by magnetic stirring to ensure that the pyrogallol particles were well-dissolved in the ethyl acetate solution. Different amounts of polyurethane and hardener were added to the ethyl acetate solutions while stirring vigorously using an automatic stirrer for 30 min to produce the coating solution, followed by further stirring for 30 min. Then, each mixture was bar-coated onto a glass substrate containing a modified LDPE film sample (30 × 34 cm2) using a plate stripe coater (PSC-300, PEMS., Yuseong-gu, Daejeon, 305-343, Korea), which was used to control the film thickness. The film thickness was maintained at about 61 ± 1 μm to aid in assessing the physical properties. The pyrogallol-coated films were dried at 45 °C for 24 h in a vacuum oven. Then, the prepared films were stored in a high barrier aluminum foil stand-up zip lock bag (9 × 17 cm2) composed of PET/AL/PE (King Packaging Co., Ltd. Dongguan, Guangdong, China) until the experiments commenced. Finally, all of the film samples were studied to evaluate their physical, mechanical, and oxygen scavenging properties.
Table 1.
Typical recipe of the PG/PU coatings with different amounts of pyrogallol
| Film Code | Pyrogallol (g) | Polyurethane (g) | Polyisocyanate (g) | Total amount (g) |
|---|---|---|---|---|
| LDPE/PG 5% | 0.5 | 6.0 | 3.5 | 10 |
| LDPE/PG 10% | 1 | 5.5 | 3.5 | 10 |
| LDPE/PG 20% | 2 | 5.0 | 3.0 | 10 |
A control LDPE film without modification with sodium carbonate or a PG coating was developed using the same processing conditions, and then placed in the same high barrier aluminum foil stand-up zip lock bag.
Oil packaging and storage
10 mL of soybean oil were poured into 125 mL capacity clear glass vials, as shown in Fig. 1. Then, two pieces of LDPE/PG films (8 × 6 cm2) with a mass of 5.0 g (4% w/v) were inserted into the glass vial. The glass vial also contained 0.5 mL of deionized water to generate a high humidity environment inside the vial during the storage period. Further, the vial was hermetically sealed with a metal cap containing a polytetrafluoroethylene (PTFE) septum, and then the samples were stored at 5, 23, and 60 °C for 30 days. The storage temperature and relative humidity in the vial were maintained throughout the storage period and analyzed using a digital temperature and humidity meter (HTC, Shanghai, China).
Fig. 1.
Airtight packaging of soybean oil with LDPE/PG oxygen scavenging films coated with different amounts of pyrogallol
Determination of oxygen content in the headspace
The oxygen concentration in the glass vials was measured from the headspace of the glass vial using an oxygen/carbon dioxide analyzer (PBI-Dansensor America Inc., NJ, USA) with a zirconium oxide sensor. The oxygen analyzer sensor was injected into the glass vial through the PTFE septum and the oxygen concentration from the vial headspace was measured. The absorption of oxygen from the headspace by the pyrogallol-coated oxygen scavenging film was evaluated on the same day of soybean oil analysis. The results obtained using the oxygen analyzer reflected the oxygen percentage in the headspace of the vial over time. The oxygen content was measured in relation to the absorbed volume of oxygen in mL over time.
Peroxide value (PV)
The PV of packed soybean oil was measured by mixing the oil sample (5.0 g) with acetic acid–chloroform solution and saturated potassium iodide solution and titrated against 0.1 N sodium thiosulphate using starch indicator and calculated as milliequivalent peroxide/kg sample.
Thiobarbituric acid value (TBA)
0.1 mL of the oil sample was mixed with 0.9 mL water and 2.0 mL TBA reagent (5.2 mg/mL solution of TBA reagent) in test tubes and heated in a water bath for 15 min. The tubes were cooled to room temperature for 10 min and then vortexed for 2 min. The absorbance was measured at 532 nm. Concentrations of TBARS were evaluated from a standard calibration curve prepared using malondialdehyde (MDA) standard.
p-Anisidine value (p-AV)
p-Anisidine in acetic acid and allowed to stand for 10 min. The absorbance of the mixture was measured at the same wavelength. The p-AV was calculated using the equation: 25 * (1.2A2 − A1)/W, where, A1, A2, and W are the absorbance before adding p-anisidine, the absorbance after adding p-anisidine at 350 nm, and sample weight, respectively.
Statistical analysis
Data analysis was performed using the IBM SPSS statics 21 software (Statistical Package for Social Science, SPSS Inc., Chicago, IL, USA). The effect of each factor was analyzed by Scheffe multiple comparisons with a significance level of p < 0.05. The experimental results are expressed as the mean ± SD. A graphical representation was obtained using Sigma-plot 12.0 Software (Systat Software Inc., Richmond, CA, USA).
Results and discussion
Oxygen scavenging phenomena
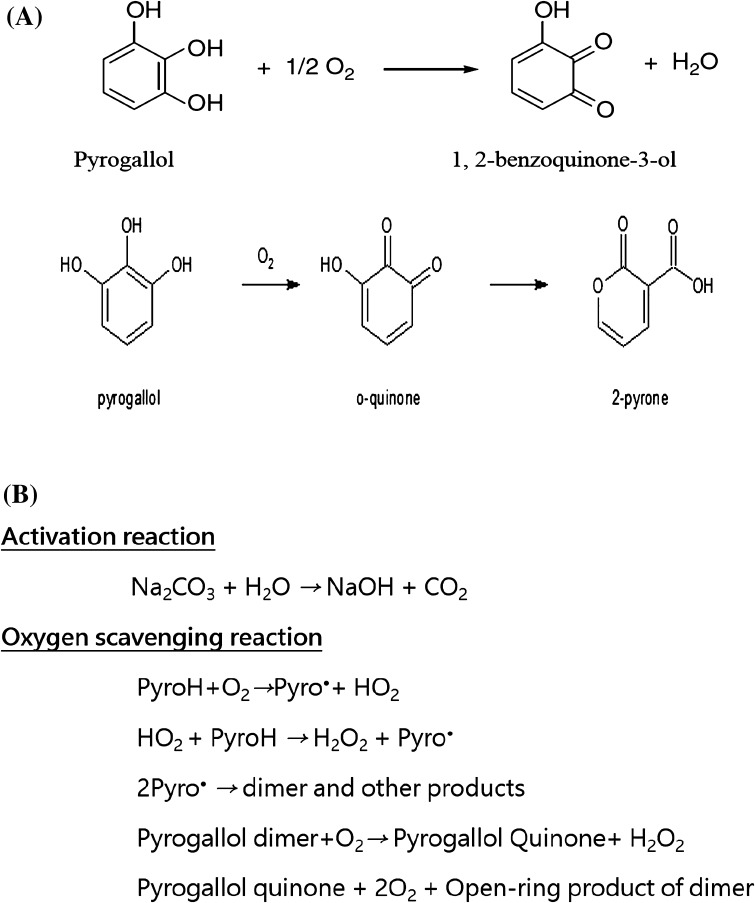
The phenomenon of oxygen scavenging is shown in Scheme 1. Briefly, oxygen free radicals are derived from non-enzymatic reactions of oxygen along with an alkaline solution including sodium carbonate [26]. The alkaline solution activates oxygen to the singlet electron state oxygen. Then, the activated oxygen undergoes subsequent decrease to reactive oxygen species, which is an oxygen free radical. Pyrogallol is an efficient free radical scavenger. It can also react irreversibly with singlet oxygen and produce pyrogallol quinone [27]. Pyrogallol can donate its electrons to scavenge the oxygen free radical. When the free radical gains the electron from pyrogallol, it returns to its ground state and the free radical is eliminated. Therefore, the oxygen content in the headspace will be reduced by these successive chemical reactions.
Scheme 1.
Oxidation mechanism of pyrogallol (A) and oxygen scavenging reaction (B)
Determination of headspace oxygen
The existing oxygen in the package responsible for the oxidation of soybean oil and therefore the determination of oxygen content in the headspace is necessary [4]. The oxygen scavenging capacity of all LDPE/PG films stored at 5, 23, and 60 °C showed significant differences (p < 0.05). When the storing temperature increased, the oxygen scavenging capacity of the LDPE/PG films also showed high effectiveness. The oxygen content (%) in the headspace of packages containing soybean oil and LDPE/PG films during the storage time were measured and presented in Table 2. There was a significant reduction in oxygen content (%) in the headspace of the package containing LDPE/PG 20% film treatment. The original oxygen content (%) in the vial headspace of 20.9% decreased to 0% by day 30 at 23 and 60 °C. The treatments containing pure LDPE with soybean oil did not show any reduction in oxygen content within the package headspace. The small reduction in headspace oxygen observed at 5 °C storage temperature in all concentrations of LDPE/PG films, was believed to be due to a lack of generation of relative humidity inside the package, and hence the oxygen scavenging reaction decreased at a low relative humidity. Moisture triggers the oxygen scavenging reaction in the LDPE/PG films. At a high oxidations temperature, accelerated changes in the physiochemical properties of oil occurred. Our prepared film has a high oxygen scavenging capacity at high temperature (60 °C), as well as at ambient temperature (23 °C). Therefore, due to the lack of oxygen in the headspace of the package, the oxidative quality of soybean oil remains stable even at high temperature over a prolonged storage period. Our results are in good agreement with Maloba et al. [4], wherein the oxidative stability of sunflower oil was increased by using an oxygen scavenging film containing polyfuryloxirane at 23 and 35 °C.
Table 2.
Oxygen content (%) in packages containing soybean oil with LDPE/PG films during 30 days storage period
| Treatments | Storage temperature (°C) | 0 Day | 7 Days | 14 Days | 21 Days | 30 Days |
|---|---|---|---|---|---|---|
| Pure LDPE | 5 | 20.9 ± 0.00a | 20.9 ± 0.00b | 20.9 ± 0.26c | 20.8 ± 0.00cd | 20.8 ± 0.00e |
| 23 | 20.9 ± 0.00a | 20.9 ± 0.00b | 20.9 ± 0.12a | 20.9 ± 0.00bc | 20.8 ± 0.00d | |
| 60 | 20.9 ± 0.00a | 20.9 ± 0.00bc | 20.9 ± 0.00a | 20.9 ± 0.00bc | 20.9 ± 0.00d | |
| LDPE/PG 5% | 5 | 20.9 ± 0.00a | 19.7 ± 0.61ab | 19.8 ± 0.25ab | 19.4 ± 0.10bc | 19.3 ± 0.32d |
| 23 | 20.9 ± 0.00a | 17.7 ± 0.30bc | 15.2 ± 0.26bc | 14.2 ± 0.20d | 13.9 ± 0.17cd | |
| 60 | 20.9 ± 0.00a | 05.2 ± 0.23b | 01.0 ± 0.20b | 0.00 ± 0.00c | 0.0 ± 0.11d | |
| LDPE/PG 10% | 5 | 20.9 ± 0.00a | 18.3 ± 0.20b | 18.3 ± 0.26b | 18.0 ± 0.23b | 18.1 ± 0.31c |
| 23 | 20.9 ± 0.00a | 14.7 ± 0.43b | 09.9 ± 0.82cd | 03.1 ± 0.54d | 0.0 ± 0.00c | |
| 60 | 20.9 ± 0.00a | 0.00 ± 0.00a | 0.0 ± 0.00b | 0.0 ± 0.00bc | 0.0 ± 0.00d | |
| LDPE/PG 20% | 5 | 20.9 ± 0.00a | 18.4 ± 0.22a | 18.6 ± 0.01b | 18.2 ± 0.11bc | 18.1 ± 0.12c |
| 23 | 20.9 ± 0.00a | 09.3 ± 0.85a | 03.6 ± 0.00bc | 0.0 ± 0.00ab | 0.0 ± 0.00c | |
| 60 | 20.9 ± 0.00a | 0.0 ± 0.00b | 0.0 ± 0.00b | 0.0 ± 0.00c | 0.0 ± 0.00d |
Results are expressed as the mean ± SD (n = 3)
a–dThe different letters within same row differ significantly (p < 0.05)
Peroxide value (PV)
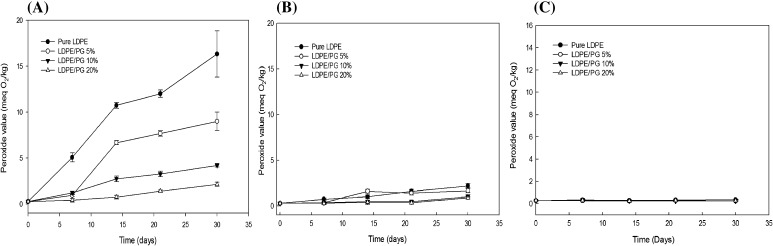
The peroxide value is a commonly used technique to measure the oxidative stability of soybean oil. The extent of peroxides shows the degree of key oxidation and therefore is directly linked to rancidity [7]. The stable increase of the peroxide value indicates the development of hydroperoxides during the oxidation of fat. Figure 2 shows the changes in PV of the soybean oil stored with the LDPE/PG film at 5, 23, and 60 °C. At the 60 °C storage condition, the sample containing pure LDPE had an initial value of 0.26 ± 0.11. However, a steady increase was noted during storage and, after the third week of storage, there was a significant increase to 10.73 ± 0.33 and finally to 16.33 ± 2.51 at the last week of storage. On the other hand, the sample containing LDPE/PG 20% had an initial value of 0.26 ± 0.11 on day one, which increased only to 2.11 ± 0.23 by the fourth week. The PV of oils at the last week of storage was 0.25 ± 0.01 and 0.86 ± 0.12, respectively, for samples LDPE/PG 20% stored at 5 and 23 °C. As can be seen, the soybean oil packaged with LDPE/PG with 10 and 20% exhibited better stability compared to pure LDPE. Oil packed with LDPE/PG 5% showed values higher than oil packed with LDPE 10 and 20%, which indicates that the film with a 5% pyrogallol coating is less effective than other concentrations at all storage conditions. However, at 23 and 60 °C LDPE/PG 5% effectively reduced the formation of peroxides, indicating better stabilization of the oil, as compared to pure LDPE and the positive control. This may be attributed to the radical scavenging efficiency of pyrogallol, which is also a phenolic compound leading to the decreased peroxide formation. Colín‐Chávez et al. [28], have studied the oxidative stability of soybean oil packaged in LDPE film extruded marigold (Tagetes erecta) extract. As a result, the PV was significantly lower in the oil packed with marigold films than that in oil packed with control films. It has been reported that the vegetable oil packaged with oxygen scavenging film reduced the peroxide value during storage [4].
Fig. 2.
Effect of LDPE/PG films on the peroxide value of soybean oil at (A) 60, (B) 23, and (C) 5 °C
Thiobarbituric acid (TBA) value
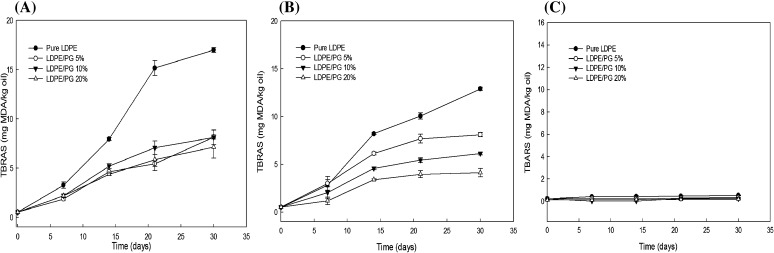
The TBA test measures the amount of malondialdehyde (MDA), which is a main secondary byproduct produced by aldehydes and ketones during the oxidation process [29]. The influence of the oxygen scavenging LDPE/PG films on the TBA value of soybean oil during a 30-day storage period at 5, 23, and 60 °C was reflected by the TBARS values, which is an index of lipid peroxidation and shown in Fig. 3. Soybean oil packaged with individual LDPE/PG films showed an increase in TBA value during storage on day 3 at 23 and 60 °C storage conditions. However, the TBA value in the soybean oil packaged with the PVA/AP film was significantly different (p > 0.05) compared to that in the pure LDPE film as a function of the storage time at 23 and 60 °C. At low temperature (5 °C), no significant difference was observed in all stored samples. The highest TBA value, 17.00 ± 0.24, was observed in the soybean oil packaged in pure LDPE during storage at 60 °C. This indicates that the effect of the LDPE/PG with 20% was much greater than that of the pure LDPE and LDPE/PG films with 5 and 10% pyrogallol retarded lipid oxidation. This observation is probably due to the lack of oxygen present in the package because of the high oxygen scavenging capacity of the film. The oxygen scavenging activities of the pyrogallol polyphenols have been attributed to assorted mechanisms, including prevention of radical chain initiation due to oxygen limitations, binding of transition metal ion catalysts, and interaction with free radicals to inhibit lipid oxidation [30]. Gaikwad et al. [31] reported that polyvinyl alcohol films containing dried apple pomace had a high free radical scavenging ability of the films with an increase of apple pomace content, which showed oxidative stability at 60 °C. The oxygen capacity of pyrogallol is very high at high temperatures while in alkaline condition [2]. Therefore, at 60 °C the LDPE/PG film showed high oxygen scavenging activity. This result suggested that lipid oxidation in soybean oil samples could be minimized by using LDPE/PG 10 and 20% films due to the demonstrated oxygen scavenging capacity.
Fig. 3.
Effect of LDPE/PG films on the TBA value of soybean oil at (A) 60, (B) 23, and (C) 5 °C
p-Anisidine value (p-Av)
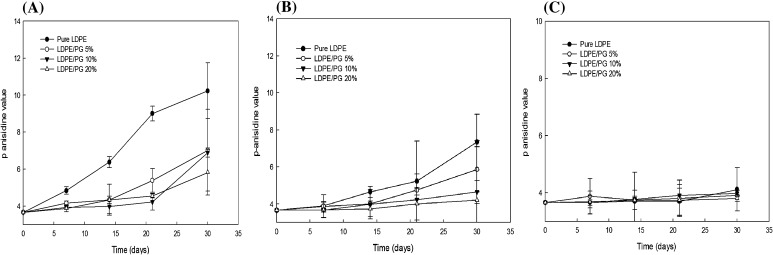
An assessment of the 2-alkenals and 2, 4-alkadienals, which are secondary oxidation products in soybean oil produced during decomposition of hydroperoxides can be estimated by the determination of p-anisidine content [32]. Our results for p-anisidine analysis are presented in Fig. 4. The trends of increasing p-anisidine content in different temperature conditions were similar to the peroxide value results. The p-Av of soybean oil was studied for 30 days without LDPE/PG films and showed the highest degradation. On day 0, the p-anisidine value of soybean oil packaged with pure LDPE film was 3.66, which reached a maximum of 7.23 and 10.23 at 23 and 60 °C, respectively. Oil packaged with LDPE/PG 10 and 20% showed a maximum of 4.65 and 4.21 anisidine, respectively. Oxygen scavenging films with 10 and 20% pyrogallol effectively reduced the oxidation rate of the oil, as detected by decreases in p-AVs and relatively low reduction compared to pure LDPE. Our results are in good agreement with the results provided by Taghvaei et al. [32], which indicate that the p-Av values were lower in soybean oil containing natural antioxidants.
Fig. 4.
Effect of LDPE/PG films on the p-Anisidine value of soybean oil at (A) 60, (B) 23, and (C) 5 °C
Overall, the results showed that, during a 30 days storage period at 5, 23, and 60 °C, the soybean oil packaged with LDPE/PG oxygen scavenging film showed a significant positive effect on soybean oil stability than samples packaged with pure LDPE. A direct and positive correlation was observed between oxygen and PV, p-AV levels, for all the tested oil samples. The presence of oxygen in the package headspace resulted in oxidation reactions, which further deteriorated the quality of soybean oil samples. Additionally, the damaging effect of increasing temperature and RH was highly correlated with PV, TBA, and p-AV levels. The protective effect of pyrogallol coated LDPE/PG films in stabilizing the soybean oil samples was prominently observed. Our results clearly confirmed that the soybean oil samples packed with LDPE/PG films, especially coated with 10 and 20% pyrogallol, showed a better stabilizing effect than oil packaged with pure LDPE under the examined experimental conditions. However, further studies are required for the industrial application of pyrogallol as a natural oxygen scavenger in order to prepare oxygen scavenging active food packaging.
Acknowledgment
The authors would like to thank One Jung Can (OJC) Manufacturing Co., Ltd., Seoul, South Korea, for financial support for this project. This research study is part of project titled “Development of oxygen scavenging package for fish cake” (Project Number: 2015-51-031).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Gaikwad KK, Singh S, Lee YS. A pyrogallol-coated modified LDPE film as an oxygen scavenging film for active packaging materials. Prog. Org. Coat. 2017;111:186–195. doi: 10.1016/j.porgcoat.2017.05.016. [DOI] [Google Scholar]
- 2.Gaikwad KK, Lee YS. Novel natural phenolic compound-based oxygen scavenging system for active packaging applications. Food Measure. 2016;10:533–538. doi: 10.1007/s11694-016-9332-1. [DOI] [Google Scholar]
- 3.Ahn BJ, Gaikwad KK, Lee YS. Characterization and properties of LDPE film with gallic‐acid‐based oxygen scavenging system useful as a functional packaging material. J. Appl. Polym. Sci. 133 (2016)
- 4.Maloba FW, Rooney ML, Wormell P, Nguyen M. Improved oxidative stability of sunflower oil in the presence of an oxygen-scavenging film. J. Am. Oil Chem. Soc. 1996;73:181–185. doi: 10.1007/BF02523892. [DOI] [Google Scholar]
- 5.Almasi H, Ghanbarzadeh B, Dehghannya J, Entezami AA, Khosrowshahi Asl A. Development of a novel controlled-release nanocomposite based on poly (lactic acid) to increase the oxidative stability of soybean oil. Food Addit. Contam. A. 2014;31:1586–1597. doi: 10.1080/19440049.2014.935962. [DOI] [PubMed] [Google Scholar]
- 6.Gaikwad KK, Singh S, Shakya BR. Studies on the development and shelf life of low calorie herbal aonla-ginger RTS beverage by using artificial sweeteners. J. Food Process. Technol. 4:200. 2. (2013)
- 7.Chandran J, Nayana N, Roshini N, Nisha P. Oxidative stability, thermal stability and acceptability of coconut oil flavored with essential oils from black pepper and ginger. J. Food Sci. Tech. Mys. doi:10.1007/s13197-016-2446-y (2016) [DOI] [PMC free article] [PubMed]
- 8.Moudache M, Colon M, Nerín C, Zaidi F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016;212:521–527. doi: 10.1016/j.foodchem.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Alasalvar C, Al-Farsi M, Quantick PC, Shahidi F, Wiktorowicz R. Effect of chill storage and modified atmosphere packaging (map) on antioxidant activity, anthocyanins, carotenoids, phenolics and sensory quality of ready-to-eat shredded orange and purple carrots. Food Chem. 2005;89:69–76. doi: 10.1016/j.foodchem.2004.02.013. [DOI] [Google Scholar]
- 10.Kostaki M, Giatrakou V, Savvaidis IN, Kontominas MG. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (dicentrarchus labrax) fillets. Food Microbiol. 2009;26:475–482. doi: 10.1016/j.fm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Bodbodak S, Rafiee, Z. Recent trends in active packaging in fruits and vegetables. Eco-Friendly Technology for Postharvest Produce Quality. 77–125 (2016)
- 12.Biji KB, Ravishankar CN, Mohan CO, Gopal TS. Smart packaging systems for food applications: a review. J. Food Sci. Tech. Mys. 2015;52:6125–6135. doi: 10.1007/s13197-015-1766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Park L, Shin Y, Lee YS. Antimicrobial seafood packaging: a review. J. Food Sci. Tech. Mys. 2016;53:2505–2518. doi: 10.1007/s13197-016-2216-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh S, Park I, Shin YJ, Lee YS. Antimicrobial properties of polypropylene films containing AgSiO2, AgZn and AgZ for returnable packaging in seafood distribution. J. Food Meas. Charact. 2016;10:781–793. doi: 10.1007/s11694-016-9363-7. [DOI] [Google Scholar]
- 15.Singh S, Gaikwad KK, Park SI, Lee YS. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int. J. Biol. Macromol. 2017;99:506–510. doi: 10.1016/j.ijbiomac.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Choi WS, Singh S, Lee YS. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘formosa’plum (Prunus salicina L.). LWT-Food. Sci. Technol. 2016;70:213–222. [Google Scholar]
- 17.Gaikwad KK, Lee YS. Effect of storage conditions on the absorption kinetics of non-metallic oxygen scavenger suitable for moist food packaging. J. Food Meas. Charact. 2017 [Google Scholar]
- 18.Byun Y, Bae HJ, Whiteside S. Active warm-water fish gelatin film containing oxygen scavenging system. Food Hydrocolloids. 2012;27:250–255. doi: 10.1016/j.foodhyd.2011.06.010. [DOI] [Google Scholar]
- 19.Cruz RS, dos Santos Pires AC, Camilloto GP. Oxygen scavengers: an approach on food preservation. INTECH Open Access Publisher (2012)
- 20.Kaur C, Kapoor HC. Antioxidants in fruits and vegetables—the millennium’s health. Int. J. Food Sci. Tech. 2001;36:703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- 21.Sánchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999;32:407–412. doi: 10.1016/S0963-9969(99)00097-6. [DOI] [Google Scholar]
- 22.Tinh TH, Nuidate T, Vuddhakul V, Rodkhum C. Antibacterial activity of pyrogallol, a polyphenol compound against vibrio parahaemolyticus isolated from the central region of thailand. Procedia Chem. 2016;18:162–168. doi: 10.1016/j.proche.2016.01.025. [DOI] [Google Scholar]
- 23.Alarcón E, Campos AM, Edwards AM, Lissi E, López-Alarcón C. Antioxidant capacity of herbal infusions and tea extracts: a comparison of ORAC-fluorescein and ORAC-pyrogallol red methodologies. Food Chem. 2008;107:1114–1119. doi: 10.1016/j.foodchem.2007.09.035. [DOI] [Google Scholar]
- 24.Jing G, Huang H, Yang B, Li J, Zheng X, Jiang Y. Effect of pyrogallol on the physiology and biochemistry of litchi fruit during storage. Chem. Cent. J. 2013;7:19. doi: 10.1186/1752-153X-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkan D, Aydemir LY, Arcan I, Yavuzdurmaz H, Atabay HI, Ceylan C, Yemenicioglu A. Development of flexible antimicrobial packaging materials against campylobacter jejuni by incorporation of gallic acid into zein-based films. J. Agr. Food Chem. 2011;59:11003–11010. doi: 10.1021/jf202584b. [DOI] [PubMed] [Google Scholar]
- 26.Abrash HI. The air oxidation of 4, 6-di (2-phenyl-2-propyl) pyrogallol. spectroscopic and kinetic studies of the intermediates. Carlsberg Res. Commun. 1977;42:11–25. doi: 10.1007/BF02906706. [DOI] [Google Scholar]
- 27.Matsuo Y, Tadakuma F, Shii T, Saito Y, Tanaka T. Selective oxidation of pyrogallol-type catechins with unripe fruit homogenate of citrus unshiu and structural revision of oolongtheanins. Tetrahedron. 2015;71:2540–2548. doi: 10.1016/j.tet.2015.03.016. [DOI] [Google Scholar]
- 28.Colín-Chávez C, Soto-Valdez H, Peralta E, Lizardi-Mendoza J, Balandrán-Quintana RR. Fabrication and properties of antioxidant polyethylene-based films containing marigold (Tagetes erecta) extract and application on soybean oil stability. Packag. Technol. Sci. 2013;26:267–280. doi: 10.1002/pts.1982. [DOI] [Google Scholar]
- 29.Mohammadi A, Jafari SM, Esfanjani AF, Akhavan S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016;190:513–519. doi: 10.1016/j.foodchem.2015.05.115. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Wang L. Sodium alginate/carboxymethyl cellulose films containing pyrogallic acid: physical and antibacterial properties. J. Sci. Food Agr. 2017;97:1295–1301. doi: 10.1002/jsfa.7863. [DOI] [PubMed] [Google Scholar]
- 31.Gaikwad KK, Lee JY, Lee YS. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J. Food Sci. Tech. Mys. 2016;53:1608–1619. doi: 10.1007/s13197-015-2104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taghvaei M, Jafari SM, Mahoonak AS, Nikoo AM, Rahmanian N, Hajitabar J, Meshginfar N. The effect of natural antioxidants extracted from plant and animal resources on the oxidative stability of soybean oil. LWT-Food Sci. Technol. 2014;56:124–130. doi: 10.1016/j.lwt.2013.11.009. [DOI] [Google Scholar]