Abstract
The objective of this study is to investigate the antiviral, anti-inflammatory, and antioxidant effects of the ethanol extract of Mentha piperita L. leaves (MPE). M. piperita L. leaves were extracted by reflux with ethanol. Total phenolic acid and total flavonoid content were determined. The antiviral activity of MPE against the respiratory syncytial virus (RSV) and the anti-inflammatory activity were evaluated in vitro. The levels of key pre-inflammatory mediators and cytokines including nitric oxide (NO), tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and prostaglandin E2 (PGE2) were determined. The antioxidant activities were also evaluated using a colorimetry method. MPE contained high levels of phenolic acid and flavonoid, showed antiviral activity against RSV with a high selectivity index, and significantly decreased the production of NO, TNF-α, IL-6, and PGE2 in lipopolysaccharide-stimulated RAW 264.7 cells. Meanwhile, MPE showed potential free-radical scavenging activities. These results indicate that Mentha piperita L. might be a good source of medicinal plants.
Keywords: Mentha piperita L., Antiviral, Anti-inflammatory, Antioxidant
Introduction
Inflammation is a complex biological response to harmful stimuli such as pathogens, damaged cells, or irritants and is a protective response involving immune cells, blood vessels, and molecular mediators [1]. Although inflammation is a defense mechanism, a series of biochemical events involving the local vascular system, the immune system, and various cells within the injured tissue propagate the inflammatory response [2]. A dose relationship exists between the viral infection and inflammation. Chronic inflammation can also be the result of a viral infection. Viruses induce disease deterioration directly and indirectly by activating inflammatory signaling pathways and cytokines, stimulating the growth of infected cells, and inhibiting apoptosis [3].
The human respiratory syncytial virus (RSV) is a syncytial virus that causes respiratory tract infections, occasionally leading to hospitalization during infancy and childhood [3]. The mechanism underlying RSV-induced diseases remains elusive. Experimental evidence has demonstrated that overexpression of inflammatory responses induced by the host plays an important role in the development of clinical manifestations of the RSV infection. In patients with primary RSV infection, alveolar macrophages and epithelial cells are likely to be infected, resulting in increased production of pro-inflammatory cytokines including interleukin (IL-1β), tumor necrosis factor (TNF-α), IL-6, prostaglandin E2 (PGE2), and chemokines (IL-8) [4]. Although these pro-inflammatory cytokines are involved in the host defense mechanism, their excessive production can lead to mutagenic effects on the host genome during persistent infection [5]. Thus, pharmacological reduction of inflammatory mediators is a preferred target in the development of therapeutic interventions aimed at limiting the inflammatory response due to RSV infection. Therefore, the search for more effective and safer anti-inflammatory and antiviral agents has become an important area of study the past two decades.
Mentha piperita L. (Peppermint), a hybrid mint, is a cross species between watermint and spearmint and belongs to the family Lamiaceae. This plant is native to Europe, Canada, and the USA and has been widely cultivated in other parts of the world. This Mentha species is famous for its flavoring and medicinal properties and is used in food, cosmetics, and medicines. It has been shown to be helpful in symptomatic relief from illnesses such as colds, cramps, indigestion, nausea, sore throat, toothache, or even cancer [6]. Many pharmacologic studies also have shown that M. piperita L. possesses antioxidant, cytotoxic, antiallergenic, antiviral, and antibacterial activities with few side effects [7, 8]. Essential oils extracted by steam distillation from the aerial parts of the plant have been reported to have anti-inflammatory, antibacterial, and antifungal properties [9, 10]. As described above, the various bioactivities of peppermint have been attributed to its different constituents. However, only a few studies have focused on the non-volatile constituents. Therefore, the present study was conducted to investigate the in vitro antiviral activity of the ethanol extract from Mentha pipertia L. leaves against RSV and to determine the anti-inflammatory and antioxidant activities of this ethanol extract.
Materials and methods
Materials
Dulbecco’s modified essential medium and other cell-culture reagents including fetal bovine serum (FBS) were obtained from GIBCO (NY, USA). Lipopolysaccharide (LPS), 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT), sodium 3′-[1-ohenylamino-carbonyl-3,4-tetrazolium]-bis[4-methoxy-6-nitro]benzene sulfonic acid (XTT), 1,1-diphenyl-2- picryl-hydrazyl (DPPH), and butylated hydroxytoluene (BHT) were purchased from Sigma-Aldrich (St. Louis, USA). All other chemicals and solvents used in this study were of reagent grade quality.
Plant material and preparation of the extract
The whole, fresh M. piperita L. was collected from Jiangyin City, Jiangsu Province, and was identified by Dr. Zhenliang Sun (Shanghai Fengxian District Central Hospital). Dried M. piperita L. leaves (500.0 g) were ground in a rotary mill and sieved (10 mesh) to obtain a fine powder as the pretreated sample. The dried powdered leaves were firstly extracted with absolute ethanol at 90 °C for 2 h, and the residue was then re-extracted twice. The filtrate was evaporated under reduced pressure to yield a dark brown extract. The extract was then dissolved in hot distilled water and washed with petroleum ether (fraction 30–60 °C) to remove non-polar impurities. The extract was then concentrated to dryness under vacuum, yielding a residue of 4.27 g. The obtained ethanol extract from M. piperita L. leaves (MPE) was dissolved in methanol or dimethyl sulfoxide (DMSO) for evaluation of bioactivity.
Total phenolic acid content assay
The total phenolic content in the MPE was measured by a colorimetric assay according to a previously published method [11]. One milliliter of MPE (2 mg/mL) was diluted in 23 mL distilled water, followed by the addition of 0.5 mL Folin–Ciocalteu reagent and 1.5 mL of Na2CO3 (2%). The samples were vortexed and then kept at room temperature for 60 min. Absorbance was measured at 760 nm and recorded. The results were expressed as µg gallic acid equivalents (GAE) per milligram of MPE.
Total flavonoid content assay
Total flavonoid content was measured according to a previously described method [12]. One milliliter of MPE (2 mg/mL) was added to a solution of 0.05 M NaNO2 (0.14 mL), and the solution was mixed well and incubated at room temperature for 6 min. Afterwards 0.14 mL Al(NO3)3 (0.1 M) was added, and then, the mixture was shaken vigorously and kept at room temperature for another 5 min. Finally, 1 mL NaOH (0.1 mM) was added, and the absorbance was then measured against a prepared standard at 510 nm. Total flavonoid content was expressed as µg rutin equivalents per milligram of MPE.
Cells and viral cultures
The human larynx epidermal carcinoma (Hep-2) cell line was provided by the Cell Bank of Shanghai Institute of Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The RSV long strain was grown and titrated in Hep-2 cells, which were then cultured in DMEM (Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS, 2 mM l-glutamine, and a 100-U/mL penicillin and streptomycin solution (Sigma, St, Louis, MO, USA). Antiviral and cytotoxicity assays were performed in a medium only containing 2% FBS. All cells were cultured at 37 °C in a humidified atmosphere of 5% CO2.
Cytotoxicity assay
Cytotoxicity of MPE on the Hep-2 cells was measured by the XTT method according to the manufacturer’s instructions [13]. Briefly, 1 × 104 cells/well cells were seeded into 96-well culture plates and incubated overnight at 37 °C under 5% CO2. The medium was replaced by different concentrations of MPE (5, 10, 50, 100, and 200 µg/mL) in triplicate. After 72-h incubation, the cytotoxicity of MPE was determined using the XTT kit. Cytotoxicity was expressed as the 50% cytotoxic concentration (CC50), which is the concentration of sample that inhibited the growth of cells up to 50%.
Antiviral activity assay
Hep-2 cell cultures were prepared in 96-well plastic plates and incubated at 37 °C in a CO2 (5%) incubator for 2 days. The medium was then removed from the cell when the cell culture became confluent. A virus suspension (0.1 mL) containing 50% tissue culture-infective dose (TCID50) and maintenance medium (0.1 mL) with an appropriate concentration of the test sample were added to the Hep-2 cell monolayers in 96-well plates, using the maximal non-cytotoxic concentration as the highest concentration. The plates were incubated at 37 °C in a humidified CO2 atmosphere for 3 days. The cell morphology was inspected daily and observed for microscopically detectable alternations, including loss of the monolayer, rounding, shrinking of the cells, granulation, and vacuolization in the cytoplasm. The cytopathic effect (CPE) was scored according to the following score: 0 = 0%, 1 = 0–25%, 2 = 25–50%, 3 = 50–75%, and 4 = 75–100%. The concentration required to cause visible changes in 50% of intact cells (CC50) was estimated from the constructed graphs. The reduction in virus multiplication was calculated as a percentage of the virus control (% virus control = CPEexp/CPEvirus control × 100). The concentration of MPE that reduced the CPE by 50% with respect to virus control was estimated from graphs and defined as 50% inhibited concentration (IC50) expressed in µg/mL. The selectivity index (SI) was calculated from the ratio CC50/IC50 [14]. Ribarivin was used as the positive control.
Anti-inflammatory activity assay
Cell culture
The murine macrophage cell line RAW 264.7 was purchased from Shanghai Institute of Cell Biology (Shanghai, China). RAW 264.7 cells were cultured in DMEM supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% FBS. Cells were incubated in a 5% CO2 atmosphere at 37 °C and were subcultured every day.
Cell viability assay
Cell viability was determined using the MTT method according to a previous study [9]. Cells were seeded and treated with the indicated concentrations of MPE with LPS for 22 h and then were incubated with a solution of 5 mg/mL MTT for the next 2 h. DMSO (1% final concentration) was added to each well, and formazan was dissolved by gentle shaking. The plates were evaluated in a microplate reader at 540 nm.
Determination of nitric oxide (NO) production
To determine NO levels, RAW 264.7 cells were seeded at a density of 2 × 105 cells/well in a 96 well plate and grown for 2 h to adhere. Then, they were treated with various concentrations of MPE for 1 h and incubated for 24 h in fresh DMEM with or without LPS (1 µg/mL). The Griess reagent was used to determine nitrite levels. Briefly, an equal volume of culture supernatant (100 µL) was mixed with the Griess reagent, and absorbance was measured at 540 nm using a microplate reader.
Determination of TNF-α, IL-6, and PGE2
The secretion of TNF-α, IL-6, and PGE2 was measured by ELISA [15]. Briefly, RAW 264.7 cells (1 × 104 cells/well) were seeded in 24-well plates, stimulated with LPS (1 µg/mL), and incubated with different concentrations of MPE for 24 h. Then, TNF-α, IL-6, and PGE2 levels in the culture medium were determined with an ELISA kit according to the manufacturer’s instructions. All experiments were performed in triplicate.
Antioxidant activity assay
Assay of DPPH radical scavenging activity
DPPH radical scavenging activity was determined according to a previously published method with slight modifications [16]. A DPPH radical solution was prepared by dissolving it in methanol (4 mg/mL). Various concentrations of MPE (1 mL) were added to a 4-mL DPPH solution. The absorbance was measured at 517 nm after incubation for 30 min at room temperature. BHT was used as the positive control. The DPPH radical scavenging effect was calculated as follows:
where Ablank is the absorbance of DPPH without sample and Asample is the absorbance in the presence of MPE.
Assay of hydroxyl radical scavenging activity
Hydroxyl radical scavenging activity was determined according to the method of Liu et al. [17]. Briefly, a reaction mixture comprising 1.0 mL of FeSO4 (9.0 mM), 1.0 mL of MPE at different concentrations, and 1.0 mL of 9.0 mM H2O2, each added sequentially, was incubated for 30 min at 37 °C, and the absorbance was recorded at 510 nm. BHT was used as the positive control. The hydroxyl radical scavenging capacity was calculated according to the following equation:
where Acontrol and Asample represent the absorbance of the blank control group and sample group at 510 nm, respectively.
Assay of superoxide anion scavenging activity
Superoxide radical scavenging activity was measured based on a previously published method [17]. Briefly, 2 mL of 0.05 M Tris–HCl buffer (pH 8.2) was incubated at 25 °C for 20 min. Different concentrations of MPE (1 mL) and 0.4 mL 1,2,3-phentriol were then added, and the mixture was shaken rapidly at room temperature. The absorbance value of the mixture was measured at 325 nm per 20 s against a blank. The ability of the solution to inhibit pyrogallol autoxidation was calculated using the following equation:
where Ssample represents the slope of the sample group and Sblank is the slope of blank control group. A decrease in Ssample indicated an increase in scavenging activity, and BHT was used as the positive control.
Assay of reducing power
Different concentrations of MPE in a phosphate buffer (2.5 mL, 0.2 mol/L, and pH 6.6) were added to 1% potassium ferricyanide (2.5 mL) to evaluate its reducing power. After incubation at 50 °C for 20 min, 1 mL 0.1% FeCl3 and 2.5 mL distilled water were added to the mixture. Then, it was centrifuged at 4000 rpm for 10 min. Absorbance was recorded at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. BHT was used as the positive control.
Statistical analysis
All results were recorded as mean ± SD. Differences between the experimental groups were determined by the Student’s t test, and a p value less than 0.05 was considered statistically significant.
Results and discussion
Determination of the total phenolic content by Folin–Ciocalteu assay
Phenolic compounds, including simple phenols, phenolic acids, hydroxycinnamic acid derivatives, and flavonoids, are bioactive metabolites widely found in plants [18]. Phenolic compounds are known to directly contribute to the antioxidant activity of natural extracts, and therefore, it is important to determine the total phenolic content in MPE. In the present study, an equation obtained from the standard substance was given as follows: absorbance = 0.0075 × Gallic acid (μg/mg) and R 2 = 0.9995. The total phenolic content was expressed as GAE, 325.84 ± 14.17 µg/mg, indicating that approximately one-third of the compounds present in the MPE were phenolic compounds. The above results suggested that the bioactivities of the MPE might be attributed to the high phenolic content of the plant.
Determination of the total flavonoids content
Flavonoids are a diverse group of phytonutrients found in almost all fruits, and they have a general structure comprising a 15-carbon skeleton with two phenyl rings and a heterocyclic ring. Pharmacological studies have demonstrated that flavonoids derived from plants have various bioactivities [19]. In the present study, the total flavonoid content in the MPE was determined by the regression equation calibration curve (Y = 0.0013x + 0.0064 and R 2 = 0.9995) and was 118.92 ± 17.09 µg/mg, expressed in rutin equivalents.
Antiviral activity
Cytotoxicity was measured using the XTT method. MPE did not show any cytotoxicity against Hep-2 cells up to a concentration of 200 μg/mL. The high CC50 shows that it is safe for use. The antiviral activity of the MPE against RSV in Hep-2 cells was determined. As shown in Table 1, MPE exhibited potent in vitro antiviral activity with an IC50 value of 10.41 µg/mL and an SI value of 21.83. Although the IC50 of MPE is lower than that of ribavirin, its SI value was statistically higher than ribavirin, indicating that M. piperita L. has a promising future as an antiviral plant resource. Previous studies have also shown that some phenolic compounds including caffeoyl acid derivatives and flavonoid glycosides had potent anti-RSV activities [20]. Our chemical analyses showed that MPE had relatively high phenolic compound and flavonoid content. These compounds may be related to the antiviral activity of M. piperita L. against RSV. Therefore, a phytochemical investigation and mechanistic study of the antiviral activity of M. piperita L. extracts may be useful as a further study.
Table 1.
Antiviral activity of MPE against RSV
| Sample | IC50 (µg/mL)a | CC50 (µg/mL)b | SIc |
|---|---|---|---|
| MPE | 10.41 ± 0.82 | 227.34 ± 9.37 | 21.83* |
| rRbavirin | 2.73 ± 0.15 | 58.68 ± 3.98 | 21.49 |
aIC50 is the concentration of the sample required to inhibit virus-induced CPE 50%
bCC50 is the concentration of the 50% cytotoxic effect
cSI = CC50/IC50
Significance is shown as * p < 0.05, compared with ribavirin
Effect of MPE on cell viability
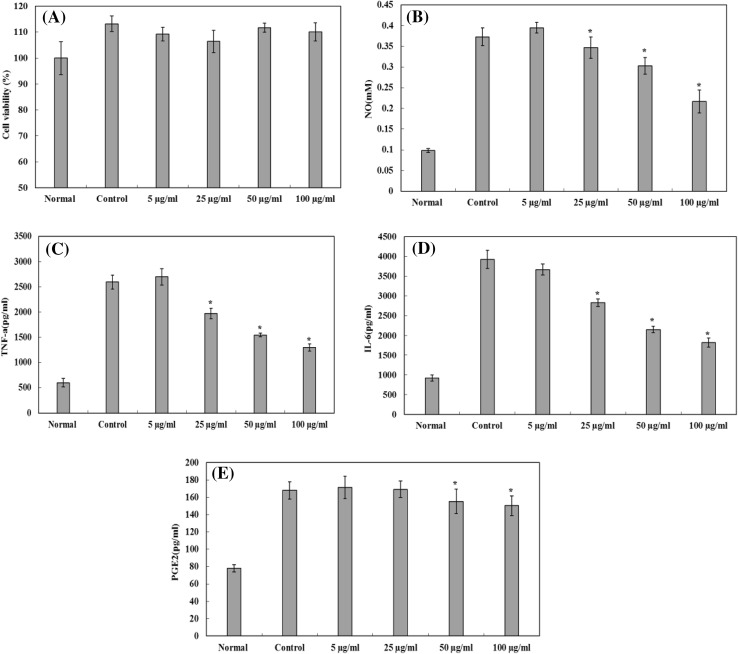
The MTT method was used to determine the effect of MPE on the viability of RAW 264.7 cells. As shown in Fig. 1A, no significant difference was observed in cell proliferation between the different groups and no cytotoxicity against RAW 264.7 cells was observed, suggesting that MPE had no adverse effects on RAW 264.7 cells.
Fig. 1.
The effect of MPE on NO, TNF-α, IL-6, and PGE2 production by LPS-stimulated RAW264.7 cells. (A) The effect of MPE on the viability of RAW 264.7 cells. (B) The inhibitory effect of MPE on NO production. (C) The inhibitory effect of MPE on TNF-α production. (D) The inhibitory effect of MPE on IL-6 production. (E) The inhibitory effect of MPE on PGE2 production. Results are expressed as mean ± SD (n = 3), and statistical differences were analyzed by Dunnett’s test (*p < 0.05)
NO is a pro-inflammatory mediator produced from arginine and synthesized by nitric oxide synthase (iNOS). NO exerts multiple modulatory effects on inflammations and plays a key role in the regulation of immune responses [21]. As shown in Fig. 1B, MPE could significantly decrease the LPS-induced NO production in a concentration-dependent manner by 7.06, 18.85, and 41.88% at 25, 50, and 100 µg/mL, respectively.
TNF-α, IL-6, and PGE2 are well known as pro-inflammatory cytokines that play important roles in the pathogenesis of diverse inflammatory and infectious disorders [22]. As shown in Fig. 1C, D, E, the treatment of cells with LPS alone substantially increased the secretion of these cytokines. As seen in Fig. 1C, D, MPE suppressed TNF-α secretion by 20.71, 34.74, and 42.95% at concentrations of 25, 50, and 100 µg/mL, respectively. In addition, MPE reduced IL-6 levels by 27.00, 43.71, and 51.85% in LPS-stimulated RAW 264.7 cells. We also found that MPE could inhibit PGE2 production compared with untreated control LPS-stimulated RAW 264.7 cells (Fig. 1E). These results indicate that after LPS stimulation with MPE intervention, TNF-α, IL-6, and PGE2 secretion was significantly decreased compared with that in cells stimulated with LPS.
Antioxidant activity assay
DPPH radical scavenging activity
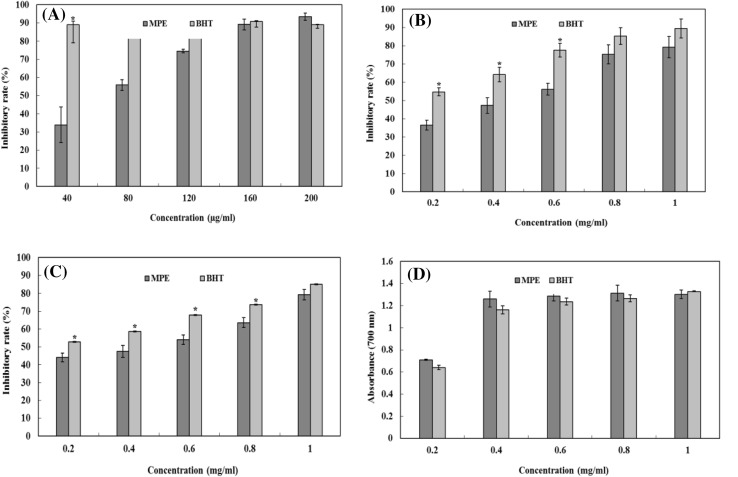
DPPH is a stable organic free radical with an absorption maximum at 517 nm. When DPPH radicals encounter a proton radical scavenger, its purple color rapidly fades. Therefore, the DPPH assay is useful for evaluating radical of scavenging activity. As shown in Fig. 2A, MPE could dose-dependently scavenge DPPH radicals. The maximum scavenging rate (93.45%) was found at a concentration of 200 µg/mL. In addition, MPE showed higher scavenging activity than BHT at 200 µg/mL. These results suggested that MPE might act as an electron or hydrogen donor to scavenge DPPH radicals.
Fig. 2.
Antioxidant activity of MPE. (A) Scavenging of DPPH radicals. (B) Scavenging of hydroxyl radicals. (C) Scavenging of superoxide radicals. (D) Reducing power. Each value is expressed as mean ± SD (n = 3), and significance is shown as *p < 0.05 compared with that of BHT
Hydroxyl radical scavenging activity
Hydroxyl radicals can easily cross cell membranes at specific sites where they can react with a range of biomolecules, causing tissue damage and cell death. Thus, removing hydroxyl radicals is important for antioxidant defense in cells [23]. As shown in Fig. 2B, MPE showed excellent radical scavenging performance in a concentration-dependent manner. The scavenging rate reached 79.27% at a concentration of 1.0 mg/mL, whereas that for BHT was 89.38%. Although the scavenging activity of MPE was lower than that of BHT, its scavenging activity at 1 mg/mL was nearly equal to that of 0.6 mg/mL of BHT, indicating that MPE is a good scavenger for hydroxyl radicals.
Superoxide anion radical scavenging activity
Superoxide anion radicals are very harmful radicals that result in the formation of other reactive oxygen species such as hydrogen peroxide or singlet oxygen in living systems [23]. As can be seen from Fig. 2C, the scavenging activity of MPE is dependent on its concentration and BHT had higher superoxide anion scavenging ability. The EC50 of MPE and BHT was 1.52 and 0.89 mg/mL, respectively. Although the activity of MPE was weaker than that of BHT, MPE was still able to scavenge superoxide radicals well at higher concentrations.
Reducing power
Reducing power is recognized as an indicator of potential antioxidant activity. A higher absorbance value denotes a stronger reducing power. As seen in Fig. 2D, the reducing power of MPE initially increased as the concentration increased and then became stable when the concentration increased beyond 0.4 mg/mL, indicating that MPE may function as a good electron and hydrogen donor.
Immunization against RSV is currently unavailable, and the few therapies that exist for the treatment of RSV infections, such as palivizumab and ribavirin, are only moderately effective or limited in efficacy [3]. Thus, there is a need to develop effective antiviral agents for the treatment of RSV infections. Recent studies have demonstrated that herbal medicines and purified natural products are rich resources for antiviral drug development. Previous studies showed that some phenolic compounds and flavonoids have potent anti-RSV activities; for instance uncinoside A and B, the two chromone glycosides isolated from Selaginella uncinata, could potently inhibit RSV infections [24]. Three bioflavonoids extracted from Radix wikstroemiae were found to have antiviral activities against RSV [25]. In addition, some tannins such as chebulagic acid and punicalagin also showed antiviral effects against RSV infections, could inactivate RSV particles, and could block viral entry-related events including binding and fusion [26]. Our chemical analysis showed that MPE had relatively high phenolic compound and flavonoid content. These compounds may be related to the antiviral activity of M. piperita.
Although inflammation is a complex protective response, prolonged inflammation results in the pathogenesis of various inflammatory diseases. Macrophages play a key role in the regulation of inflammation and the immune response by releasing inflammatory mediators such as pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6), NO, and PGE2, which recruit additional immune cells to the site of infection or tissue injury [15, 27]. Previous reports have shown that exposure of macrophages to a virus resulted in the release of various pro-inflammatory cytokines [28]. Several studies have speculated that symptoms associated with some respiratory complications are the result of increased levels of pro-inflammatory cytokines rather than direct effects of virus replication [29, 30]. This view is further substantiated by the fact that the ethanol extract of Echinacea purpurea can alleviate “cold and flu,” and possibly other respiratory disorders by inhibiting viral growth and the secretion of pro-inflammatory cytokines [31]. Phytochemical investigation showed that eriocitrin, rosmarinic acid, luteolin 7-O-rutinoside, and hesperidin were major representative constituents of M. piperita, and these compounds may play an important role in the anti-inflammatory and antiviral effects. Therefore, a phytochemical investigation is necessary to be performed in the further study.
This study provides the first evidence that M. piperita L. extract has antiviral activity against RSV in Hep-2 cells. Previous studies have shown that M. piperita L. has antiviral activity against influenza A, herpes simplex virus, vaccinia virus, and human immunodeficiency virus-1 (HIV-1) [7]. Meanwhile, MPE was found to suppress NO, TNF-α, IL-6, and PGE2 production in macrophages, suggesting a linked pathway in viral infections. RSV infections are known to induce TNF-α secretion, which can exacerbate illness and cause significant weight loss [32]. Therefore, M. piperita L. could enhance innate immunity, which is beneficial to counteract RSV infection in addition to directly interfering with viral entry without exacerbating the illness during the management of RSV infection. In addition, according to a previous study, the LD50 of peppermint extract is 24.36 g kg−1 d−1, which is equivalent to 284.2 times the daily dosage of a 70 kg body weight adult, suggesting that peppermint is considerably safe for the treatment of viral infections [33].
In many inflammatory disorders, there is excessive phagocyte activation and production of hydroxyl, superoxide anion, and non-free-radical species (H2O2) that are harmful to tissues. Release of these free radicals initiates lipid peroxidation, resulting in membrane destruction and subsequently provokes an inflammatory response by producing mediators and chemostatic factors. The antioxidant activity of MPE has the capacity to reduce cellular damage that causes lipid peroxidation and inflammation and may have a positive effect in preventing the progression of many human diseases caused by free radicals. Determination of the phenolic compound and flavonoid content showed that the phenolic compounds represented the major chemical constituents of M. piperita L. A positive correlation was seen between antioxidant activity and the polyphenolic content of MPE, which may be the main contributor to antioxidant activity of this plant. Polyphenols may also contribute to the anti-inflammatory and antiviral activity of M. piperita L. The biological activities of these compounds in the treatment and management of oxidants and inflammatory disorders as well as viral infections are not achieved by a single group of compounds. Some of these molecules might act synergistically to exert the observed bioactivities.
The results obtained in this study support the traditional use of peppermint resources. The ethanol extract from the leaves of M. piperita L. was effective against RSV and could suppress the production of TNF-α, IL-6, NO, and PGE2. The anti-inflammatory activity could be beneficial in fighting RSV infections. Furthermore, the ethanol extract of M. piperita L. leaves with a high amount of phenolic compounds exhibited notable antioxidant activity. These bioactivities may be attributed to the synergistic action of the constituents in M. piperita L. The results obtained in this study may serve as a valuable research reference for clinical research on the treatment of inflammation and RSV infections.
Acknowledgements
The research is supported by the scientific research program of Jilin Provincial Education Bureau (No. [2015]382), Youth Funds of Jilin Agricultural Science and Technology College (2017116), Key Discipline Project for Traditional Chinese medicine specialty of Jilin Province, National Nature Science Foundation of China (81403264), Shanghai Science and Technology Development Funds (14YF1411500), and Shanghai University of TCM funds, and was partly supported by Shanghai Fengxian District Science and Technology Project (20151205 and 20141001) and Shanghai Municipal Health and Family Planning Commission Project (201540027 and 20174Y0236).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Odeyemi S, Afolayan A, Bradely, G. In vitro anti-inflammatory and free radical scavenging activities of crude saponins extracted from Albuca bracteata jacq. Bulb. Afr. J. Tradit. Complement Altern. Med. 12: 34–40 (2015).
- 3.Xu JJ, Liu Z, Tang W, Wang W, Wang GC, Chuang HY, Liu QY, Zhuang L, Li MM, Li YL. Tangeretin from citrus reticulate inhibits respiratory syncytial virus replication and associated inflammation in vivo. J Agr. Food Chem. 2015;63:9520–9527. doi: 10.1021/acs.jafc.5b03482. [DOI] [PubMed] [Google Scholar]
- 4.Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Curr. Opin. Virol. 2013;3:468–474. doi: 10.1016/j.coviro.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur. Respir. J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- 6.Shah PP, Mello PMO. A review of medicinal uses and pharmacological effects of Mentha piperita. Nat. Prod. Res. 2004;3:214–221. [Google Scholar]
- 7.McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) Phytother. Res. 2006;20:619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Sun ZL, Jia AR, Shi YP, Li RH, Yang PM. Extraction, Preliminary characterization and evaluation of in vitro antitumor and antioxidant activities of polysaccharides from Mentha piperita. Int. J. Mol. Sci. 2014;15:16302–16319. doi: 10.3390/ijms150916302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Z, Wang H, Wang J, Zhou L, Yang P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLOS ONE 9(12): e114767. doi:10.1371/journal.pone.0114767. [DOI] [PMC free article] [PubMed]
- 10.Inoue T, Sugimoto Y, Masuda H, Kamei C. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol. Pharm. Bull. 2002;25:256–259. doi: 10.1248/bpb.25.256. [DOI] [PubMed] [Google Scholar]
- 11.Bursal E, Köksal E, Gülçin İ, Bilsel G, Gören AC. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res. Int. 2013;51:66–74. doi: 10.1016/j.foodres.2012.11.022. [DOI] [Google Scholar]
- 12.Yang X, Yu W, Ou Z, Ma H, Liu W, Ji X. Antioxidant and immunity activity of water extract and crude polysaccharide from Ficus carica L. fruit. Plant Food Hum. Nutri. 64: 167–173 (2013). [DOI] [PubMed]
- 13.Lin TJ, Wang KC, Lin CC, Chiang LC, Chang JS. Anti-viral activity of water extract of Paeonia lactiflora pallas against human respiratory syncytial virus in human respiratory tract cell lines. Am. J. Chinese Med. 2013;41:585–599. doi: 10.1142/S0192415X13500419. [DOI] [PubMed] [Google Scholar]
- 14.Ma SC, Du J, But PPH, Deng XL, Zhang YW, Ooi VEC, Xu HX, Lee SHS, Lee SF. Antiviral Chinese medicinal herbs against respiratory syncytial virus. J. Ethnopharmacol. 2002;79:205–221. doi: 10.1016/S0378-8741(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim MJ, Kim KBWR, Jeong DH, Ahn DH. Anti-inflammatory activity of ethanolic extract of Sargassum sagamianum in RAW 264.7 cells. Food Sci. Biotechnol. 2013;22:1113–1120. doi: 10.1007/s10068-013-0191-9. [DOI] [Google Scholar]
- 16.Liu X, Sun Z, Zhang M, Meng X, Xia X, Yuan W, Feng X, Liu C. Antioxidant and antihyperlipidemic activities of polysaccharides from sea cucumber Apostichopus japonicus. Carbohyd. Polym. 2012;90:1664–1670. doi: 10.1016/j.carbpol.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Zhang M, Guo K, Jia A, Shi Y, Gao G, Sun Z, Liu C. Cellulase-assisted extraction, characterization, and bioactivity of polysaccharides from Polygonatum odoratum. Int. J. Biol. Macromol. 2015;75:258–268. doi: 10.1016/j.ijbiomac.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 19.Lyu SY, Park WB. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch. Pharm. Res. 2005;28:573–581. doi: 10.1007/BF02977761. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YB, Wu P, Zhang XL, Xia C, Li G, Ye WC, Wang GC, Li YY. Phenolic compounds from the flowers of Bombax malabaricum and their antioxidant and antiviral activities. Molecules. 2005;20:19947–19957. doi: 10.3390/molecules201119660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moffat FLJ, Han T, Li ZM, Peck MD, Jy NW, Ahn YS, Chu AJ, Bourguignon LY. Supplemental l-arginine HCl augments bacterial phagocytosis in human polymorphonuclear leukocytes. J. Cell Physiol. 1996;168:26–33. doi: 10.1002/(SICI)1097-4652(199607)168:1<26::AID-JCP4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Eigler A, Greten TF, Sinha B, Haslberger C, Sullivan GW, Endres S. Endogenous adenosine curtails lipopolysccharide-stimulated tumour necrosis factor systhesis. Scand. J. Immunol. 1997;45:132–139. doi: 10.1046/j.1365-3083.1997.d01-377.x. [DOI] [PubMed] [Google Scholar]
- 23.Balavigneswarana CK, Sujin Jeba Kumara T, Moses Packiaraja R, Veeraraja A, Prakasha S. Anti-oxidant activity of polysaccharides extracted from Isocrysis galbana using RSM optimized conditions. Int. J. Biol. Macromol. 2013;60:100–108. doi: 10.1016/j.ijbiomac.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Ma LY, Ma SC, Wei F, Lin RC, But PP, Lee SH, Lee SF. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003;51:1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Zhang X, Wang Y, Ye W, Ooi VE, Chung HY, Li Y. Antiviral biflavonoids from Radix Wikstroemiae (Liaogewanggen) Chin. Med. 2010;5:23. doi: 10.1186/1749-8546-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang KC, Chang JS, Lin LT, Chiang LC, Lin CC. Antiviral effect of cimicifugin from Cimicifuga foetida against human respiratory syncytial virus. Am. J. Chin. Med. 2012;40:1033–1045. doi: 10.1142/S0192415X12500760. [DOI] [PubMed] [Google Scholar]
- 27.Jo WS, Choi YJ, Kim HJ, Nam BH, Lee GA, Seo SY, Lee SW, Jeong MH. Methanolic extract of Asterina pectinifera inhibits LPS-induced inflammatory mediators in murine macrophage. Toxicol. Res. 2010;26:37. doi: 10.5487/TR.2010.26.1.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J. Exp. Med. 1996;184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaller M, Hogaboam CM, Lukacs N, Kunkel SL. Respiratory viral infections drive chemokine expression and exacerbate the asthmatic response. J. Allergy Clin. Immunol. 2006;118:295–302. doi: 10.1016/j.jaci.2006.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szretter KJ, Gangapper S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma M, Anderson SA, Schoop R, Hudson JB. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009;83:165–170. doi: 10.1016/j.antiviral.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Choi J, Callaway Z, Kim HB, Fujisawa T, Kim CK. The role of TNF-α in eosinophilic inflammation associated with RSV bronchiolitis. Pediat. Allerg. Imm. 2010;21:474–479. doi: 10.1111/j.1399-3038.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Sun R. Experimental comparison study in mice’s acute toxicity of different composition in Herba Menthae. Chin. J. Pharmconvigilance. 2012;9:65–68. [Google Scholar]