Abstract
This study investigated the effects of chronic administration of red ginseng extract (RGE) and black ginseng extract (BGE) on memory impairment in aged (18-month-old) mice. RGE and BGE (200 mg/kg) were orally administered for 16 weeks. Aging induced DNA damage; however, RGE and BGE protected DNA from damage and allowed for DNA recovery in blood lymphocytes. Choline acetyltransferase, vesicular acetylcholine transporter, growth-associated protein 43, synaptosomal-associated protein 25, nerve growth factor, and brain-derived neurotrophic factor protein expression were significantly increased after treatment with RGE and BGE. These data suggest that chronic administration of red ginseng and black ginseng may decrease the cognitive deficits associated with normal aging.
Keywords: Aging, Antioxidant, Brain, Ginseng, Memory
Introduction
Neurodegenerative diseases associated with aging are characterized by severe damage of the cholinergic system, which leads to cognitive and memory impairments. Moreover, cognitive decline with aging is associated with the deterioration of neuronal transmission in the hippocampus due to dysregulation of genes that are responsible for synaptic protein synthesis, such as growth-associated protein (GAP-43) and synaptosomal-associated protein (SNAP-25) [2, 3]. Neurotrophic factors, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF) play a crucial role in growth, survival, and differentiation of neurons, and are strongly influenced in the aging process [4]. Memory loss with aging is considered to occur early on as a result of changes in synaptic function. However, the relationship between synaptic plasticity-related proteins and age-related memory deficits remains largely unknown. It is therefore necessary to investigate the candidate substances that prevent or delay aged-related memory deficits and to identify the mechanisms for enhancing healthy aging [5].
Ginseng is a traditional herbal medicine popularly known as a tonic, and has been used widely to treat or prevent many diseases. Red ginseng is made by steaming raw ginseng. Black ginseng is produced by repeated steaming, has a transformed specific ginsenoside profile, and has more potent biological and pharmacological activities than red ginseng. In brief, the contents of ginsenosides Rg1, Re, Rf, Rb1, Rc, and Rd are decreased in black ginseng during the steaming process, whereas the contents of ginsenosides Rg3, Rh1, Rh2, Rh4, Rg5, Rg6, and F4 are increased as result of heat transformation during the steaming process [6, 7].
Red ginseng is believed by some to be effective for treating age-related neurodegenerative diseases; however, most studies have focused primarily on the protective effects of a major ginsenoside [8, 9]. While black ginseng has recently been reported to be effective against many diseases [10, 11], studies on its protective effects on memory loss with natural aging have not been conducted. Therefore, this study investigated the effects of red ginseng extract (RGE) and black ginseng extract (BGE) against cognitive decline and oxidative stress in aged mice.
Materials and methods
Sample preparation
Red and black ginseng were obtained from Daeduck Bio Corp. (Daejeon, Korea). In brief, to prepare the ginseng extracts, red and black ginseng were pulverized and extracted three times with 80% ethanol by ultrasonication for 1 h. The obtained extract was filtered, concentrated in vacuo, and lyophilized.
Animals and treatment
12-week-old male ICR mice were obtained from Daehan BioLink (Eumseong, Korea). Mice had ad libitum access to food and water, and were housed under a controlled temperature (22 ± 2 °C), humidity (55 ± 10%), and a 12-h light/dark cycle (lights on at 08:00). Mice were housed in the laboratory until the age of 18 months. RGE or BGE (200 mg/kg, respectively) were administrated orally for 16 weeks. All the experimental procedures were approved by the Institutional Animal Care and Use Committee of Chungnam National University (CNU-00245).
Blood and brain tissue preparation
Whole blood was obtained from a cardiac puncture. Whole brains were carefully removed from the skull, and the cerebral cortex and hippocampus were dissected. The cerebral cortex of right brains were homogenized and centrifuged to obtain the supernatant, which was used for enzyme analysis.
Assay of cholinergic activity
Acetylcholinesterase (AChE) activity was measured by the modified method of Ellman [12]. Choline acetyltransferase (ChAT) activity was analyzed using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Comet assay
The comet assay was determined using a method previously described [13], with minor modifications. Whole blood was resuspended in 0.75% low-melting point agarose in phosphate buffer saline. This suspension was poured onto a 1% agarose-precoated slide and then covered. The slides were lysed in lysis buffer for 1 h at 4 °C and then incubated to unwind in an electrophoretic alkaline buffer for 20 min at 4 °C. Electrophoresis of slides was carried out at 300 mA and 25 V, and then rinsed in Tris buffer. Images from 50 cells from each slide were analyzed using a fluorescence microscope (Leica, Germany). Tail ratio, tail moment, and tail length were determined as a measure of DNA damage using Komet 4.0 image analysis software (Kinetic Imaging, Liverpool, UK).
Western blot analysis
Proteins in the hippocampus were extracted using a lysis buffer (PRO-PREP™, iNtRON Biotechnology, Seongnam, Korea) and centrifuged. After protein quantification, samples were separated on 10–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane membrane (0.45 μm, Millipore, MA, USA). After blocking in a 5% nonfat dry milk for 1 h, the membrane was incubated with the primary antibody overnight at 4 °C. The membrane was then incubated with horseradish peroxidase-linked secondary antibodies for 1 h. Blots were detected using the enhanced chemiluminescent substrate detection kit (WEST-ONE™, iNtRON Biotechnology).
Statistical analysis
Data were analyzed using the SPSS software package, version 20 (IBM Corporation, Armonk, NY, USA). Differences between groups were analyzed using a one-way ANOVA followed by the Duncan’s multiple range test, and results were considered statistically significant at p < 0.05.
Results and discussion
Cholinergic activity
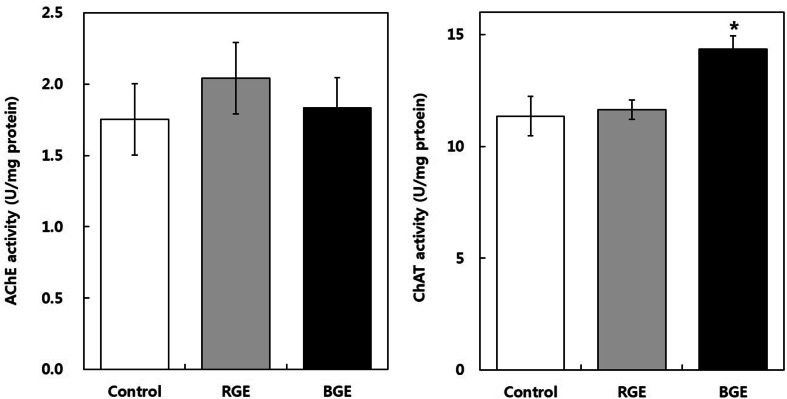
The cortical AChE and ChAT activities are shown in Fig. 1. In the control group, AChE and ChAT activities were 1.76 ± 0.46 U/mg protein and 11.34 ± 0.88 U/mg protein, respectively. In the RGE-treated group, AChE and ChAT activities were not significantly different to the control group. However, ChAT activity in BGE-treated group (14.00 ± 0.46 U/mg protein) was significantly higher than that in the control group (11.34 ± 0.88 U/mg protein) (p < 0.05). Similarly, levels of AChE and ChAT have been found to be decreased in patients with severe Alzheimer’s disease (AD), and an age-related decline in ChAT activity was also found in a rodent experiment [14, 15]. Moreover, ginsenoside Rg5 has been found to significantly increased ChAT activity in the hippocampus and cortex of streptozotocin-induced memory deficit rats [16]. Therefore, long-term intake of BGE might have a beneficial effect on cognitive function in aged mice by regulation of the cholinergic system.
Fig. 1.
Effect of administration of red and black ginseng extract (RGE, BGE, 200 mg/kg, p.o., 16 weeks) on acetylcholinesterase (A) and choline acetyltransferase (B) activities in the cerebral cortex of aged mice. Values are expressed as mean ± SD. (n = 5)
Comet assay in peripheral lymphocytes
The results of the comet assay for DNA damage in peripheral lymphocytes of aged mice are shown in Table 1. The values for DNA damage in the ginseng-treated groups were significantly lower than those in the control group (p < 0.05). Previous work has reported that various ginsenosides, such as Rb1, Rg2, Rg1, Rc, Re, Rh1, Rg3, and Rd, have a radical scavenging activity and antioxidant defense system [17]. In addition, heat-treated ginseng has stronger O2 −, ONOO−, and ·OH-scavenging activities than white ginseng [18]. These data suggest that aging induces DNA damage, but that RGE and BGE may protect DNA from this damage or be used as a strategic herb for degenerative diseases caused by aging.
Table 1.
Effect of RGE and BGE on comet parameters of the lymphocytes of peripheral blood in aged mice
| Group | Tail ratio (% DNA) | Olive tail moment | Tail Length (μm) |
|---|---|---|---|
| Control | 10.85 ± 1.07a | 5.48 ± 1.27a | 16.56 ± 2.52a |
| RGE | 7.02 ± 0.73b | 2.71 ± 0.30b | 11.35 ± 1.54b |
| BGE | 6.01 ± 0.91b | 2.76 ± 0.85b | 9.70 ± 1.20b |
RGE: red ginseng extract, BGE: black ginseng extract, 200 mg/kg, p.o., respectively
aValues are expressed as means ± SD (n = 5)
bValues with different superscripts within the same row are significantly different at p < 0.05
Western blot analysis in the hippocampus
The neuropathological feature of AD is the degeneration of the cholinergic system; enhancement of cholinergic function may have beneficial effects in patients with mild to moderate AD [19]. The hippocampal ChAT and VAChT protein expression in aged mice was significantly enhanced after treatment with RGE (92 and 74%, respectively), and BGE (102 and 62%, respectively) compared to the aged control mice [Fig. 2(A)]. Therefore, increased expression of major cholinergic markers, ChAT and VAChT, which are involved in the synthesis and translocation of acetylcholine (ACh), might be a potential therapy for alleviating the process and symptoms of AD.
Fig. 2.
Western blot analysis in the hippocampus of aged mice-administrated ginseng extracts for 16 weeks. Lane 1, Control; Lane 2, RGE (red ginseng extract, 200 mg/kg, p.o.); Lane 3, BGE (black ginseng extract, 200 mg/kg, p.o.). β-Actin was used as an internal control for protein expression. Results are expressed as mean ± SD. (n = 5). **p < 0.05, ***p < 0.001 compared with control group
In the previous rodent studies, the expression of target proteins such as VAChT, SNAP-25, and BDNF in aged control group (20–24 months) was significantly reduced compared to young control group (4–6 month) [20, 21]. Loss of synaptic plasticity can result in cognitive impairment and contribute to the pathogenesis of neurodegenerative disease [22]. The hippocampal GAP-43 and SNAP-25 protein levels were significantly greater in the RGE (77, 145%) and BGE group (76, 153%), respectively, compared to the control group [Fig. 2(B)] (p < 0.001). The results of this study therefore suggest that red and black ginseng may be a useful natural herbal medicine for age-related memory loss via an enhancement of hippocampal neuroplasticity.
As shown in Fig. 2(C), the levels of NGF and BDNF in the hippocampus were significantly greater by treatment with RGE (44, 28%) and BGE (59, 33%), respectively, compared to the control group (p < 0.01). Ginsenosides Rb1 and Rg1 have been reported to facilitate ACh release and increased ACh receptors, ChAT, NGF mRNA, and hippocampal synaptic density [23]. Recently, treatment with ginsenosides Rg5 and Rh3 isolated from heat-processed ginseng was found to result in memory improvements in mice via inhibition of AChE and by increasing BDNF expression [24]. Based on these results, RGE and BGE might be a useful candidate for the prevention of age-related memory impairments.
Compliance with ethical standards
Conflict of interests
The authors declare no conflict of interests.
References
- 1.Harman D. The free radical theory of aging. Antioxid. Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- 2.Gureviciene I, Gurevicius K, Tanila H. Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J. Neural. Transm. (Vienna) 2009;116:13–22. doi: 10.1007/s00702-008-0149-x. [DOI] [PubMed] [Google Scholar]
- 3.Sabel M, Bele S, Gass P, Sommer C, Kiessling M. Developmental expression of GAP-43 and SNAP-25 in heterotopic rat cortical grafts. Neurosci. Lett. 1995;189:151–154. doi: 10.1016/0304-3940(95)11478-F. [DOI] [PubMed] [Google Scholar]
- 4.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukitt-Hale B, Lau FC, Joseph JA. Berry fruit supplementation and the aging brain. J. Agric. Food Chem. 2008;56:636–641. doi: 10.1021/jf072505f. [DOI] [PubMed] [Google Scholar]
- 6.Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z, Lee MR, Li Z, Li JJ, Sung CK. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J. Pharm. Biomed. Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 8.Rausch WD, Liu S, Gille G, Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol. Exp. 2006;66:369–375. doi: 10.55782/ane-2006-1625. [DOI] [PubMed] [Google Scholar]
- 9.Lee Mi-Ra, Yun Beom-Sik, Liu Lei, Zhang Dong-Liang, Wang Zhen, Wang Chun-Ling, Li-Juan Gu, Wang Chun-Yan, Mo Eun-Kyung, Sung Chang-Keun. Effect of black ginseng on memory improvement in the amnesic mice induced by scopolamine. J. Ginseng Res. 2010;34:51–58. doi: 10.5142/JGR.2010.34.1.051. [DOI] [Google Scholar]
- 10.Kim JH, Pan JH, Cho HT, Kim YJ. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLos One. 2016;11:e0146843. doi: 10.1371/journal.pone.0146843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CH, Kim JM, Kim DH, Park SJ, Liu X, Cai M, Hong JG, Park JH, Ryu JH. Effects of Sun ginseng on memory enhancement and hippocampus neurogenesis. Phytother. Res. 2013;27:1293–1299. doi: 10.1002/ptr.4873. [DOI] [PubMed] [Google Scholar]
- 12.Ellman GL, Courtney KD. Andres VJr., Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 13.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Giacobini E. Cholinergic function and Alzheimer’s disease. Int. J. Ger. Psychiatry. 2003;18:S1–S5. doi: 10.1002/gps.935. [DOI] [PubMed] [Google Scholar]
- 15.Yegla B, Parikh V. Effects of sustained proNGF blockade on attentional capacities in aged rats with compromised cholinergic system. Neuroscience. 2014;261:118–132. doi: 10.1016/j.neuroscience.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu S, Gu J, Feng L, Liu J, Zhang M, Jia X, Liu M, Yao D. Ginsenoside Rg5 improves cognitive dysfunction and beta-amyloid deposition in STZ-induced memory impaired rats via attenuating neuroinflammatory responses. Int. Immunopharmacol. 2014;19:317–326. doi: 10.1016/j.intimp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J. Agric. Food Chem. 2003;51:2555–2558. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- 18.Kang KS, Kim HY, Pyo JS, Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat-processing. Biol. Pharm. Bull. 2006;29:750–754. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 19.Krall WJ, Sramek JJ, Cutler NR. Cholinesterase inhibitors: a therapeutic strategy for Alzheimer disease. Ann. Pharmacother. 1999;33:441–450. doi: 10.1345/aph.18211. [DOI] [PubMed] [Google Scholar]
- 20.Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging. 2009;30:1877–1884. doi: 10.1016/j.neurobiolaging.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Li Q, Pei X, Zhang Z, Yang R, Wang J, Li Y. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6 J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav. Brain Res. 2009;201:311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol. Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim EJ, Jung IH, Van Le TK, Jeong JJ, Kim NJ, Kim DH. Ginsenosides Rg5 and Rh3 protect scopolamine-induced memory deficits in mice. J. Ethnopharmacol. 2013;146:294–299. doi: 10.1016/j.jep.2012.12.047. [DOI] [PubMed] [Google Scholar]