Abstract
Changes in contents of sugars, organic acids, limonoids, phenolics contents, and antioxidant capacities of lemon slices dried at different temperatures were evaluated. Air drying (AD) promoted losses of sugars, citric acid, ascorbic acid, extractable phenolics (EPs), and non-extractable phenolics (NEPs), while it introduced an increase in limonoids. Phenolics of lemon were mainly presented in their extractable form. Hesperidin and eriocitrin were the main EPs; protocatechuic acid and poncirin were the predominant NEPs. The decrease in extractable phenolic acid, EP, and NEP content in lemon is lower at low drying temperatures, while the increase in non-extractable phenolic acid content is higher at high drying temperatures. The antioxidant capacity of EP was higher than that of NEP. Phenolics contributed to antioxidant capacities of lemon slices, and flavonoids were the main contributors among phenolics. Considering limonoids contents and the high levels of EP, NEP, and antioxidant capacities, AD at 60 °C could be an appreciate treatment for dehydrating lemon slices.
Keywords: Lemon slices, Drying, Phenolic, Limonoids, Antioxidant capacity
Introduction
Lemon (Citrus limon L. Burm. f) is acid citrus distributed in semi-arid and coastal areas with excellent quality. The annual citrus fruit world production is over 100 million metric tonnes, and lemon and lime fruit is one of the main citrus fruits, with a world annual production of over 6.2 million tonnes [1]. In 2014, the annual production of lemon and lime fruits in China reached 2.16 million tonnes, accounting for 34.9% of the world annual production of lemon and lime fruits [2].
Phytochemical analysis studies showed that lemon fruit is a rich source of nutrients and bioactive compounds, such as citric acid, ascorbic acid, limonoids, and phenolics, which exhibit multiple biological activities, thus promoting numerous health benefits [3, 4]. The chemical compositions of lemon fruits are different owing to different genotypes and harvest times [5, 6], geographical locations [7], extraction methods [8], cultivation methods [9], and processing technologies [10, 11]. Lemon fruits are typically consumed in their fresh form, as dried slices or as powder, for flavoring tea, and as a condiment in various commercial foods, beverages, and the fragrance industry. Drying reduces the moisture content and hence inhibits microbial growth and forestalls certain biochemical changes. However, it leads to loss of bioactive compounds and may pose an adverse effect on the nutrients, physical properties, and antioxidant activity of agricultural products. In previous studies, drying characteristics, mathematical models, kinetics, mass transfer, and some quality attributes of lemon slices were investigated [12–16]. Furthermore, the kinetics of quality attributes and the physico-chemical and technological properties of lemon peels in air and under microwave drying were investigated [17, 18]. García-Salas et al. [19] characterized the stability of phenolic compounds of whole-lemon and their stability under different storage conditions. Previous studies also focused on the effect of heat on phenolics and limonoids contents and the antioxidant capacities of citrus peels or pomace [20, 21]; nevertheless, to the best of our knowledge, no studies have been conducted to determine the effect of drying temperature on sugars, organic acids, phenolics, limonoids, and antioxidant activity of lemon slices. Thus, the objective of the present study was to evaluate the impact of different drying temperatures on the sugars, organic acids, phenolics, limonoids contents, and antioxidant activity of lemon slices.
Materials and methods
Raw materials
Lemon fruits were collected from the citrus resources nursery of the Hunan Horticultural Institution (113°36′E, 28°49′N) at an optimum stage of ripening and those showing defects were discarded. The selected lemon fruits were washed thoroughly to remove dust and other foreign materials and stored in a refrigerator at 4 °C before drying.
Drying process
The selected lemon fruits were washed, cut horizontally into 0.5-cm-thick slices using a multiple slicing machine (Beijing Meikeda Science and Technology Development Ltd., Co), and 1 kg of lemon slices was put on a tray to form a 0.5-cm-thick layer and dried at 50, 60, 70, 80, and 90 °C in a tray dryer (DHG-9053A) equipped with controls for temperature and airflow velocity. The dryer air velocity was set at 1.5 m/s. Dehydration was continued until a moisture content of 8% was achieved. The dried samples were stored at 4 °C for 24 h in sealed fresco bags to equilibrium the moisture of the dried samples. Then, the samples were ground and stored in plastic bags at − 20 °C.
Analysis of soluble sugars
The contents of soluble sugars in the samples were analyzed according to the procedure described by Gao et al. [22] with some modifications. In brief, a total of 6 g of milled fresh lemon samples or 1.5 g of dried lemon fruit powder was immersed in 20 mL of distilled water and homogenized for 2 min. The homogenized samples were extracted by ultrasonic-assisted extraction for 30 min at room temperature, and then centrifuged with 8000×g for 10 min. The supernatant was separated, and the residue was re-extracted under the same conditions. The two filtrates were combined to measure 50 mL. The supernatants were filtered through 0.45-μm cellulose filters before high performance liquid chromatography (HPLC) analysis. Samples were analyzed using a Shimadzu HPLC system. HPLC analysis was performed using Shimadzu (Kyoto, Japan) equipment including a LC-20A pump, a refractive index detector (RID), a DUG-20A analytical degasser, and a SIL-20A injector with a 20-μL loop on a YMC-Pack Polyamine II/S column (250 mm × 4.6 mm, 5 μm). The mobile phase used was 80% acetonitrile and deionized water at a flow rate of 1.0 mL min−1 at 35 °C, and the injection volume was 20 μL. The separation was performed in the isocratic elution mode.
Analysis of organic acids
The organic acids were analyzed using the method reported by Uckoo et al. [9] with slight modifications. In brief, 4 g of milled fresh lemon samples or 1 g of lemon slices powder sample was extracted twice with 15 mL of 3% (w/v) meta-phosphoric acid (MPA) at 25 °C for 1 h. After centrifugation at 8000×g for 10 min, the supernatants were combined to measure 50 mL. The HPLC system constituted a Waters HPLC series connected to a PDA detector. A C18 column from Waters was used for the separations. Elution was conducted at 25 °C, and the mobile phase was 3 mM phosphoric acid at a flow rate of 0.5 mL min−1 under isocratic conditions. The detection was set at dual wavelengths of λ223 nm and λ254 nm with a total analysis time of 10 min. Peaks were identified by comparing and matching the ultraviolet spectra as well as the retention time of the individual standards.
Preparation of EP and NEP
The extraction procedure used was adapted from Arranz et al. [23]. Milled fresh lemon samples (6 g) or dried lemon powder (1.5 g) was extracted twice with 20 mL of 80% (v/v) methanol in an ultrasound bath at 25 °C for 30 min. After centrifugation at 5000×g for 10 min, the supernatants were combined to measure 50 mL for measuring EP and antioxidant activity. The lemon residues were reserved for extracting NEP compounds. The lemon residues were hydrolyzed with 20 mL of methanol/H2SO4 (90:10, v/v) for 20 h at 85 °C. Samples were centrifuged at 4 °C with 5000×g for 10 min, and NEP in supernatants were determined using the same as those for EP.
Determination of individual phenolic compounds
Extractable and non-extractable individual phenolics of lemon were quantified by a UPLC (Waters Acquity H-class) equipped with an ultraviolet–visible detector according to method proposed by Li et al. [24]. Separation was performed using an acquity UPLC BEH C18 column (50 mm × 2.1 mm, 1.8 μm) with a column temperature of 30 °C. The mobile phase comprised A: 0.5% formic acid in water and B: acetonitrile with a gradient elution flow rate of 0.3 mL min−1 with 95% A for 0–7 min, 95–88% A for 7–8 min, 88–83% A for 8–12 min, 83–83% A for 12–17 min, 83–70% A for 17–18 min, 70–70% A for 18–26 min, and 70–30% A for 26–42 min. The identification and quantification of the peaks were performed by comparing the retention times obtained herein with those from authentic standards and spiking the suspected compounds with standards. Protocatechuic acid, syringic acid, taxifolin, eriocitrin, narirutin, naringin, hesperidin, and poncirin were detected at 283 nm. Neochlorogenic acid, chlorogenic acid, p-coumaric acid, rhoifolin, rutin, luteolin, apigenin, diosmetin, and nobiletin were detected at 330 nm. The results are expressed in mg/100 g dry weight (DW).
Determination of limonoids
Limonoids were extracted and analyzed as described by Li et al. [25]. For extracting limonin, nomilin, and obacunone, lyophilized or AD powder (1.5 g) was extracted twice with 50 mL dichloromethane in an ultrasonic bath at 25 °C for 30 min. The liquid extracts were collected and centrifuged at 5000×g for 10 min. The supernatants were concentrated to dryness in a rotary vacuum evaporator at 35 °C and acetonitrile was added to 25 mL. Samples were analyzed using a Waters UPLC system equipped with a T3 column (100 mm × 2.1 mm, 1.8 μm). The mobile phase used was 80% acetonitrile and deionized water at a flow rate of 0.5 mL min−1 at 35 °C, and the injection volume was 20 μL. The separation was performed in the isocratic elution mode.
Ferric reducing/antioxidant power assay
The ferric reducing/antioxidant power (FRAP) assay was used as described by Zhang et al. [26]. Extracts (0.5 mL) were allowed to react with 2.0 mL of a working FRAP solution at 37 °C for 10 min in the dark. The results are expressed in μM of Trolox equivalents (TE)/g DW.
Scavenging 1,1-diphenyl-2-picrylhydrazyl radical assay
Scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical capacity was measured as described by Wang et al. [27]. A 4-mL aliquot of a DPPH working solution was added to 0.4 mL of lemon extract diluted with 80% methanol, and the mixture was kept in the dark at 25 °C for 20 min. The reduction ratio of the purple DPPH radical to the yellow form was calculated from the standard curve. The results are expressed as μM of TE/g DW.
Statistical analysis
All results are reported as mean ± standard deviation (SD) values for triplicate experiments. Statistical analysis was performed using OriginPro 8.0 software. Tukey’s multiple range test was used for evaluating significant differences between means at p < 0.05.
Results and discussion
Sugars and organic acids
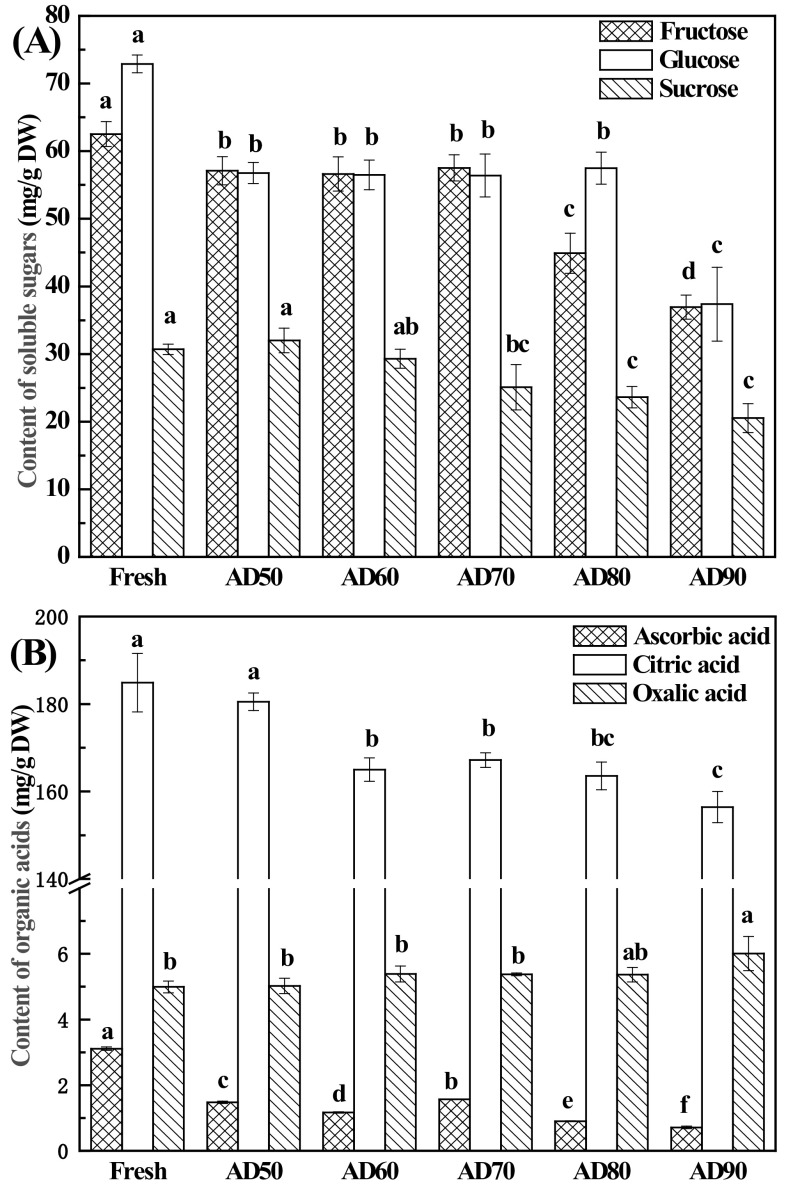
Sugars and organic acids of fruits are important factors for both fresh and processed products. Herein, the impact of drying temperature on the contents of soluble sugars and organic acids of lemon slices were investigated. As showed in Fig. 1(A), fructose, glucose, and sucrose were found in lemon slices, even after drying. Fructose and glucose were the main sugars; this coincides with the result reported by Ting and Attaway [28]. The contents of fructose and glucose in lemon slices decreased significantly after AD treatments, especially at high temperatures (p < 0.05). However, little differences in fructose or glucose content were observed between AD at 50, 60, and 70 °C (p > 0.05). The level of sucrose in lemon slices did not change significantly after dehydration via AD at 50 °C or 60 °C, whereas further elevating drying temperatures could promote losses of sucrose in lemon slices. It is known that reducing sugars are one of the substrates of the Maillard reaction, which could explain the decreasing contents of fructose and glucose, whereas the contents of sucrose did not change during AD at 50 °C or 60 °C. Besides, sucrose can be hydrolyzed to fructose and glucose via acid catalysis under a high temperature condition, and this can explain the loss of sucrose during high-temperature drying treatments. Citric, oxalic, and ascorbic acids were noted in fresh and AD lemon slices [Fig. 1(B)]. Citric acid was found to be the main predominant organic acid in all dried lemon samples, which ranged from 156.4 to 184.9 mg/g DW. However, contents of ascorbic acid (0.7–3.1 mg/g DW) and oxalic acid (5.0–6.0 mg/g DW) were far lower than that of citric acid. The contents of citric acid and ascorbic acid obtained herein were higher than those of lemon slices dehydrated using a heat pump dryer reported by Fu et al. [29], which contained 133.7 mg/g DW of citric acid and 0.95 mg/g DW of ascorbic acid. Compared with fresh samples, AD at 60–90 °C caused a significant decrease in citric acid content, while AD at 50 °C did not introduce significant change of citric acid. AD at 50–90 °C caused a significant decrease in ascorbic acid content compared with that of fresh samples. This demonstrates the significant impact of temperature on ascorbic acid degradation, the major loss being obtained at the highest tested temperature. Similar results were reported by others for AD of Galega kale [30]. However, oxalic acid behaved differently with citric acid and ascorbic acid. The contents of oxalic acid in the samples dried at 50–80 °C exhibited no significant difference from the value in fresh samples (p > 0.05), while AD at 90 °C caused a significant increase in comparison with other dehydration treatments.
Fig. 1.
Effect of drying temperature on the content of soluble sugars and organic acids in lemon slices. Mean values in the same type index for each treatment with different letters are significantly different (p < 0.05)
Limonin, nomilin, and obacunone
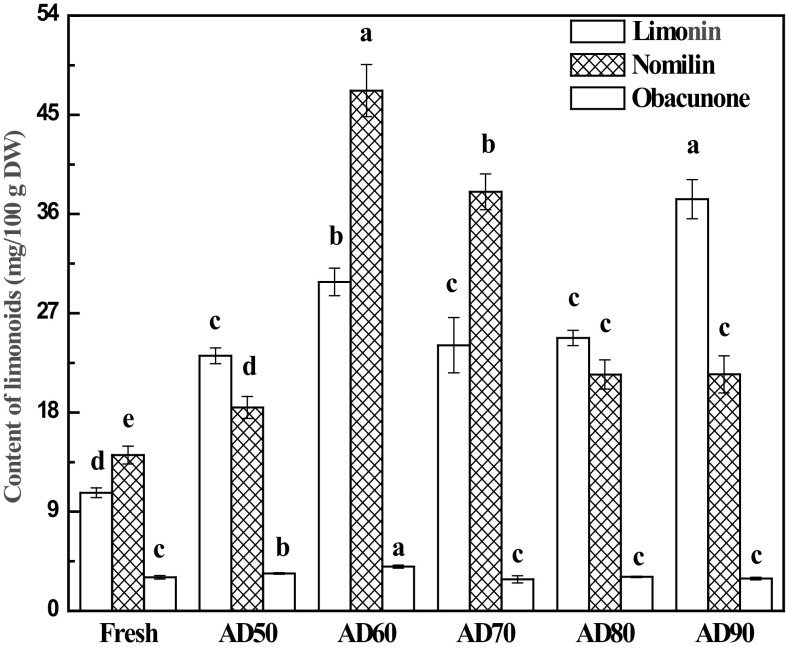
Contents of limonin, nomilin, and obacunone in fresh and AD lemon slices are presented in Fig. 2. The limonin, nomilin, and obacunone contents of fresh lemon slices were 10.7, 14.1 and 3.0 mg/100 g DW, respectively. These observed values for lemon slices were lower than those reported by Hasegawa and Mannners [31] in citrus limon var shiikuwasha seeds (400 mg/100 g DW of limonin, 300 mg/100 g DW of nomilin, and 60 mg/100 g of obacunone) and by Sun et al. [32] in physiologically dropped un-matured citrus (50.4–496.5 mg/100 g DW of limonin and 14.8–22.3 mg/100 g DW of nomilin). Russo et al. [33] found 8.4, 18.7, 6.9, 1.5 mg/100 g DW of obacunone in lemon juice, seeds, peels, and pulps, respectively. Both the contents of limonin and nomilin in lemon slices increased significantly after AD at 50–90 °C compared with fresh samples. For example, the contents of limonin and nomilin of samples undergoing AD at 60 °C increased 3.3 and 2.8 times, respectively, compared with those of fresh samples. Kuljarachanan et al. [20] found that both the limonin and nomilin contents of lime residues increased significantly when fresh lime waste was exposed at 60 °C for 8 h compared with those of fresh one. Increases in limonoids contents due endogenous enzymes in citrus tissues have been reported in previous studies, in which limonin D-ring-lactone hydrolase, which catalyzes the reversible lactonization–hydrolysis of the open and closed A-ring lactone to limonin, has been extracted and purified [34]. Besides, it has been proved that limonin D-ring-lactone hydrolase from citrus fruit is a heat-stable enzyme [35]. Herein, the increase of limonin and nomilin contents might be related to the endogenous enzymes mentioned above, which were not completely inactivated in the initial drying process.
Fig. 2.
Effect of drying temperature on the contents of limonin, nomilin, and obacunone in lemon slices. Mean values in the same type index for each treatment with different letters are significantly different (p < 0.05)
Limonoid and nomilin in lemon slices behaved differently in terms of their response to different drying temperatures, as showed in Fig. 2. Samples undergoing AD at 60 °C exhibited the highest limonoid content, followed by those undergoing AD at 70 °C. Meanwhile, samples undergoing AD at 50 °C exhibited the lowest limonoid content among the samples undergoing AD at different temperatures, while samples undergoing AD at 50 and 60 °C exhibited lower nomilin content than samples undergoing AD at 90 °C. This observation agrees with a previously reported result, which also exhibited lower limonoid content in lime residues when samples were subjected to higher temperatures [21]. Additionally, AD at 50 and 60 °C introduced a significant increase in obacunone content, while drying temperature treatments at 70–90 °C did not exhibit a significant change in obacunone level in lemon slices compared with that of fresh samples. Obacunone naturally occurs in all citrus fruit tissues, and the amount varies greatly depending on the variety and part of the fruit [11, 33]. The differences might result from the variety and the tissue parts used for analysis. The lemon slices used herein were a combination of different tissues, i.e., flavedo, pulps, and seeds.
EP compounds
In total, 11 EP compounds were identified in fresh and AD samples: three phenolic acids (protocatechuic acid, neochlorogenic acid, and chlorogenic acid), three flavones (rutin, rhoifolin, and luteolin), and five flavanones (taxifolin, eriocitrin, narirutin, naringin, and hesperidin) (Table 1). Rutin, rhoifolin, eriocitrin, naringin, and hesperidin have already been reported in lemon fruits [7, 19]. Among these compounds, hesperidin (601.0–1138.2 mg/100 g DW) and eriocitrin (602.0–724.0 mg/100 g DW) were found to be the major EPs, followed by rutin (72.4–181.6 mg/100 g DW) and naringin (49.5–52.0 mg/100 g DW). The result of hesperidin content was similar to that of lemon peels (942 mg/100 g DW) reported by Wang et al. [36]. Extractable hesperidin and eriocitrin occupied 55.9 and 30.8%, respectively, of the total individual phenolics in fresh samples. The predominant extractable hesperidin and eriocitrin reported here agrees with those reported by González-Molina et al. [5] and Barreca et al. [37] in lemon juice. In fresh lemon samples, 10 EP compounds were identified. In AD lemon samples, 11 EP compounds were identified: the ten phenolic identified in fresh samples in addition to p-coumaric acid. García-Salas et al. [19] also reported rutin, rhoifolin, eriocitrin, naringin, and hesperidin in whole-lemon powder obtained by freezing and vacuum drying. In addition, neohesperidin was identified as the main phenolic compound and its content (109 μg/g) was 13.3 times that of hesperidin. This difference may be ascribed to the origin of the raw material.
Table 1.
Extractable and non-extractable phenolic acid, flavone, flavanone, polymethoxyflavonoid contents of fresh and air dried lemon slices
| Phenolic compounds | Extractable phenolics (mg/100 g DW) | Non-extractable phenolics (mg/100 g DW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | AD50 | AD60 | AD70 | AD80 | AD90 | Fresh | AD50 | AD60 | AD70 | AD80 | AD90 | |
| Phenolic acids | ||||||||||||
| Protocatechuic acid | ND | ND | ND | ND | ND | ND | 277.6 ± 8.8b | 238.4 ± 8.2c | 260.0 ± 26.4bc | 289.7 ± 7.6b | 311.9 ± 8.5a | 318.2 ± 11.8a |
| Neochlorogenic acid | 10.9 ± 0.2a | 10.1 ± 0.1ab | 9.5 ± 0.5b | 8.3 ± 0.5c | 6.2 ± 0.1d | 5.0 ± 0.3e | ND | ND | ND | ND | ND | ND |
| Chlorogenic acid | 9.2 ± 0.1a | 9.0 ± 0.1ab | 8.6 ± 0.4b | 8.7 ± 0.3b | 7.0 ± 0.3c | 7.8 ± 0.5c | ND | ND | ND | ND | ND | ND |
| p-Coumaric | ND | ND | ND | ND | ND | 2.1 ± 0.1 | ND | ND | ND | ND | ND | ND |
| Syringic acid | ND | ND | ND | ND | ND | ND | 18.2 ± 0.6e | 20.1 ± 0.7d | 19.5 ± 1.2de | 22.0 ± 1.1c | 26.3 ± 0.8b | 30.1 ± 0.8a |
| Total phenolic acids | 20.1a | 19.1b | 18.1c | 17.0d | 13.1e | 12.8f | 295.8b | 258.6d | 279.5c | 311.7b | 338.2a | 348.3a |
| Flavone | ||||||||||||
| Rutin | 158.3 ± 5.5b | 72.4 ± 3.1c | 72.6 ± 2.3c | 76.3 ± 4.4c | 175.0 ± 6.5a | 181.6 ± 9.1a | ND | ND | ND | ND | ND | ND |
| Rhoifolin | 3.9 ± 0.2d | 5.5 ± 0.1c | 6.3 ± 0.4b | 6.0 ± 0.4b | 6.8 ± 0.4b | 7.9 ± 0.5a | 16.6 ± 0.9c | 17.7 ± 0.42bc | 19.0 ± 0.5a | 18.8 ± 0.8ab | 16.4 ± 1.3c | 17.7 ± 0.4bc |
| Luteolin | 5.2 ± 0.2c | 4.9 ± 0.3c | 5.7 ± 0.3b | 6.8 ± 0.2a | 6.0 ± 0.2b | 5.7 ± 0.1b | 11.2 ± 0.3c | 10.7 ± 1.3c | 14.0 ± 0.2a | 13.1 ± 0.3b | 7.0 ± 0.8d | 14.6 ± 1.4ab |
| Apigenin | ND | ND | ND | ND | ND | ND | 6.3 ± 0.3b | 6.3 ± 0.2b | 8.2 ± 0.3a | 6.4 ± 0.5b | 6.5 ± 0.4b | 9.1 ± 0.7a |
| Diosmetin | ND | ND | ND | ND | ND | ND | 61.6 ± 1.2a | 60.5 ± 1.1a | 60.9 ± 1.0a | 55.5 ± 2.5b | 46.8 ± 1.9c | 58.1 ± 5.2ab |
| Flavanone | ||||||||||||
| Taxifolin | 10.4 ± 0.1c | 9.6 ± 0.1d | 10.0 ± 0.5cd | 9.6 ± 0.2d | 11.8 ± 0.5b | 14.4 ± 0.7a | ND | ND | ND | ND | ND | ND |
| Eriocitrin | 626.4 ± 13.9cd | 694.7 ± 36.8b | 666.6 ± 37.0bc | 602.0 ± 19.5d | 620.5 ± 30.4cd | 724.0 ± 6.2a | 11.5 ± 1.7c | 12.2 ± 0.5c | 16.2 ± 0.5a | 14.4 ± 0.5b | 12.1 ± 1.0c | 13.3 ± 0.6b |
| Narirutin | 31.8 ± 1.0b | 26.60 ± 0.9d | 32.5 ± 1.7ab | 27.8 ± 1.5c | 30.8 ± 1.3b | 34.8 ± 1.8a | ND | ND | ND | ND | ND | ND |
| Naringin | 49.5 ± 1.2a | 50.8 ± 0.3a | 49.7 ± 1.3a | 51.1 ± 2.0a | 52.0 ± 1.6a | 49.6 ± 2.4a | ND | ND | ND | ND | ND | ND |
| Hesperidin | 1138.2 ± 50.9a | 802.1 ± 18.8b | 759.9 ± 57.6bc | 692.6 ± 32.9c | 608.0 ± 18.6d | 601.0 ± 31.3d | 34.7 ± 1.2a | 29.2 ± 1.5b | 37.3 ± 2.2a | 28.2 ± 0.5b | 17.4 ± 2.3c | 20.4 ± 1.2c |
| Poncirin | ND | ND | ND | ND | ND | ND | 137.8 ± 17.8ab | 161.2 ± 7.6a | 135.9 ± 1.0b | 125.6 ± 3.6b | 96.1 ± 2.2c | 59.5 ± 1.4d |
| PMF | ||||||||||||
| Nobiletin | ND | ND | ND | ND | ND | ND | 2.8 ± 0.2b | 3.2 ± 0.2b | 3.7 ± 0.4ab | 3.7 ± 0.4ab | 3.6 ± 0.3ab | 4.0 ± 0.2a |
| Total flavonoids | 2023.7a | 1666.4b | 1603.2b | 1472.2c | 1510.9c | 1618.9b | 282.5a | 301.0a | 295.3a | 265.76b | 206.0c | 196.6c |
| Total phenolics | 2043.8a | 1685.5b | 1621.3b | 1489.2c | 1524.0c | 1631.7b | 578.3a | 559.6b | 574.8a | 577.5a | 544.1c | 544.9c |
ND means not detected; AD50, AD60, AD70, AD80, and AD90 represent air drying at 50, 60, 70, 80, and 90 °C, respectively; PMF: polymethoxyflavonoid; Mean values in the same row for each treatment with different superscripts are significantly different (p < 0.05)
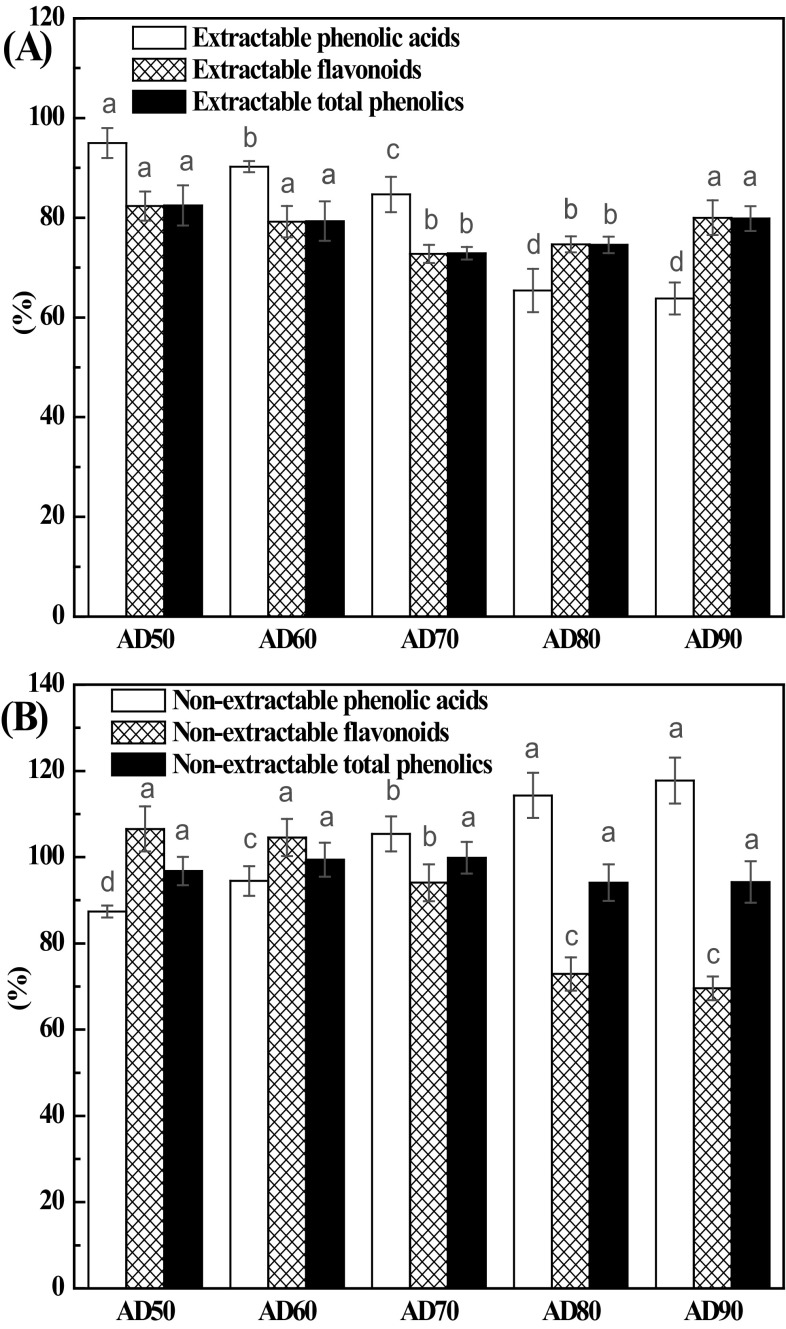
The extractable phenolic acid, flavonoid, and phenolics contents in fresh lemon slices were 20.1, 2023.7, and 2043.8 mg/100 g DW, respectively. Thus, phenolic acids in fresh lemon slices were mainly present in their non-extractable form (87.8%), whereas flavonoids were predominantly present in their extractable form (93.6%). AD of lemon slices promoted loss of extractable phenolic acids, flavonoids, and phenolics, compared with those of fresh samples [Fig. 3(A)]. Generally, AD at 50 °C exhibited the highest retention ratio of extractable phenolic acids, flavonoids, and phenolics. As drying temperature increased from 50 to 90 °C, the retention ratio of extractable phenolic acids decreased gradually from 95.0 to 63.8%. However, the retention ratio of extractable flavonoids and EP first decreased and then increased in the temperature-rise period. Loss of EP during drying processes may be mainly attributed to oxidative reactions. Since lemon slices exposed to oven drying are susceptible to both non-enzymatic and enzymatic oxidative reactions, AD, especially at low temperatures, did not immediately deactivate degradative enzymes, such as polyphenol oxidase and peroxidase. Therefore, they are able to degrade phenolic compounds, especially in the initial drying process. Nesrine et al. [18] observed that both total phenols and total flavonoids of lemon peels exhibited a decreasing trend when they were dried at 40–60 °C, and their degradations followed the first-order kinetic models. The change trend of EP content indicates that AD temperature poses different influences in EPs. Typically, the contents of extractable phenolic acids in AD lemon slices decreased as drying temperature increased from 50 to 90 °C. Extractable p-coumaric acid was only detected in samples undergoing AD at 90 °C (Table 1). Compared with fresh lemon slices, the ones undergoing AD showed a significant decrease in the content of extractable neochlorogenic acid (p < 0.05) and its content exhibited a decreasing tread as drying temperature increased from 50 to 90 °C. A similar phenomenon was observed for extractable chlorogenic acid. Since lemon slices exhibited high level of acids, the decline of these two hydroxycinnamic acids could be attributed to cleavage of the esterified bond, induced by acid catalysis under high-temperature conditions. Xu et al. [38] found that the amount of free chlorogenic acid in huyou (Citrus paradisi Changshanhuyou) peels decreased with increasing heating time (30–90 min) at temperatures of 90–150 °C, and Madrau et al. [39] observed that both the neochlorogenic acid and chlorogenic acid contents in apricots decreased significantly as the apricots were subjected to hot-air drying at 55 or 75 °C. The extractable hesperidin content was found to decrease as drying temperature increased from 50 to 90 °C, which decreased from 29.5 to 47.2% compared to fresh samples. Degradation of hesperidin at high temperatures was also observed in drying of immature calamondin [40]. This degradation could be attributed to acid hydrolysis under high temperatures, since lemon slices contained abound organic acids, especially citric acid. Additionally, AD at 50–70 °C could cause a significant decrease in the extractable rutin content of lemon slices (p < 0.05), ranging from 54.2 to 57.5%. Meanwhile, AD at 80 or 90 °C could introduce a 9.5% or 12.8% increase of extractable rutin, respectively, compared with that of fresh samples. Chen et al. [41] found that AD at 50 and 60 °C promoted loss of rutin in orange (C. sinensis (L.) Osbeck) peels, while AD at 80–100 °C could induce an increase. However, extractable rhoifolin content showed an increasing trend as drying temperature elevated from 50 to 90 °C, which increased from 41.0 to 102.6% compared with fresh samples. Lou et al. [42] found that the rhoifolin content of immature kumquat remained unchanged as it was subjected to AD at 130 °C for 1 h, while it significantly increased when the immature kumquat was exposed for 1.5 or 2 h at 130 °C. A similar trend was noted for the content of extractable taxifolin. The levels of extractable naringin were similar in all dried samples, as well as in the fresh ones.
Fig. 3.
Contents relative to fresh lemon slices of extractable phenolic acids, flavonoids, phenolics (A), non-extractable phenolic acids, flavonoids, and phenolics (B) in AD lemon slices. Mean values in the same type index for each treatment with different letters are significantly different (p < 0.05)
NEP compounds
10 NEP compounds were identified in fresh lemon slices (Table 1): two phenolic acids (protocatechuic acid and syringic acid), four flavones (rhoifolin, luteolin, apigenin, and diosmetin), three flavanones (eriocitrin, hesperidin, and poncirin), and one polymethoxyflavonoid (Nobiletin). For NEPs, protocatechuic acid and poncirin were identified as the predominant phenolics, accounting for 48.0 and 23.8%, respectively, of the total individual phenolics in fresh samples. Zhang et al. [43], however, reported that ferulic acid rather than protocatechuic acid, was the main bound phenolic acid in the peels of 14 wild mandarin genotypes and 2 cultivars. The content of protocatechuic acid (277.6–318.2 mg/100 g DW) of the lemon slices used in this study, however, was much higher than that of wild mandarin peels reported by Xi et al. [44]. These differences may be attributed to the different genetic backgrounds of the citrus species and/or environmental factors. Neochlogenic acid, chlorogenic acid, taxifolin, rutin, narirutin, and naringin were found exclusively as EP, whereas protocatechuic acid, syringic acid, apigenin, diosmetin, poncirin, and nobiletin were found exclusively as NEP. Additionally, eriocitrin, hesperidin, rhoifolin, luteolin, and poncirin were found in both their extractable and non-extractable forms. To the best of our knowledge, there are no available reports of extractable and non-extractable individual phenols, flavonoids, and flavanones for lemon slices.
Herein, the non-extractable phenolic acid, flavonoid, and phenolic contents in fresh lemon slices were 295.8, 282.5, and 578.3 mg/100 g DW, respectively. The retention ratio of non-extractable phenolic acids increased from 87.4 to 117.8% as the drying temperature increased from 50 to 90 °C [Fig. 3(B)]. In contrast, the retention ratio of non-extractable flavonoids decreased from 106.5 to 69.6%. NEP in lemon slices behaved differently at different drying temperatures. Compared with fresh samples, AD at 50 °C caused a decrease in amount of non-extractable protocatechuic acid, while AD at 70–90 °C induced an increase. Furthermore, this amount increased as drying temperature increased from 50 to 90 °C. A similar phenomenon was observed for non-extractable syringic acid. This indicates that high temperatures could cause the release of these two non-extractable phenolic acids from the cell structure. Xu et al. [38] also found that ester and glycoside of cinnamics (caffeic, p-coumaric, ferulic, and sinapic) and benzoics (p-hydroxybenzoic and vanillic) in Huyou peel showed a decreasing tread as it exposed to 120 °C for 2 h. Compared with fresh lemon slices, the ones undergoing AD at 50 °C increased in the content of non-extractable poncirin it decreased as the drying temperature was increased from 60 to 90 °C. The non-extractable hesperidin contents of samples undergoing AD 60 °C showed maximum value, and then decreased significantly as the drying temperature increased from 70 to 90 °C. Non-extractable nobiletin content showed an increasing trend as the drying temperature elevated from 50 to 90 °C. Lou et al. [40] found that ester linkage nobiletin was released from immature calamondin peels as the drying temperature increased from 130 to 150 °C. The contents of non-extractable luteolin and apigenin were the highest in samples undergoing AD at 90 °C. Compared with fresh samples, all AD treatments induced an increase in non-extractable rhoifolin and taxifolin contents. Jeong et al. [45] found that several low-molecular-weight phenolic compounds were newly formed as citrus peels were dried at 150 °C for 0.5 h.
Antioxidant capacities
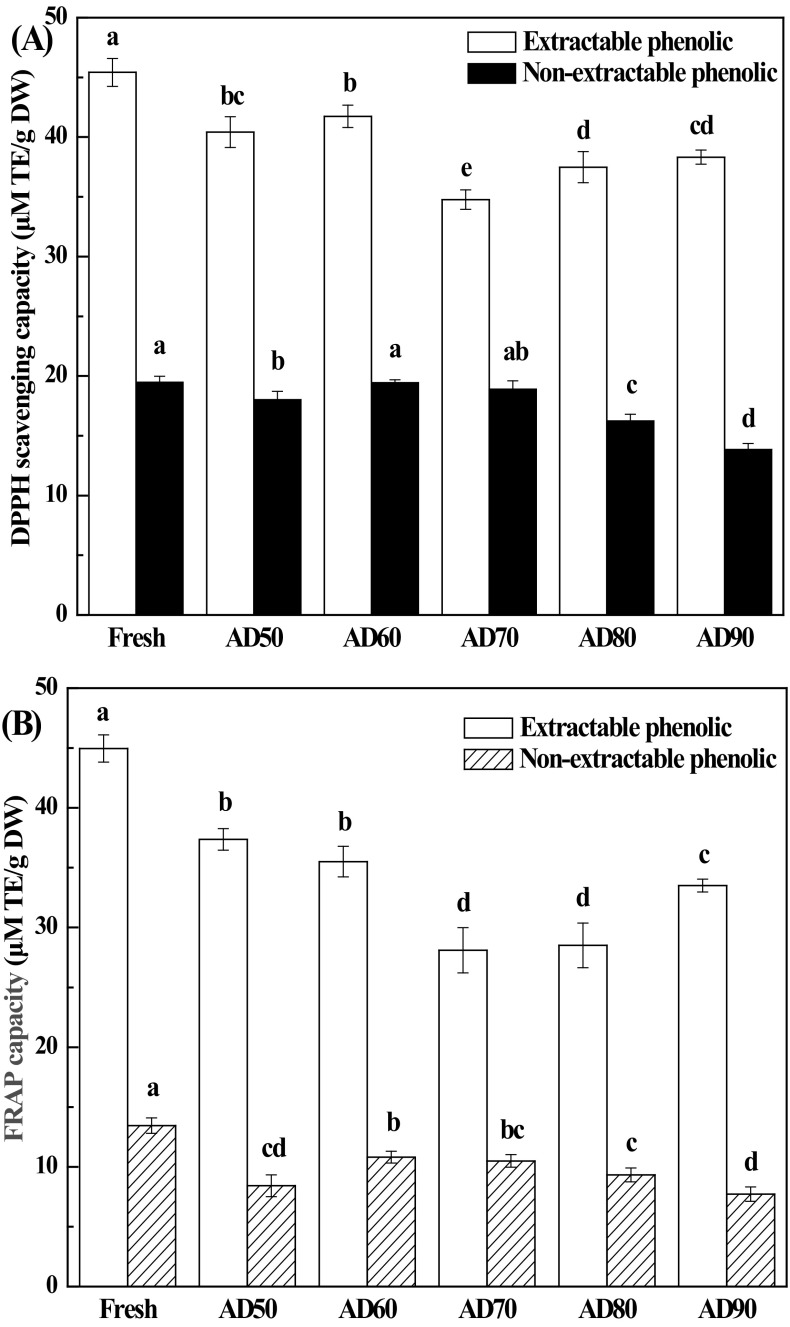
Antioxidant capacities of fresh and AD lemon slices were evaluated using DPPH and FRAP assays in both extractable and non-extractable extracts. The DPPH values for EP and NEP ranged from 34.5 to 45.4 μM TE/g DW and from 13.8 to 19.4 μM TE/g DW, respectively [Fig. 4(A)]. The levels of DPPH antioxidant capacity detected herein for EP agreed with those reported by Zhang et al. [43] in mandarin peels (29.4–51.1 μM TE/g DW), while they were much higher compared to those reported by Xi et al. [44] in mandarin pulps (9.10–19.75 μM TE/g DW). Generally, AD led to a decrease in scavenging DPPH capacities of EP extracts, ranging from 8.1 to 24.0%, compared with FD lemon slices. In addition, scavenging DPPH capacities of EP extracts did not change significantly as the drying temperature was increased from 50 to 60 °C (p > 0.05), while it increased significantly as the temperature was further increased to 90 °C (p < 0.05). Similar results were obtained by other authors working with lime [46]. The antioxidant capacity loss of extractable phenolics in AD lemon slices could be attributed to the reduction of ascorbic acid and the total phenolics, as confirmed in Figs. 1(B) and 3(A). Levels of DPPH antioxidant capacity for EP maintained the DPPH value of fresh lemon slices as it dried at 50–70 °C, while it decreased significantly as the drying temperature was increased to 90 °C.
Fig. 4.
Effect of drying temperature on the scavenging DPPH radical (A) and FRAP (B) of extractable and non-extractable phenolics in lemon slices. Mean values in the same type index for each treatment with different letters are significantly different (p < 0.05)
The FRAP values for EP and NEP ranged from 28.1 to 45.0 μM TE/g DW and from 7.7 to 13.4 μM TE/g DW, respectively [Fig. 4(B)]. A higher FRAP value was found in fresh lemon slices both for EP and NEP. The FRAP value of EP in lemon slices first decreased and then increased as the drying temperature elevated from 50 to 90 °C, while the changes in FRAP values of NEP showed the opposite trend. The levels of FRAP antioxidant capacity detected herein were lower than those reported by García-Salas et al. [19] (70.9 μM TE/g DW) in lemon powder.
In summary, AD process induced increases in limonin, nomilin, and obacunone, especially at 50–70 °C. AD promoted losses of EP and NEP in lemon slices, compared with fresh samples. The decrese in extractable phenolic acid, EP, and NEP content in lemon is lower at low drying temperatures, while the increase in non-extractable phenolic acid content is higher at high drying temperatures. The antioxidant capacities of EPs extracts in lemon slices was higher than those of NEPs. Phenolics contributed to antioxidant capacities of lemon slices, and flavonoids were the main contributors among phenolics to antioxidant capacity. Considering the high levels of limonoids, EPs, NEPs, and antioxidant capacities in lemon slices, AD at 60 °C could be an appreciate treatment for dehydration of lemon slices.
Acknowledgements
This work was supported by the Natural Science Foundation of Hunan Province, China (2016JJ6077), Key R & D Plan of Hunan Province, China (2017NK2110), and National Natural Science Foundation of China (31501543).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.United States Department of Agriculture-Foreign Agricultural Service Citrus: World Markets and Trade, January 2014.
- 2.FAO. 2014. http://www.fao.org/faostat/en/#data/QC.
- 3.González-Molina E, Domínguez-Perles R, Moreno DA, García-Viguera C. Natural bioactive compounds of Citrus limon for food and health. J. Pharmaceut. Biomed. 2010;51:327–345. doi: 10.1016/j.jpba.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Liu YQ, Heying E, Tanumihardjo SA. History, global distribution, and nutritional importance of citrus fruits. Comp. Rev. Food Sci. F. 2012;11:530–545. doi: 10.1111/j.1541-4337.2012.00201.x. [DOI] [Google Scholar]
- 5.González-Molina E, Moreno DA, García-Viguera C. Genotype and harvest time influence the phytochemical quality of Fino lemon juice (Citrus limon (L.) Burm. F.) for industrial use. J. Agr. Food Chem. 2008;56:1669–1675. doi: 10.1021/jf073282w. [DOI] [PubMed] [Google Scholar]
- 6.González-Molina E, Moreno DA, García-Viguera C. Comparison of ‘Verna’ lemon juice quality for new ingredients and food products. Sci. Hortic. 2009;120:353–359. doi: 10.1016/j.scienta.2008.11.010. [DOI] [Google Scholar]
- 7.Amenta M, Ballistreri G, Fabroni S, Romeo FV, Spina A, Rapisarda P. Qualitative and nutraceutical aspects of lemon fruits grown on the mountainsides of the Mount Etna: A first step for a protected designation of origin or protected geographical indication application of the brand name ‘Limone dell’Etna’. Food Res. Int. 2015;74:250–259. doi: 10.1016/j.foodres.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Ledesma-Escobar CA, Priego-Capote F, Luque De Castro MD. Effect of sample pretreatment on the extraction of lemon (Citrus limon) components. Talanta. 2016;153:386–391. doi: 10.1016/j.talanta.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Uckoo RM, Jayaprakasha GK, Patil BS. Phytochemical analysis of organic and conventionally cultivated Meyer lemons (Citrus meyeri Tan.) during refrigerated storage. J. Food Compos. Anal. 2015;42:63–70. doi: 10.1016/j.jfca.2015.01.009. [DOI] [Google Scholar]
- 10.Interdonato R, Rosa M, Nieva CB, González JA, Hilal M, Prado FE. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ. Exp. Bot. 2011;70:204–211. doi: 10.1016/j.envexpbot.2010.09.006. [DOI] [Google Scholar]
- 11.Russo M, Bonaccorsi I, Inferrera V, Dugo P, Mondello LG. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in orange’s by-products. J. Funct. Foods. 2015;12:150–157. doi: 10.1016/j.jff.2014.11.008. [DOI] [Google Scholar]
- 12.Chen HH, Hernandez CE, Huang TC. A study of the drying effect on lemon slices using a closed-type solar dryer. Sol. Energy. 2005;78:97–103. doi: 10.1016/j.solener.2004.06.011. [DOI] [Google Scholar]
- 13.Darvishi H, Khoshtaghaza MH, Minaei S. Drying kinetics and colour change of lemon slices. Int. Agrophys. 2014;28:1–6. doi: 10.2478/intag-2013-0021. [DOI] [Google Scholar]
- 14.Sadeghi M, Mirzabeigi Kesbi O, Mireei SA. Mass transfer characteristics during convective, microwave and combined microwave-convective drying of lemon slices. J. Sci. Food Agr. 2013;94:471–478. doi: 10.1002/jsfa.5786. [DOI] [PubMed] [Google Scholar]
- 15.Torki-Harchegani M, Ghanbarian D, Sadeghi M. Estimation of whole lemon mass transfer parameters during hot air drying using different modelling methods. Heat Mass Transfer. 2015;51:1121–1129. doi: 10.1007/s00231-014-1483-1. [DOI] [Google Scholar]
- 16.Torki-Harchegani M, Ghasemi-Varnamkhasti M, Ghanbarian D, Sadeghi M, Tohidi M. Dehydration characteristics and mathematical modelling of lemon slices drying undergoing oven treatment. Heat Mass Transfer. 2016;52:281–289. doi: 10.1007/s00231-015-1546-y. [DOI] [Google Scholar]
- 17.Ghanem N, Mihoubi D, Kechaou N, Mihoubi NB. Microwave dehydration of three citrus peel cultivars: Effect on water and oil retention capacities, color, shrinkage and total phenols content. Ind. Crop. Prod. 2012;40:167–177. doi: 10.1016/j.indcrop.2012.03.009. [DOI] [Google Scholar]
- 18.Nesrine GR, Catherine B, Nabil K, Nourhène BM, Ghanem RN, Bonazzi C, Kechaou N, Boudhrioua MN. Effect of air-drying temperature on kinetics of quality attributes of lemon (Citrus limon cv. lunari) peels. Dry. Technol. 2015;33:1581–1589. doi: 10.1080/07373937.2015.1012266. [DOI] [Google Scholar]
- 19.García-Salas P, Gómez-Caravaca AM, Arráez-Román D, Segura-Carretero A, Guerra-Hernández E, García-Villanova B, Fernández-Gutiérrez A. Influence of technological processes on phenolic compounds, organic acids, furanic derivatives, and antioxidant activity of whole-lemon powder. Food Chem. 2013;141:869–878. doi: 10.1016/j.foodchem.2013.02.124. [DOI] [PubMed] [Google Scholar]
- 20.Kuljarachanan T, Devahastin S, Chiewchan N. Evolution of antioxidant compounds in lime residues during drying. Food Chem. 2009;113:944–949. doi: 10.1016/j.foodchem.2008.08.026. [DOI] [Google Scholar]
- 21.Wuttipalakorn P, Srichumpuang W, Chiewchan N. Effects of pretreatment and drying on composition and bitterness of high-dietary-fiber powder from lime residues. Dry. Technol. 2009;27:133–142. doi: 10.1080/07373930802566036. [DOI] [Google Scholar]
- 22.Gao QH, Wu CS, Wang M, Xu BN, Du LJ. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agr. Food Chem. 2012;60:9642–9648. doi: 10.1021/jf3026524. [DOI] [PubMed] [Google Scholar]
- 23.Arranz S, Saura Calixto F. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010;51:313–318. doi: 10.1016/j.jcs.2010.01.006. [DOI] [Google Scholar]
- 24.Li LG, Xi WP, Zhang YM, Jiao B, Zhou ZQ. A study of the flavonoids in different tissues of the basic citrus types native to China. Sci. Agr. Sinica. 2013;46:4753–4762. [Google Scholar]
- 25.Li SJ, Wang Z, Ding F, Sun D, Ma ZC, Cheng YJ, Xu J. Content changes of bitter compounds in ‘Guoqing No.1’ Satsuma mandarin (Citrus unshiu Marc.) during fruit development of consecutive 3 seasons. Food Chem. 2014;145:963–969. doi: 10.1016/j.foodchem.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Jiang L, Ye S, Ye YB, Ren FZ. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010;48:1461–1465. doi: 10.1016/j.fct.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Wang RR, Ding SH, Zhao DD, Wang ZF, Wu JH, Hu XS. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci. Biotechnol. 2016;25:137–143. doi: 10.1007/s10068-016-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting SV, Attaway JA. Citrus fruits. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Eds.; Academic Press: London, Vol. 2: 107–169 (1971).
- 29.Fu MQ, Xiao GS, Wu JJ, Chen YL, Yu YS, Chen WD, Xu YJ. Effects of modified atmosphere packaging on the quality of dried lemon slices. Effects of modified atmosphere packaging on the quality of dried lemon slices. J. Food Process. Pres. doi:10.1111/jfpp.13043 (2016).
- 30.Oliveira SM, Ramos IN, Brandão TRS, Silva CLM. Effect of air-drying temperature on the quality and bioactive characteristics of dried galega kale (Brassica oleracea L.var. acephala). J. Food Process. Pres. 39: 2485–2496 (2015).
- 31.Hasegawa S, Manners GD. A new normal phase liquid chromatographic method for the analysis of limonoids in citrus. Phytochem. Analysis. 1999;10:76–81. doi: 10.1002/(SICI)1099-1565(199903/04)10:2<76::AID-PCA439>3.0.CO;2-7. [DOI] [Google Scholar]
- 32.Sun YJ, Shen Y, Liu DH, Ye XQ. Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. LWT-Food Sci. Tech. 2015;60:1269–1275. doi: 10.1016/j.lwt.2014.09.001. [DOI] [Google Scholar]
- 33.Russo M, Bonaccorsi I, Torre G, Sarò M, Dugo P, Mondello L. Underestimated sources of flavonoids, limonoids and dietary fibre: Availability in lemon’s by-products. J. Funct. Foods. 2014;9:18–26. doi: 10.1016/j.jff.2014.04.004. [DOI] [Google Scholar]
- 34.Maier VP, Hasegawa S, Hera E. Limonin D-ring-lactone hydrolase. A new enzyme from citrus seeds. Phytochemistry. 1969;8:405–407. doi: 10.1016/S0031-9422(00)85439-4. [DOI] [Google Scholar]
- 35.Hasegawa S. Metabolism of limonoids. Limonin D-ring lactone hydrolase. J. Agr. Food Chem. 1976;24:24–26. doi: 10.1021/jf60203a024. [DOI] [PubMed] [Google Scholar]
- 36.Wang YC, Chuang YC, Hsu HW. The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan. Food Chem. 2008;106:277–284. doi: 10.1016/j.foodchem.2007.05.086. [DOI] [Google Scholar]
- 37.Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem. 2011;129:417–422. doi: 10.1016/j.foodchem.2011.04.093. [DOI] [PubMed] [Google Scholar]
- 38.Xu GH, Ye XQ, Chen JC, Liu DH. Effect of heat treatment on the phenolic compounds and antioxidant capacity of ctrus peel extract. J. Agr. Food Chem. 2007;55:330–335. doi: 10.1021/jf062517l. [DOI] [PubMed] [Google Scholar]
- 39.Madrau MA, Piscopo A, Sanguinetti AM, Del Caro A, Poiana M, Romeo FV, Piga A. Effect of drying temperature on polyphenolic content and antioxidant activity of apricots. Eur. Food Res. Technol. 2009;228:441–448. doi: 10.1007/s00217-008-0951-6. [DOI] [Google Scholar]
- 40.Lou SN, Lin YS, Hsu YS, Chiu EM, Ho CT. Soluble and insoluble phenolic compounds and antioxidant activity of immature calamondin affected by solvents and heat treatment. Food Chem. 2014;161:246–253. doi: 10.1016/j.foodchem.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Chen ML, Yang DJ, Liu SC. Effects of drying temperature on the flavonoid, phenolic acid and antioxidative capacities of the methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels. Int. J. Food Sci. Tech. 46: 1179–1185 (2011).
- 42.Lou SN, Lai YC, Huang JD, Ho CT, Ferng LHA, Chang YC. Drying effect on flavonoid composition and antioxidant activity of immature kumquat. Food Chem. 2015;171:356–363. doi: 10.1016/j.foodchem.2014.08.119. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YM, Sun YJ, Xi WP, Shen Y, Qiao LP, Zhong LZ, Ye XQ, Zhou ZQ. Phenolic compositions and antioxidant capacities of Chinese wild mandarin (Citrus reticulata Blanco) fruits. Food Chem. 2014;145:674–680. doi: 10.1016/j.foodchem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Xi WP, Zhang YM, Sun YJ, Shen Y, Ye XQ, Zhou ZQ. Phenolic composition of Chinese wild mandarin (Citrus reticulata Balnco.) pulps and their antioxidant properties. Ind. Crop. Prod. 2014;52:466–474. doi: 10.1016/j.indcrop.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Jeong SM, Kim SY, Kim DR, Jo SC, Nam KC, Ahn DU, Lee SC. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agr. Food Chem. 2004;52:3389–3393. doi: 10.1021/jf049899k. [DOI] [PubMed] [Google Scholar]
- 46.Esparza-Martínez FJ, Miranda-López R, Guzman-Maldonado SH. Effect of air-drying temperature on extractable and non-extractable phenolics and antioxidant capacity of lime wastes. Ind. Crop. Prod. 2016;84:1–6. doi: 10.1016/j.indcrop.2016.01.043. [DOI] [PubMed] [Google Scholar]