Abstract
Probiotic characteristics of Bacillus subtilis P223 isolated from kimchi were investigated in this study. Spore cells of B. subtilis P223 showed high tolerance to artificial gastric juice (pH 2.5, 0.3% pepsin, 3 h) and bile salts (0.3% oxgall, 24 h). Spore cells of B. subtilis P223 showed more adherence to intestinal cells (HT-29 cells) than vegetative cells. In addition, B. subtilis P223 showed high autoaggregation ability, similar to a commercial strain (Bacillus clausii ATCC 700160). Moreover, its coaggregation abilities with pathogens were strong. The adherence of three pathogens (Salmonella enteritidis ATCC 13076, Listeria monocytogenes ATCC 15313, and Escherichia coli ATCC 25922) to HT-29 cells was inhibited by B. subtilis P223. It was found that B. subtilis P223 could not produce β-glucuronidase, a carcinogenic enzyme. However, it had amylase and protease activities. Antibiotic susceptibility was measured using disk diffusion assay. It was revealed that B. subtilis P223 was only resistant to streptomycin among eight kinds of antibiotics. In addition, B. subtilis P223 showed no hemolysis activity. It did not have enterotoxin genes. Results of this study suggest that B. subtilis P223 isolated from kimchi has potential as a probiotic strain.
Keywords: Probiotics, Bacillus, Spore, Kimchi, Safety
Introduction
Probiotics are defined as ‘live microorganisms which, when administered in adequate amounts, confer a health benefit on the host’ [1]. Probiotics can improve intestinal health, enhance immune response, and reduce serum cholesterol levels [2]. They can also help overcome lactose intolerance, Helicobacter pylori infections, and inflammatory bowel disease [3]. However, probiotics should be confirmed to have specific characteristics such as resistance to gastric and bile acid with adhesion activity to human epithelial cells to exert their probiotic potential [4]. Safety assessment of probiotics such as antibiotics resistance and hemolysis should also be tested for probiotics [5]. The majority of probiotics commercialized are isolated from human gastrointestinal tract and dairy products [2].
Kimchi is a Korean traditional vegetable fermented food made of cabbage or radish with ground red pepper, garlic, and diverse seasoning ingredients [6]. Kimchi is fermented in low temperature, usually under 10 °C. Diverse bacteria increase during fermentation [7]. Kimchi is known to have many beneficial effects to human body, including anti-cancer [8], anti-mutagenic [9], anti-oxidative [10], and anti-aging [11]. Its beneficial effects are due to components in kimchi, including fibers, vitamins, phytochemicals, and minerals derived from ingredients and bacteria in kimchi [12]. Many studies have reported lactic acid bacteria as probiotics isolated from kimchi. However, few research studies have reported probiotic Bacillus strains isolated from kimchi [13–15]. Many Bacillus species have been isolated from fermented soybean food and applied in industrial products as probiotics for many years [5, 16]. Bacillus species known as probiotics include B. subtilis, B. cereus, B. clausii, B. coagulans, and B. licheniformis [16]. Bacillus spp. produce spores when strains are exposed to extreme conditions of environment [17]. These spores are heat-stable therefore they can be stored during long period. They are resistant to low pH environment such as gastric acid [16, 18]. Therefore, Bacillus spp. have advantages over non-spore forming probiotics such as Lactobacillus, Bifidobacterium, Leuconostoc, and Pediococcus [5]. Enterogermina® is a probiotic product that contains B. clausii spores in water. It is expected to improve the delivery of spores to gastrointestinal (GI) tract [16].
The aim of this study was to identify B. subtilis P223 isolated from kimchi and investigate its probiotic properties, including its resistance to acid and bile salts, enzymatic activity, adhesion ability, and auto/co-aggregation. In addition, antibiotic susceptibility, hemolysis, and toxic gene analysis were determined to assess its safety.
Materials and methods
Bacterial strains and growth conditions
B. subtilis P223 was isolated from kimchi. As a control, B. clausii ATCC 700160 used as commercial probiotic strain was obtained from Korean Collection for Type Cultures (KCTC, Daejeon, Korea). B. subtilis P223 and B. clausii ATCC 700160 were cultured in tryptic soy broth (TSB; Becton–Dickinson, Sparks, MD, USA) with shaking at 150 rpm. B. subtilis P223 and B. clausii ATCC 700160 were incubated at 37 and 30 °C, respectively. To obtain spore cells, B. subtilis P223 was incubated at 37 °C for 72 h while B. clausii ATCC 700160 was incubated at 30 °C for 7 days. After incubation, strains were heated at 80 °C for 30 min to kill vegetative cells. Staphylococcus aureus ATCC 6538, S. enteritidis ATCC 13076, L. monocytogenes ATCC 15313, and E. coli ATCC 25922 were incubated in TSB at 37 °C.
Identification of Bacillus strain
B. subtilis P223 was identified based on 16S rRNA sequencing analysis by Bionics Inc. (Seoul, Korea). Sequencing results were analyzed by searching database of National Center for Biotechnology Information Nucleotide using BLAST web site (http://blast.ncbi.nlm.nih.gov).
Resistance to artificial gastric juice and bile salts
Resistance of Bacillus strains to artificial gastric juice and bile salts was determined following protocol published by Jeon et al. [14]. Resistance of Bacillus to artificial gastric juice was assessed using TSB containing 0.3% (w/v) pepsin (Sigma-Aldrich, St. Louis, MO, USA) adjusted to pH 2.5 with 0.1 M HCl and incubated for 3 h with shaking (150 rpm). Resistance of Bacillus to bile salts was assessed by incubating in TSB containing 0.3% (w/v) oxgall (Becton–Dickinson, Sparks, MD, USA) for 24 h with shaking (150 rpm). Survival rate was determined by counting viable cells on TSA plates.
Adhesion capacity to HT-29 cells
Human colon adenocarcinoma cell (HT-29, KCLB 30038) was used to test the adhesion capacity of Bacillus strains. Briefly, 1 × 105 cells/well of HT-29 cells were seeded into 24-well cell culture plate and incubated at 37 °C for 24 h. After incubation, Bacillus strain was inoculated to HT-29 cells and incubated at 37 °C for 2 h. Non-adherent bacterial cells were removed by washing three times with phosphate-buffered saline (PBS; Gibco Life Technologies, Invitrogen, Carlsbad, CA, USA). Adherent cells were then detached using 1% (v/v) Triton X-100 (Sigma) solution. The number of adherent cells was counted by viable cell count method.
Autoaggregation and coaggregation
Autoaggregation and coaggregation abilities of bacteria were measured as described by Tareb et al. [19] with some modifications. Cultured bacteria were centrifuged to obtain bacterial cells. Cells were washed twice with PBS and resuspended in PBS to reach absorbance value of 0.3 ± 0.05 at 600 nm.
To determine autoaggregation percentage, bacterial suspension was incubated at 37 °C for 4 and 24 h. Absorbance at 600 nm was measured at 0, 4, and 24 h after incubation. The percentage of autoaggregation was expressed as follows:
where A0 and At represented the absorbance at 0 h and at indicated incubation time (4 and 24 h), respectively.
For coaggregation, each Bacillus bacterial suspension (2 mL) was mixed with each pathogen (2 mL). The mixture (4 mL) was incubated at 37 °C for 4 and 24 h. The percentage of coaggregation was expressed as follows:
where AP and AB represented the absorbance of the pathogen and the Bacillus strain at 0 h, respectively, and Amix represented the absorbance of the mixed culture after indicated incubation time.
Inhibition of adherence of pathogens to HT-29 cells
Inhibitory activity of bacterial adhesion was performed using the method of Lee et al. [4]. Briefly, HT-29 cells (1 × 105 cells/well) were seeded into 24-well cell culture plates and incubated for 24 h. About 106 cells/well of pathogen and mix culture (pathogens with Bacillus strain) was added onto the plate and incubated at 37 °C for 2 h. After the incubation, non-adherent bacterial cells were washed thrice with PBS. HT-29 cells were detached by adding 1 mL of 1% (v/v) Triton X-100 solution. The solution was diluted and spread onto XLD (Becton–Dickinson, Sparks, MD, USA) agar for S. enteritidis, Oxford agar (Becton–Dickinson) with supplement for L. monocytogenes, and EMB (Becton–Dickinson) agar for E. coli to count viable cell numbers.
Enzyme production
API ZYM kit (BioMerieux, Lyon, France) was used to determine enzymatic activity. Bacillus strains were centrifuged (14,240×g, 5 min, 4 °C) to obtain cell pellet. The pellet was washed and suspended with suspension medium (BioMerieux, Lyon, France). Then 106 CFU/mL of bacteria were inoculated into each cupule. After incubation at 37 °C for 4 h, ZYM A and ZYM B reagents were dropped to each cupule. Color change was detected to assess the level of enzyme activity after 5 min of reaction.
Amylase activity was measured using modified method of Bernfeld [20]. Briefly, 1 mL culture supernatant and 1 mL of 1% soluble starch solution were mixed at 25 °C for 3 min. DNS solution was added to the mixture followed by boiling for 15 min. After cooling on ice, 9 mL of distilled water was added to the mixture. The absorbance of the solution was then measured at 540 nm. One unit of amylase activity indicated the release of 1 mg of maltose from starch in 3 min.
Protease activity of Bacillus was determined using the method described by Lee et al. [21] with some modifications. Briefly, 1 mL of the culture supernatant was reacted with 1 mL of 0.6% casein solution (in 0.05 M potassium phosphate buffer, pH 7.5) at 37 °C for 10 min. Two milliliters of 0.4 M TCA solution was added to the solution to stop the reaction. The solution was filtered with 0.45 µm membrane filter. After filtering, 5 mL of 0.4 M sodium carbonate and 1 mL of 3×Folin and Ciocalteu’s phenol reagent (Sigma Aldrich, St. Louis, MO, USA) were added to the filtered solution followed by incubation at room temperature for 20 min. Absorbance of the solution was measured at wavelength of 660 nm. One unit of protease activity indicated the released of 1 µM of tyrosine from casein per minute.
Antibiotic susceptibility
Antibiotic susceptibility of Bacillus was tested using disk diffusion method according to CLSI performance standards for antimicrobial susceptibility testing [22]. Eight kinds of antibiotics were used: ampicillin (10 µg), gentamicin (10 µg), kanamycin (30 µg), streptomycin (10 µg), tetracycline (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), and doxycycline (30 µg). Bacillus strains (1 × 106 CFU/mL) were spread onto TSA plates. Antibiotics were loaded onto paper disks. The diameter of inhibition zone by each antibiotic was detected after incubation at 37 °C for 24 h.
Hemolysis
Columbia blood agar plate (Becton–Dickinson, Sparks, MD, USA) containing 5% (w/v) sheep blood was used to determine hemolysis. Bacillus strains were streaked onto blood agar plate and incubated 37 °C for 24 h. Plate with clear zone around the strain indicated β-hemolysis. Green-hued zone around the strain indicated α-hemolysis while no change around the strain indicated γ-hemolysis.
Detection of toxic genes
Bacillus strains were inoculated in 10 mL of TSB and incubated overnight at 37 °C. All culture medium was centrifuged (14,240×g, 4 °C, 10 min), washed with PBS, and the pellet was suspended in 200 µL of PBS. DNA was extracted from bacteria using AccuPrep ® Genomic DNA Extraction Kit (Bioneer Corp., Daejeon, Korea). Purity and concentration of DNA were assessed using a Nanodrop spectrophotometer. Primers used in this work are listed in Table 1 [23]. All primers were synthesized by Bionics Inc. (Seoul, Korea). PCR mixture (Solgent Co., Ltd., Daejeon, Korea), 1 µg of DNA, and water were used for PCR reactions. A total of 20 µL of PCR reaction mixture was processed according to the annealing temperature of each primer. PCR products were subjected to 1.2% (w/v) agarose gel electrophoresis.
Table 1.
Oligonucleotide primer pair sequence
| Primer | Sequence (5′-3′) | Size (bp) | Reference |
|---|---|---|---|
| hblA-F | AAGCAATGGAATACAATGGG | 1154 | [23] |
| hblA-R | AGAATCTAAATCATGCCACTGC | ||
| hblC-F | GATACTCAATGTGGCAACTGC | 740 | |
| hblC-R | TTGAGACTGCTCGTCTAGTTG | ||
| hblD-F | ACCGGTAACACTATTCATGC | 829 | |
| hblD-R | GAGTCCATATGCTTAGATGC | ||
| nheA-F | GTTAGGATCACAATCACCGC | 755 | |
| nheA-R | ACGAATGTAATTTGAGTCGC | ||
| nheB-F | TTTAGTAGTGGATCTGTAGC | 743 | |
| nheB-R | TTAATGTTCGTTAATCCTGC | ||
| nheC-F | TGGATTCCAAGATGTAACG | 683 | |
| nheC-R | ATTACGACTTCTGCTTGTGC |
Statistical analysis
Obtained data are expressed as mean ± standard deviation of triplicates. Differences between groups were analyzed using Student’s t test and analysis of variance (ANOVA) was used to analyze differences in several groups. Duncan’s post hoc tests were performed to evaluate significant difference (p < 0.05) using SPSS 24 software (SPSS Inc, Chicago, IL, USA).
Results and discussion
Isolation and identification of Bacillus strain
Bacillus strain was isolated from kimchi by heating at 80 °C for 30 min and identified by 16S rRNA gene sequence analysis. By comparing sequencing results to nucleotide sequence database of National Center for Biotechnology Information Nucleotide using BLAST, P223 was identified as B. subtilis, sharing 99% sequence identities (similar to GenBank accession number, HQ202545.1) with 16S rRNA of known B. subtilis strains (data not shown). Our results indicated that B. subtilis P223 could be investigated for probiotic use because it is generally recognized as safe (GRAS) [16].
Resistance to artificial gastric juice and bile salts
Tolerance of B. subtilis P223 to artificial gastric juice (pH 2.5, 0.3% pepsin, 3 h) and bile salt (0.3% oxgall, 24 h) was measured to evaluate the potential of this Bacillus strain as probiotic. Results for the number of cells after treated with artificial gastric juice and bile salts are shown in Table 2. Vegetative cells of B. subtilis P223 were reduced by 2.73 log CFU/mL whereas B. clausii ATCC 700160 did not grow under acidic condition. On the other hand, the number of spore cells of B. subtilis P223 treated with artificial gastric acid showed no significant (p > 0.05) difference with the initial cell number while B. clausii ATCC 700160 was only reduced by 0.38 log CFU/mL. In the presence of artificial bile salts for 24 h, the number of Bacillus strains of both vegetative and spore cells was increased more than that in the control. Lee et al. [15] have reported that the number of vegetative cells of B. polyfermenticus KU3 is decreased by 3.18 log CFU/mL when it is treated with artificial gastric juice (pH 2.5, 0.1% pepsin, 2 h) and by 1.26 log CFU/mL when it is treated with artificial bile salts (0.3% oxgall, 24 h) while the number of spore cells of the strain is decreased only by 0.08 log CFU/mL in the presence of artificial gastric juice and by 0.16 log CFU/mL when treated with artificial bile salts. Our results are similar to results of a previous study showing that vegetative cells of B. megaterium JHT3 and B. subtilis DET6 had poor resistance to artificial gastric acid whereas their spore cells were resistant to gastric conditions [24].
Table 2.
Probiotic characterization of Bacillus strains
| Treatment | Viable cell number (Log CFU/mL) | |||
|---|---|---|---|---|
| Vegetative cell | Spore cell | |||
| B. clausii ATCC 700160 | B. subtilis P223 | B. clausii ATCC 700160 | B. subtilis P223 | |
| Resistance to artificial gastric juice and bile salts | ||||
| Initial cell number | 6.82 ± 0.35a1 | 6.88 ± 0.18b | 5.69 ± 0.21b | 5.04 ± 0.57a |
| 0.3% (w/v) pepsin, pH 2.5, 3 h | ND2 | 4.15 ± 0.22a | 5.31 ± 0.16a | 5.38 ± 0.59a |
| 0.3% (w/v) oxgall, 24 h | 7.50 ± 0.49a | 7.14 ± 0.32b | 7.86 ± 0.10c | 7.07 ± 0.36b |
| Adhesion to HT-29 cell | ||||
| Initial cell number | 7.57 ± 0.78a | 6.96 ± 0.09a | 5.57 ± 0.08a | 4.63 ± 0.05a |
| Adhesion cell number | 5.00 ± 0.05b | 4.52 ± 0.09b | 4.39 ± 0.08b | 3.36 ± 0.10b |
| β-glucuronidase | – | – | NT3 | NT |
| Hemolysis | – | – | NT | NT |
| Toxin genes (hblA, hblC, hblD, nheA, nheB, nheC) |
– | – | NT | NT |
1a–c Different superscript letters in the same row indicate statistical differences in each characteristic (p < 0.05). Values are expressed as mean ± SD (n = 3)
2Not detected
3Not tested
Adhesion capacity to HT-29 cells
Adhesion ability of Bacillus to human epithelial cells was determined for its potential to colonize human intestine epithelial cells. Capacity for adhesion was tested for vegetative and spore cells (Table 2). The number of vegetative cells of B. clausii ATCC 700160 and B. subtilis P223 was reduced by 2.57 and 2.44 log CFU/well, respectively, while the number of their spore cells was reduced by 1.18 and 1.27 log CFU/well, respectively. Lee et al. [15] have reported that B. polyfermenticus SCD was inoculated at a concentration of 8.06 ± 0.02 log CFU/well to Caco-2 cells. After 2 h of incubation, 6.85 ± 0.08 log CFU/well of cells were found to be attached to intestinal cells [15]. Additionally, L. rhamnosus GG was inoculated to HT-29 cells at a concentration of 8.0 log CFU/well. After 2 h of incubation, 6.93 log CFU/well of bacterial cells were attached [7]. Therefore, the capacity for adhesion of B. subtilis P223 was sufficient to colonize intestinal epithelial cells.
Autoaggregation and coaggregation activities
To investigate colonization of bacteria to intestinal cells and its inhibition on GI tract infection by pathogens, autoaggregation and coaggregation were performed as described previously [25]. After 4 h of incubation, B. clausii ATCC 700160 and B. subtilis P223 showed autoaggregation abilities of 85.10 ± 1.56% and 88.13 ± 3.55%, respectively (Table 3). After 24 h incubation, the value of B. clausii ATCC 700160 was increased while the value of B. subtilis P223 remained the same. Among different pathogens, S. aureus ATCC 6538 showed the highest activity after 4 h incubation while S. aureus ATCC 6538 and L. monocytogenes ATCC 15313 showed the highest activity after 24 h incubation. However, autoaggregation abilities of pathogens were much lower than those of Bacillus strains after 4 or 24 h incubation. Patel et al. [24] have reported that autoaggregation activity of B. subtilis DET6 is about 60% after 1 h incubation at 37 °C. Autoaggregation percentages of L. rhamnnosus GG are reported to be 14.2 ± 4.4% and 48.2 ± 3.5% after 4 and 24 h of incubation at room temperature, respectively [19].
Table 3.
Autoaggregation and coaggregation activities of Bacillus strains and pathogens
| Microorganisms | 4 h | 24 h |
|---|---|---|
| Autoaggregation activity (%) | ||
| Bacillus | ||
| B. clausii ATCC 700160 | 85.10 ± 1.56d1 | 93.42 ± 0.86e |
| B. subtilis P223 | 88.13 ± 3.55e | 86.03 ± 2.46d |
| Pathogens | ||
| S. aureus ATCC 6538 | 29.42 ± 2.14c | 66.35 ± 5.16c |
| S. enteritidis ATCC 13076 | 8.88 ± 0.94a | 21.19 ± 4.69a |
| L. monocytogenes ATCC 15313 | 12.26 ± 1.59b | 66.16 ± 3.67c |
| E. coli ATCC 25922 | 13.26 ± 3.38b | 51.90 ± 3.41b |
| Coaggregation activity (%) | ||
| B. clausii ATCC 700160 with | ||
| S. aureus ATCC 6538 | 62.37 ± 4.83e1 | 72.52 ± 2.48c |
| S. enteritidis ATCC 13076 | 50.32 ± 3.66ab | 63.08 ± 2.46a |
| L. monocytogenes ATCC 15313 | 51.73 ± 3.32bc | 72.20 ± 3.25c |
| E. coli ATCC 25922 | 51.81 ± 3.11bc | 70.16 ± 2.73bc |
| B. subtilis P223 with | ||
| S. aureus ATCC 6538 | 57.09 ± 3.48d | 68.96 ± 3.24b |
| S. enteritidis ATCC 13076 | 46.86 ± 4.43a | 61.03 ± 4.98a |
| L. monocytogenes ATCC 15313 | 54.07 ± 2.49cd | 79.19 ± 3.07d |
| E. coli ATCC 25922 | 49.74 ± 2.45ab | 70.19 ± 1.46bc |
1a–e Different superscript letters in the same row indicate statistical differences in each characteristic (p < 0.05). Values are expressed as mean ± SD (n = 3)
Coaggregation activity of B. clausii ATCC 700160 (62.37 ± 4.83%) and B. subtilis P223 (57.09 ± 3.48%) showed the highest properties with S. aureus ATCC 6538 after 4 h of incubation. After 24 h of incubation, S. aureus ATCC 6538 and L. monocytogenes ATCC 15313 showed higher coaggregation activities compared to other pathogens. In comparison with autoaggregation results, autoaggregation properties of pathogens affected coaggregation activities of Bacillus. Coaggregation percentages of Bacillus subtilis KATMIRA1933 in the presence of S. aureus, S. enteritica, L. monocytogenes, and E. coli were 34, 47.4, 48.2, and 50.3%, respectively [26].
Inhibition of Bacillus on adherence of pathogens to HT-29 cells
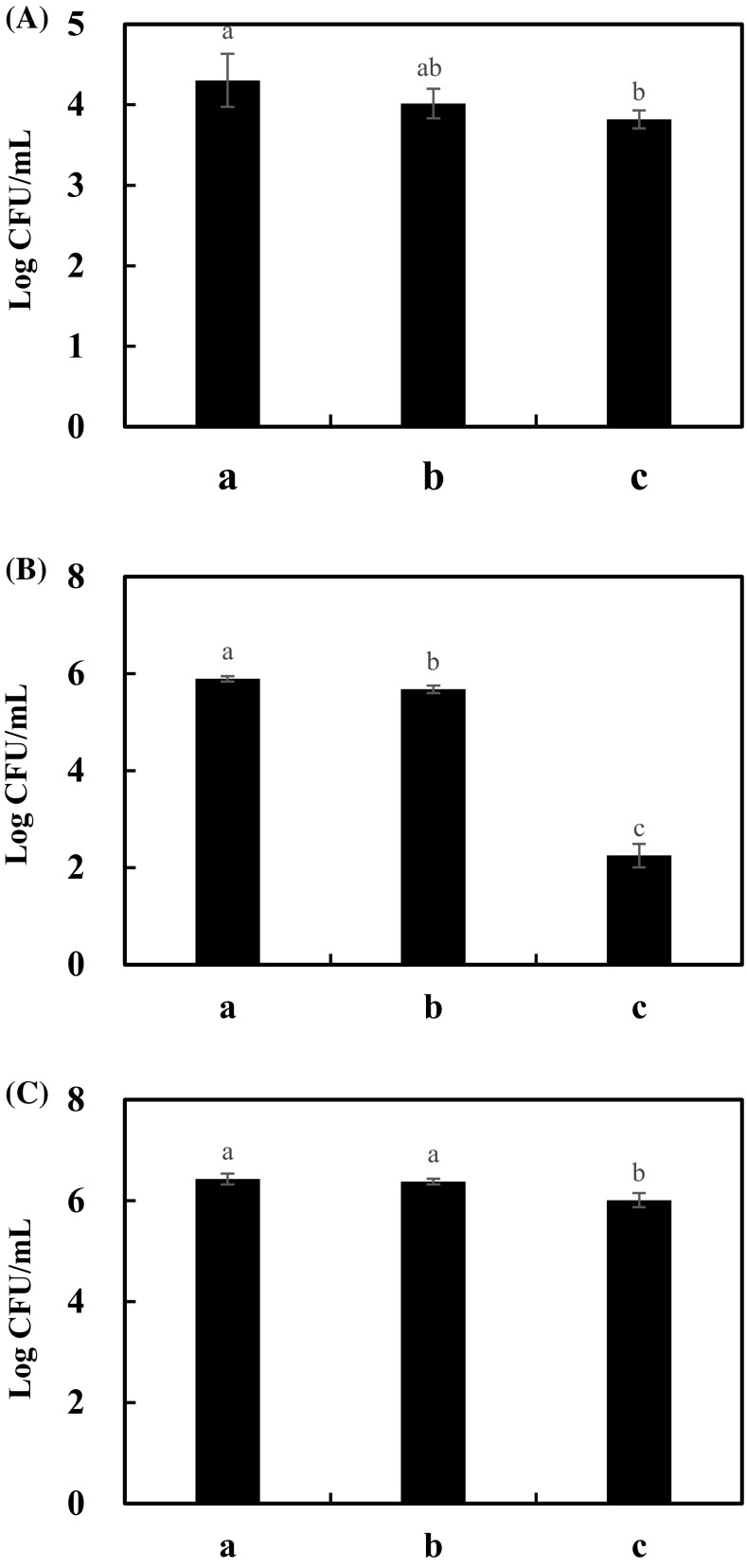
Adhesion to HT-29 cells was measured by counting viable cells (Fig. 1). Adherence of S. enteritidis ATCC 13076 to HT-29 cells was reduced by 0.29 and 0.49 CFU/mL when it was added with B. clausii ATCC 700160 and B. subtilis P223, respectively. When L. monocytogenes ATCC 15313 single culture was added to HT-29 cells, 5.90 log CFU/mL of L. monocytogenes ATCC 15313 were attached to HT-29 cells. However, when B. clausii ATCC 700160 and B. subtilis P223 was added into each well, the number of L. monocytogenes ATCC 15313 cells attached to HT-29 cells was decreased to 0.21 and 3.64 log CFU/mL, respectively. The adherence of E. coli ATCC 25922 to HT-29 cells was decreased more when it was incubated with B. subtilis P223 (0.42 log CFU/mL) compared to that when it was incubated with B. clausii ATCC 700160 (0.05 log CFU/mL). These results indicated that B. subtilis P223 could inhibit the adherence of pathogens (both Gram-positive and Gram-negative) to intestinal cells more than B. clausii ATCC 700160.
Fig. 1.
Inhibition activity of Bacillus strains against adherence of pathogens to intestinal cells. (A) S. enteritidis; (B) L. monocytogenes; (C) E. coli. a, pathogen; b, pathogen with B. clausii ATCC 700160; c, pathogen with B. subtilis P223. Different letters on each bar represent significant difference between values (p < 0.05)
Production of enzymes
Bacillus strains were tested for production of enzyme using API zym kit. B. clausii ATCC 700160 and B. subtilis P223 did not produce β-glucuronidase, a carcinogenic enzyme (Table 2). Amylase activity of B. clausii ATCC 700160 (1.32 ± 0.11 U/mL) was higher than that of B. subtilis P223 (0.73 ± 0.10 U/mL). Protease activity of B. subtilis P223 (448.08 ± 12.42 U/mL) was similar to that of B. clausii ATCC 700160 (431.63 ± 13.53 U/mL). Amylase and protease activities are necessary for probiotics with digestive effect as starter of fermented foods because these enzymes can generate amino acids, sugars, organic acids, and diverse small compounds [5].
Antibiotic susceptibility of Bacillus strains
Antibiotic susceptibility of probiotics should be measured for safety purpose. Antibiotic resistance gene transmission can occur due to transposons, plasmids, and bacterial gene mutation, leading to new antibiotic resistant strains [27]. B. clausii ATCC 700160 and B. subtilis P223 were found to be only resistant to streptomycin among 8 kinds of antibiotics (data not shown). This is similar to B. subtilis KATMIRA1933 and B. amyloliquefaciens B-1895. They are also resistant to streptomycin and intermediately resistant to chloramphenicol [26].
Hemolysis and enterotoxin production
B. clausii ATCC 700160 and B. subtilis P223 did not show hemolysis on sheep blood agar (Table 2). Similarly, B. polyfermenticus CJ6 does not cause hemolysis on horse blood agar [28]. α-Hemolysis and no-hemolysis are considered to be safe whereas β-hemolysis is considered harmful [5].
For detection of enterotoxins, PCR and electrophoresis were performed. B. cereus KCCM 11341 carried enterotoxin genes (hblA, hblC, hblD, nheA, and nheC; data not shown). However, B. clausii ATCC 700160 and B. subtilis P223 did not carry any of these six genes (Table 2). B. cereus probiotics from products Subtyl and BiosubtylDL were found to carry enterotoxin genes, whereas B. subtilis PY79 did not carry such genes [29].
References
- 1.FAO/WHO, Guideline for the Evaluation of Probiotics in Food. London, Ontario, Canada (2002)
- 2.Almudena GR, Dolores GL, Adelaida EF, Teresa R, Begoña B, M.Victeroa MA. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 44: 220–225 (2014) [DOI] [PubMed]
- 3.Angmo K, Kumari A, Savitri, Bhalla TC. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci. Technol. 2016;66:428–435. doi: 10.1016/j.lwt.2015.10.057. [DOI] [Google Scholar]
- 4.Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT- Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- 5.Lee S, Lee J, Jin YI, Jeong JC, Chang YH, Lee Y, Jeong Y, Kim M. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT-Food Sci. Technol. 2016;79:518–524. doi: 10.1016/j.lwt.2016.08.040. [DOI] [Google Scholar]
- 6.Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci. Technol. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- 7.Lee KW, Shim JM, Park SK, Heo HJ, Kim HJ, Ham KS, Kim JH. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT-Food Sci. Technol. 2016;71:130–137. doi: 10.1016/j.lwt.2016.03.029. [DOI] [Google Scholar]
- 8.Kim B, Song JL, Ju JH, Kang SA, Park KY. Anticancer effects of kimchi fermented for different times and with added ingredients in human HT-29 colon cancer cells. Food Sci. Biotechnol. 2015;24:629–633. doi: 10.1007/s10068-015-0082-3. [DOI] [Google Scholar]
- 9.Choi SM, Jeon YS, Rhee SH, Park KY. Fermentation characteristics and antimutagenicity of kimchi that prepared with different ratio of seed in red pepper powder. J. Cancer Prev. 2002;7:51–59. [Google Scholar]
- 10.Park JM, Shin JH, Gu JG, Yoon SJ, Song JC, Jeon WM, Suh HJ, Chang UJ, Yang CY, Kim JM. Effect of antioxidant activity in kimchi during a short-term and over-ripening fermentation period. J. Biosci. Bioeng. 2011;112:356–359. doi: 10.1016/j.jbiosc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Kim B, Park KY, Kim HY, Ahn SC, Cho EJ. Anti-aging effects and mechanisms of kimchi during fermentation under stress-induced premature senescence cellular system. Food Sci. Biotechnol. 2011;20:643–649. doi: 10.1007/s10068-011-0091-9. [DOI] [Google Scholar]
- 12.Lee H, Yoon H, Ji Y, Kim H, Park H, Lee J, Shin H, Holzapfel W. Functional properties of Lactobacillus strains isolated from kimchi. Int. J. Food Microbiol. 2011;145:155–161. doi: 10.1016/j.ijfoodmicro.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Khan I, Kang SC. Probiotic potential of nutritionally improved Lactobacillus plantarum DGK-17 isolated from Kimchi–A traditional Korean fermented food. Food Control. 2016;60:88–94. doi: 10.1016/j.foodcont.2015.07.010. [DOI] [Google Scholar]
- 14.Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic in cottage cheese. Food Sci. Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee NK, Son SH, Jeon EB, Jung GH, Lee JY, Paik HD. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods. 2015;14:513–518. doi: 10.1016/j.jff.2015.02.019. [DOI] [Google Scholar]
- 16.Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214–220. doi: 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Setlow P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014;196:1297–1305. doi: 10.1128/JB.01455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang H, Zhang L, Liu W, Zhang Y, Zhang X, Sun T. In vitro assessment of probiotic properties of Bacillus isolated from naturally fermented congee from inner Mongolia of China. World J. Microb. Biot. 2010;26:1369–1377. doi: 10.1007/s11274-010-0309-7. [DOI] [Google Scholar]
- 19.Tareb R, Bernardeau M, Gueguen M, Vernoux JP. In vitro characterization of aggregation and adhesion properties of viable and heat-killed forms of two probiotic Lactobacillus strains and interaction with foodborne zoonotic bacteria, especially Campylobacter jejuni. J. Med. Microbiol. 2013;62:637–649. doi: 10.1099/jmm.0.049965-0. [DOI] [PubMed] [Google Scholar]
- 20.Bernfeld P. Methods in Enzymology. Amylase, α and β. pp 149–158. Colowick SP, Kaplan NO (ed), Academic Press.0 New York, USA (1955)
- 21.Lee MS, Lee NK, Chang KH, Choi SY, Song CK, Paik HD. Isolation and characterization of a protease-producing bacterium, Bacillus amyloliquefaciens P27 from meju as a probiotic starter for fermented meat products. Korean J. Food Sci. An. 2010;30:804–810. doi: 10.5851/kosfa.2010.30.5.804. [DOI] [Google Scholar]
- 22.Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement M100-S24. Clinical and Laboratory Standards Institute. Wayne, PA, USA (2014)
- 23.Chon JW, Kim JH, Lee SJ, Hyeon JY, Song KY, Park C, Seo KH. Prevalence, phenotypic traits and molecular characterization of emetic toxin-producing Bacillus cereus strains isolated from human stools in Korea. J. Appl. Microbiol. 2012;112:1042–1049. doi: 10.1111/j.1365-2672.2012.05277.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel AK, Ahire JJ, Pawar SP, Chaudhari BL, Chincholkar SB. Comparative accounts of probiotic characteristics of Bacillus spp. isolated from food wastes. Food Res. Int. 2009;42:505–510. doi: 10.1016/j.foodres.2009.01.013. [DOI] [Google Scholar]
- 25.Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008;226:1065–1073. doi: 10.1007/s00217-007-0632-x. [DOI] [Google Scholar]
- 26.AlGburi A, Volski A, Cugini C, Walsh EM, Chistyakov VA, Mazanko MS, Bren AB, Dicks LM, Chikindas ML. Safety Properties and Probiotic Potential of Bacillus subtilis KATMIRA1933 and Bacillus amyloliquefaciens B-1895. Adv. Microbiol. 2016;6:432–452. doi: 10.4236/aim.2016.66043. [DOI] [Google Scholar]
- 27.Teuber M, Meile L, Schwarz F. Acquired antibiotic resistance in lactic acid bacteria from food. A. Van. Leeuw. J. Microb. 1999;76:115–137. doi: 10.1023/A:1002035622988. [DOI] [PubMed] [Google Scholar]
- 28.Jung JH, Chang HC. Bacillus polyfermenticus CJ9, isolated from meju, showing antifungal and antibacterial activities. Kor. J. Microbiol. Biotechnol. 2009;37:340–349. [Google Scholar]
- 29.Duc LH, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl. Environ. Microb. 2004;70:2161–2171. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]