Abstract
A novel process for producing wheat gluten enzyme hydrolyzates (WGEHs) was developed, using combinations of Flavourzyme 500MG, Alcalase 2.4L, Protamex, and Marugoto E at the high pressure of 300 MPa, and the resultant hydrolyzates were analyzed for electrophoretic and hydrolytic properties. It was found that multiple-enzyme treatments increased the proportion of the electrophoretic bands less than 5 kDa in the hydrolyzates greatly both at ambient pressure and 300 MPa compared with one-enzyme hydrolysis. The contents of total soluble solids in the WGEHs increased considerably up to 89.75% according to the increase in the number of enzymes used at 300 MPa compared with 79.37% found for the ambient-pressure hydrolysis. These characteristics together with the contents of soluble nitrogen and free amino acids clearly indicated that the high-pressure enzymatic process of this study is an efficient method for obtaining WGEHs with increased degree of hydrolysis.
Keywords: Production, Wheat gluten enzyme hydrolyzate, High pressure, Electrophoretic property, Hydrolytic property
Introduction
Wheat gluten (WG) or simply gluten is composed of 70% ethanol-extractable gliadins (β- and γ-gliadin) having a molecular mass range of 42,000–47,000 Da and heterogeneous unextractable glutenin having an average molecular mass of 2–3 million Da and containing small molecules approximately 50,000 Da as well as very large ones [1]. Glutenin and gliadin are rich in glutamic acid, glutamine, and proline [2], which suggests a way of using WG as seasoning agent after an appropriate hydrolytic procedure. It is well known that intact WG as one of the most common food allergens causes celiac disease—a small intestinal allergenic disease and a food-sensitive enteropathy triggered by ingesting WG proteins and related proteins from barley, rye, and some varieties of oat.
Protein allergenicity is decreased generally upon hydrolysis of soybean, buckwheat, and egg. Thus, enzymatic hydrolysis of WG has been expected as a method resolving gluten allergenicity and producing valuable food materials for use as condiments [3–5]. Until now, some studies dealing with the enzymatic hydrolysis of WG and characterization for the resultant hydrolyzates have been reported [6, 7]. However, these reports were conducted at ambient pressure and had some limitations with respect to reaction conditions such as reaction time and temperature, duration of hydrolysis, and product yield according to the catalytic properties of the proteases used. Moreover, high salinity might be necessary for the reaction mixture to restrain microbial contamination during the enzymatic hydrolysis [8]. Thus implementation of a new process for producing the degradation products with low salt concentration within a short time is strongly demanded to meet the growing need for low-salt seasonings.
It is acknowledged that reaction rate of an enzyme increases with increasing reaction temperature until thermal inactivation of the enzyme becomes predominant. In the case of some pressure-tolerant enzymes such as trypsin, high-pressure treatment to a certain critical temperature reduces the thermal inactivation of these enzymes by preferential hydration that is crucial for maintaining structure, function, and stability of globular proteins [9–12]. Thus these enzymes can be used efficiently at elevated temperatures, which might result in an increase in reaction rate and product yield. In addition, the protease reaction at high pressure does not require a high salt concentration for restraining microbial contamination, which is expected to be more favorable for the action of the enzymes used [8].
In light of the above literature survey, an application of high pressure at an elevated temperature to the enzymatic hydrolysis of WG, using pressure-tolerant proteases, can provide considerable merits in producing wheat gluten enzyme hydrolyzates (WGEHs) with improved sensory and functional properties, and enhanced degree of hydrolysis. In one previous report [13], pressure-tolerance of various proteases including reagent-grade enzymes such as trypsin and thermolysin, and commercial enzymes such as Flavourzyme 500MG and Alcalase 2.4L was confirmed at a medium high pressure of 300 MPa. In another previous report [9], it was found that the thermal inactivation of pressure-tolerant proteases such as Marugoto E was retarded at higher temperatures including 50 °C. In the present study, a novel enzymatic process for producing WGEHs from WG suspension was tried to be developed using combinations of some pressure-tolerant proteases at 300 MPa at a relatively high operating temperature of 50 °C, which seems to be the advanced feature of this study succeeding the previous ones. In addition, the characteristics of the resultant WGEHs were determined together with those produced at ambient pressure.
Materials and methods
Reagents and materials
Flavourzyme 500MG (aminopeptidase, from Aspergillus oryzae), Alcalase 2.4L (subtilisin, from Bacillus licheniformis), Marugoto E, and Protamex (from B. licheniformis and B. amyloliquefaciens) that are abbreviated as F, A, M, and P respectively were used solely or in combination with other(s) to produce WGEHs. Out of these enzymes, F, A, and P were products of Novozymes A/S (Bagsvaerd, Denmark), while M was a product of Supercritical Technology Research Corporation (Hiroshima, Japan). They were selected for producing WGEHs because of their relative pressure-tolerance at 300 MPa [13]. WG (food additive-grade) was obtained from a local market in Seoul, Korea. Mini-PROTEAN® TGX SDS-PAGE gels (12%, 10 wells, 30 μL), 10 × Tris/glycine/SDS buffer, and Bio-Safe™ Coomassie G-250 Stain (phosphoric acid, less than 5%) from Bio-Rad (Hercules, CA, USA) were used for SDS-PAGE. All other chemicals were guaranteed reagents from various suppliers and double distilled water was used throughout this study.
Production of WGEHs
WGEHs were produced according to the method previously reported with slight modification [14]. That is, a vinyl pouch containing 12% (w/v) WG suspension in distilled water was treated as follows inside a single-vessel (150-mL capacity) high-pressure equipment (DP-SHPL-015L-400, Dima Puretech, Incheon, Korea). After adjusting the temperature of the suspension in the pouch to 50 °C, single or combined enzyme treatment was done at the dosage for F, A, M, and P of 0.48 g, 66.7 μL (density; 1.2 g/mL), 0.156 g, and 0.12 g per 100 mL-suspension, respectively. Afterward, the pouch was submerged in the pressure vessel equilibrated with distilled water that was used as the pressure-transmitting fluid at 50 °C, followed by closing the vessel cover. Pressure was built up carefully for minimizing adiabatic heating until the pre-set pressure of 300 MPa was reached. The vessel was maintained at this pressure for 1 h at 50 °C for WG hydrolysis. For comparison, the enzymatic hydrolysis of WG at ambient pressure was also conducted for 1 h at 50 °C. Finally, the reaction mixture obtained was heated for 10 min at 90 °C, immediately followed by centrifugation at 16,300×g for 30 min at 10 °C. The supernatant was designated as the corresponding WGEHs.
Determination of indices of enzymatic hydrolysis
Individual 10-mL samples of WGEHs and WG suspensions were added to pre-weighed aluminum dishes overlaid with sea sand and then measured until constant mass using a dry oven at 105 °C. Total soluble solids (TSS) were calculated as follow:
The Kjeldahl method was used with 5 mL samples to determine total water-soluble nitrogen (TWSN) and total nitrogen (TN). To measure trichloroacetic acid (TCA)-soluble nitrogen (TCASN), TCA was added to each sample at 10% concentration and the mixture was centrifuged at 12,300×g for 20 min at 10 °C after setting still at room temperature for 30 min. Ten milliliters of the resultant supernatant were used to measure TCASN according to the Kjeldahl method. Degree of hydrolysis (DH) was calculated from the contents of TCASN and TN as follows [15]:
SDS-PAGE for samples
SDS-PAGE was conducted using 12% Mini-PROTEAN® TGX slab gel installed in a Power Pac™ Basic Power Supply (Bio-Rad). For use as running buffer, 25 mM Tris-192 mM glycine (pH 8.3, containing 0.1% SDS) was used. Samples were prepared by mixing 10 μL of individual hydrolyzates and 10 μL of the sample buffer that was made by mixing 950 μL of 62.5 mM Tris–HCl (pH 6.8, containing 25% glycerol, 2% SDS, and 0.01% bromophenol blue) and 50 μL of 2-mercaptoethanol. To the vertical slab gel in the electrophoresis unit filled with the running buffer, 20 μL aliquots of samples and 5 μL aliquots of Precision Plus Protein™ Dual Standards (Bio-Rad) that included 12 recombinant proteins (2–250 kDa) showing 9 blue- and 3 pink-stained bands were loaded in the corresponding wells. The electrophoresis unit was connected to the power supply, and electrophoresis was conducted at a constant voltage of 80 V for 1 min and then at a constant voltage of 200 V for 40–60 min. The developed gel was dipped into Bio-Safe™ Coomassie G-250 Stain in a container on an undulating shaker for 90 min and washed with distilled water twice for 2 h. SDS-PAGE electropherograms were obtained using a Gel Doc™ EZ Imager (Bio-Rad) that was operated by Image Lab (version 4.1, Bio-Rad).
Determination of free amino acids
Free amino acids in WGEHs were determined with Agilent 7890B GC-FID (Agilent Technologies, Santa Clara, CA, USA) for the samples prepared by chloroformate derivatization using KG0-7167 Easy-fast Amino Acid Sample Testing Kit (Phenomenex, Torrance, CA, USA). A ZB-AAA column (10 m × 0.25 mm × 0.20 μm) of the same company was used for analysis. Column injection was done in split mode (10:1) at 250 °C using 2 μL of sample and the carrier gas was helium flowing at 1.5 mL/min. The oven temperature was increased from 110 to 320 °C at 35 °C/min and the FID temperature was set at 320 °C. Amino acid standards (Phenomenex) at 200 μM were used and norvaline at 200 μM eluting at 2.80 min was used as internal standard.
Data analysis
When necessary, data were presented as mean ± standard deviation (SD) of three replicate determinations. Analysis of variance (ANOVA) was applied to the data of TSS and soluble nitrogen to determine significant differences at p < 0.05 using SAS (SAS Institute, Cary, NC, USA).
Results and discussion
Production of WGEHs according to protease combinations
The electropherograms of WGEHs obtained with two different media for enzymatic hydrolysis, 0.1 M sodium phosphate buffer (pH 7.0) and distilled water, were very similar irrespective of the number of proteases used and operating pressures (data not shown). Taking into account convenience and applicability, distilled water was selected as the medium for further study.
For the production of WGEHs in distilled water, various protease combinations using F as compulsory element for two-, three-, and four-enzyme hydrolysis because of its exo-type action mechanism were used at ambient pressure and 300 MPa. As shown in lanes 2 and 3 of Fig. 1D, the electropherograms of the unhydrolyzed WG suspension treated at ambient pressure and 300 MPa were almost similar. Thus it was presumed that changes in protein structure including association, dissociation, and rearrangement did not take place meaningfully during the high-pressure treatment of WG. On the whole, the molecular masses of the bands of WGEHs after enzymatic hydrolysis were present less than 50 kDa irrespective of types of protease combination. However, the electropherograms of the hydrolyzates in Fig. 1 revealed an abundance of far migrating bands approximately below 5 kDa in the cases of multiple-enzyme treatments [16]. Compared with one-enzyme hydrolysis, the relative proportion of the bands less than 5 kDa were drastically increased by the enzymatic hydrolysis using combinations of two or more enzymes at both operating pressures (Fig. 1A–D), which indicated that the hydrolytic efficiency of multiple-enzyme treatments was better than that of single-enzyme treatment. However, no meaningful differences in band pattern were found between the hydrolyzates produced at ambient pressure and 300 MPa at the same enzyme combination. This fact might suggest that some portions of low molecular mass peptides produced by the hydrolysis at 300 MPa moved faster than bromophenol blue tracking dye having the molecular mass of 670 Da during SDS-PAGE and were not reflected in the corresponding electropherograms. When analyzed for the peptide profiles by HPLC, most of the peptides in the hydrolyzates had shorter retention time than standard peptides such as angiotensin II (FW 1031) and Leu enkephalin (FW 569) (data not shown).
Fig. 1.
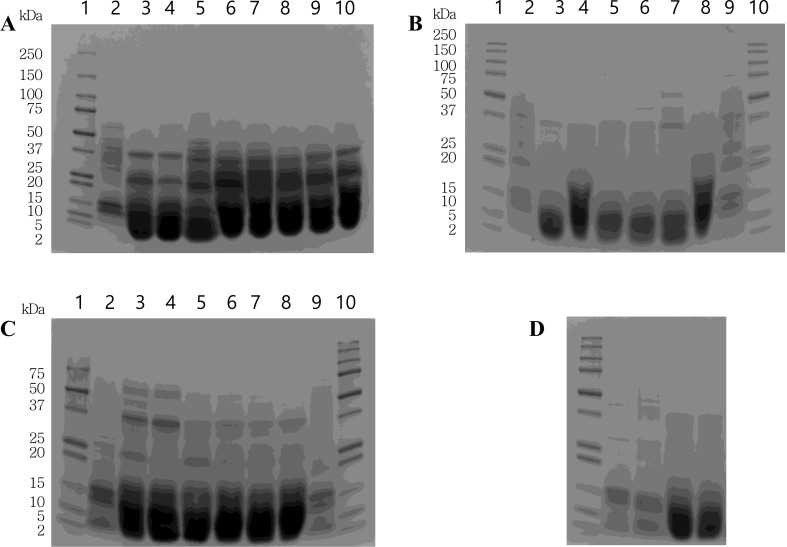
Electropherograms of WGEHs produced in distilled water for 1 h at 50 °C by one- (A), two- (B), three- (C), and four-enzyme (D) hydrolysis at ambient pressure and 300 MPa. Lane description. (A): 1 molecular mass markers; 2 12% WG without enzyme treatment (ambient pressure); 3 F (ambient pressure); 4 F (300 MPa); 5 A (ambient pressure); 6 A (300 MPa); 7 P (ambient pressure); 8 P (300 MPa); 9 M (ambient pressure); 10 M (300 MPa). (B): 1 molecular mass markers; 2 12% WG without enzyme treatment (ambient pressure); 3 F–A (ambient pressure); 4 F–A (300 MPa); 5 F–P (ambient pressure); 6 F–P (300 MPa); 7 F–M (ambient pressure); 8 F–M (300 MPa); 9 12% WG without enzyme treatment (300 MPa); 10 molecular mass markers. (C): 1 molecular mass markers; 2 12% WG without enzyme treatment (ambient pressure); 3 F–A–P (ambient pressure); 4 F–A–P (300 MPa); 5 F–A–M (ambient pressure); 6 F–A–M (300 MPa); 7 F–P–M (ambient pressure); 8 F–P–M (300 MPa); 9 12% WG without enzyme treatment (300 MPa); 10 molecular mass markers. (D): 1 molecular mass markers; 2 12% WG without enzyme treatment (ambient pressure); 3 12% WG without enzyme treatment (300 MPa); 4 F–A–P–M (ambient pressure); 5 F–A–P–M (300 MPa). For all hydrolytic processes, F, A, P, and M denote Flavourzyme 500MG, Alcalase 2.4L, Protamex, and Marugoto E, respectively
Determination of TSS in WGEHs
It has been reported that enzymatic hydrolysis of proteinaceous substrates increases soluble fractions and thus TSS [17–20]. Based on this, TSS was used as an important index of protein hydrolysis in this study. The contents of TSS were determined for the WGEHs produced at ambient pressure and 300 MPa. As shown in Table 1, the contents of TSS in the hydrolyzates increased according to the increase in the number of enzymes of the protease combinations at both operating pressures with the resultant TSS contents of 79.37 and 89.75% in the case of four-enzyme hydrolysis at ambient pressure and 300 MPa, respectively. This trend is well evidenced by ANOVA applied to TSS contents in Table 1. Also, the TSS contents in the hydrolyzates were remarkably higher than those of 4.89 and 6.16% respectively found for the blanks undergoing treatments at ambient pressure and 300 MPa irrespective of types of protease combination. Meanwhile, the TSS contents in the hydrolyzates produced at 300 MPa were mostly higher and significantly different at p < 0.05 by ANOVA than those in the hydrolyzates produced at ambient pressure at the same protease combination, which indicated that hydrolytic efficiency was better at the 300 MPa-procedure. In addition, the TSS contents found for the WGEHs produced at 300 MPa were considerably higher than those found for anchovy fine powder enzyme hydrolyzates produced at 300 MPa [14]. At this stage, the number of protease combinations was reduced on the basis of one per number of enzymes used as follows. In the cases of two-, three-, and four-enzyme hydrolysis, F–A, F–A–M, and F–A–P–M respectively, which were found to maintain the contents of TSS in the hydrolyzates at relatively higher levels, were used. On the other hand, F was selected for one-enzyme hydrolysis as described above.
Table 1.
Changes in TSS of WGEHs according to protease combinations
| Protease combination | TSS (%) | |
|---|---|---|
| Ambient pressure | 300 MPa | |
| Blank1 | 4.89 ± 0.09hB | 6.16 ± 0.10jA |
| F2 | 42.00 ± 0.18fB | 67.23 ± 0.24gA |
| A | 25.58 ± 0.21gB | 43.65 ± 0.50iA |
| P | 50.85 ± 0.36eA | 49.43 ± 0.33hB |
| M | 49.48 ± 0.38eB | 74.08 ± 0.84fA |
| F–A3 | 75.78 ± 3.15cA | 80.84 ± 1.15dA |
| F–P | 76.06 ± 0.36cB | 81.06 ± 0.27dA |
| F–M | 69.10 ± 0.88 dB | 78.05 ± 1.15eA |
| F–A–P4 | 77.10 ± 0.68bcB | 83.04 ± 0.63cA |
| F–A–M | 76.84 ± 0.65bcB | 84.32 ± 0.45bA |
| F–P–M | 78.29 ± 0.79abB | 84.19 ± 0.26bA |
| F–A–P–M5 | 79.37 ± 0.90aB | 89.75 ± 0.82aA |
For all hydrolytic processes, F, A, P, and M respectively denote Flavourzyme 500MG, Alcalase 2.4L, Protamex, and Marugoto E, while TSS means total soluble solids. Values of TSS are mean ± SD (n = 3). Means within the same column with different lowercase letters are significantly different at p < 0.05 by ANOVA. Means within the same row with different capital letters are significantly different at p < 0.05 by ANOVA
1Twelve percent WG suspension
2One-enzyme hydrolysis
3Two-enzyme hydrolysis
4Three-enzyme hydrolysis
5Four-enzyme hydrolysis
Determination of soluble nitrogen in WGEHs
For the WGEHs that were prepared using the above protease combinations, the changes in soluble nitrogen were determined (Table 2). Although the contents of TWSN and TCASN increased simultaneously according to the increase in the number of enzymes used at ambient pressure and 300 MPa, the contents of soluble nitrogen in the hydrolyzates were considerably higher and significantly different at p < 0.05 by ANOVA in the case of the enzymatic hydrolysis at 300 MPa at the same protease combination. The blank that underwent the high-pressure treatment at 300 MPa had the similar contents of TWSN and TCASN to that treated at ambient pressure. The TCASN/TWSN ratios for the WGEHs produced at ambient pressure and 300 MPa were in the ranges of 48.98–96.40 and 92.93–97.56%, respectively. This fact meant that most soluble nitrogenous compounds in the WGEHs produced at 300 MPa were present as low molecular mass peptides irrespective of the number of enzymes used for hydrolysis [21, 22]. As shown in Table 2, DH was calculated from the contents of TCASN and TN for the WGEHs produced at ambient pressure and 300 MPa. It is generally acknowledged that DH can be defined as the ratio of the number of peptide bonds hydrolyzed to the number of total peptide bonds [23]. In the case of the ambient-pressure hydrolysis, DH increased from 1.74% for the blank to 64.01% for the hydrolyzate produced with the enzyme combination of F–A–P–M. On the other hand, DH increased from 3.03% for the blank to 71.98% for the hydrolyzate produced using the same enzyme combination at 300 MPa. Although DH increased according to the increase in the number of enzymes used at both operating pressures, the degree of increase was more conspicuous in the case of the high-pressure hydrolysis. This fact seemed to indicate that the high-pressure hydrolysis at 300 MPa is an efficient procedure for producing peptide-based flavoring components from WG because a DH value of 30% or more is generally required for protein hydrolyzates to elicit taste and flavor [24]. This fact further suggested that the current high-pressure procedure could be exploitable for producing enzyme hydrolyzates with higher degree of hydrolysis from other proteinaceous substrates in the food industry.
Table 2.
Changes in soluble nitrogen and DH of WGEHs according to protease combinations
| Protease combination | Ambient pressure | 300 MPa | ||||
|---|---|---|---|---|---|---|
| TWSN (%) | TCASN (%) | DH (%)1 | TWSN (%) | TCASN (%) | DH (%) | |
| Blank2 | 0.049 ± 0.006 dB | 0.030 ± 0.002 dB | 1.74 | 0.056 ± 0.003eA | 0.052 ± 0.003eA | 3.03 |
| F | 0.600 ± 0.012cB | 0.400 ± 0.002cB | 23.70 | 0.990 ± 0.004dA | 0.920 ± 0.011dA | 53.81 |
| F–A | 0.980 ± 0.060bA | 0.480 ± 0.007bB | 28.13 | 1.086 ± 0.029cA | 1.024 ± 0.017cA | 59.63 |
| F–A–M | 1.140 ± 0.034aB | 1.091 ± 0.012aB | 63.54 | 1.230 ± 0.023bA | 1.200 ± 0.013bA | 70.18 |
| F–A–P–M | 1.140 ± 0.037aB | 1.099 ± 0.013aB | 64.01 | 1.290 ± 0.018aA | 1.230 ± 0.016aA | 71.98 |
The content of TN in 12% WG suspension without enzyme treatment was 1.717 ± 0.011%. For all hydrolytic processes, F, A, P, and M respectively denote Flavourzyme 500MG, Alcalase 2.4L, Protamex, and Marugoto E, while TWSN, TCASN, and DH respectively mean total water-soluble nitrogen, trichloroacetic acid-soluble nitrogen, and degree of hydrolysis. Values of TWSN and TCASN are mean ± SD (n = 3). Means within the same column with different lowercase letters are significantly different at p < 0.05 by ANOVA. Means within the same row with different capital letters are significantly different at p < 0.05 by ANOVA
1TCASN/TN × 100
2Twelve percent WG suspensions that underwent pressure treatments without enzymes at ambient pressure and 300 MPa, respectively
Free amino acids in WGEHs
The contents of free amino acids instead of total amino acids are generally more important for evaluating hydrolytic procedures using proteolytic enzymes. Accordingly, the contents of free amino acids were determined for the WGEHs that were produced by one-, two-, three-, and four-enzyme hydrolysis at 300 MPa, together with the blank (12% WG suspension) that underwent the high-pressure treatment at 300 MPa without enzymes. As shown in Table 3, the contents of total free amino acids increased from 222 for the blank to 29,297 mg/kg for the WGEH produced using the protease combination of F–A–P–M at 300 MPa, which coincided with the result of a previous report on pressurized beef [25]. In a separate analysis on free amino acids in the WGEHs produced using the protease combination of F–A–P–M at ambient pressure and 300 MPa, the content of total free amino acids was slightly higher in the case of the 300 MPa-hydrolysis (data not shown). In the blank of Table 3, aspartic acid, asparagine, tryptophan, leucine, and some uncommon amino acids were individually present in the range of 10–30 mg/kg. After the high-pressure enzymatic hydrolysis, the contents of total free amino acids increased drastically at all protease combinations and the degree of increase in each amino acid was mostly correlated with the number of proteases used. By the high-pressure enzymatic hydrolysis, the contents of leucine, valine, isoleucine, serine, phenylalanine, tyrosine, methionine, lysine, and histidine increased significantly up to several hundred-fold or more. Furthermore, alanine, glycine, serine, aspartic acid, glutamic acid, and glutamine which have sweet or savory taste also increased meaningfully.
Table 3.
Contents of free amino acids in WGEHs produced at 300 MPa
| Retention time (min) | Content (mg/kg)a | ||||
|---|---|---|---|---|---|
| Blank | F | F–A | F–A–M | F–A–P–M | |
| Alanine (2.38) | 7 | 618 | 636 | 770 | 713 |
| Sarcosine (2.44) | 4 | 7 | 8 | 11 | 11 |
| Glycine (2.49) | 2 | 180 | 236 | 349 | 336 |
| α-Aminobutyric acid (2.58) | 0 | 16 | 15 | 14 | 15 |
| Valine (2.68) | 6 | 1845 | 1998 | 2578 | 2919 |
| Leucine (2.87) | 10 | 4381 | 4530 | 5811 | 6773 |
| Isolucine (2.93) | 4 | 1914 | 1966 | 2532 | 3605 |
| Threonine (3.08) | NDb | 1 | 8 | 12 | 19 |
| Serine (3.14) | 2 | 816 | 959 | 1169 | 1206 |
| Proline (3.26) | 3 | 283 | 255 | 569 | 487 |
| Asparagine (3.59) | 12 | 24 | 18 | 21 | 22 |
| Aspartic acid (3.83) | 18 | 219 | 212 | 299 | 242 |
| Methionine (3.88) | ND | 660 | 786 | 1115 | 1076 |
| 4-Hydroxyproline (4.01) | ND | 40 | 47 | 69 | 73 |
| Glutamic acid (4.16) | 6 | 161 | 185 | 227 | 197 |
| Phenylalanine (4.22) | 8 | 2479 | 2767 | 3717 | 3994 |
| α-Aminoadipic acid (4.44) | 31 | 61 | 61 | 89 | 80 |
| α-Aminopimelic acid (4.62) | 17 | 365 | 439 | 789 | 995 |
| Glutamine (4.68) | ND | 155 | 238 | 384 | 522 |
| Ornithine (4.95) | 26 | 51 | 77 | 94 | 99 |
| Glycine-proline (5.12) | ND | 9 | 8 | 14 | 13 |
| Lysine (5.36) | 4 | 517 | 540 | 703 | 597 |
| Histidine (5.55) | 4 | 1556 | 1650 | 2277 | 2072 |
| Hydroxylysine (5.74) | 31 | 270 | 336 | 651 | 676 |
| Tyrosine (5.82) | 6 | 1194 | 1219 | 1691 | 1612 |
| Proline-hydroxyproline (6.06) | ND | 30 | 34 | 41 | 42 |
| Tryptophan (6.14) | 21 | 471 | 576 | 878 | 714 |
| Cystine (6.75) | ND | 170 | 140 | 215 | 187 |
| Total free amino acids | 222 | 18,493 | 19,944 | 27,089 | 29,297 |
Blank indicates 12% WG suspension. For all hydrolytic processes, F, A, P, and M respectively denote Flavourzyme 500MG, Alcalase 2.4L, Protamex, and Marugoto E
aWet sample basis
bNot detected
It was concluded that the high-pressure hydrolysis using proteases at 300 MPa accelerates WG degradation conspicuously compared with the ambient-pressure counterpart. Thus the process of this study seemed to be competent for producing flavoring components from WG. Further studies on sensory properties and functionality for the hydrolyzates produced might be required shortly.
Acknowledgements
This study was conducted as one part (E0143053307) of the research project of Development of High Pressure Process for Foods, Korea Food Research Institute, Republic of Korea.
References
- 1.Jones RW, Babcock GE, Taylor NW, Senti FR. Molecular weights of wheat gluten fractions. Arch. Biochem. Biophys. 1961;94:483–488. doi: 10.1016/0003-9861(61)90076-5. [DOI] [PubMed] [Google Scholar]
- 2.Rombouts I, Lamberts L, Celus I, Lagrain B, Brijs K, Delcour JA. Wheat gluten amino acid composition analysis by high-performance anion-exchange chromatography with integrated pulsed amperometric detection. J. Chromatogr. A. 2009;1216:5557–5562. doi: 10.1016/j.chroma.2009.05.066. [DOI] [PubMed] [Google Scholar]
- 3.Hacini-Rachinel F, Vissers YM, Doucet-Ladeveze R, Blanchard C, Demont A, Perrot M, Panchaud A, Prioult G, Mercenier A, Nutton S. Low-allergenic hydrolyzed egg induces oral tolerance in mice. Int. Arch. Allergy Immunol. 2014;164:64–73. doi: 10.1159/000363110. [DOI] [PubMed] [Google Scholar]
- 4.Sung D, Ahn KM, Lim S-Y, Oh S. Allergenicity of an enzymatic hydrolysate of soybean 2S protein. J. Sci. Food Agric. 2014;94:2482–2487. doi: 10.1002/jsfa.6583. [DOI] [PubMed] [Google Scholar]
- 5.Sung D-E, Lee J, Han Y, Oh S, Do JR. Effects of enzymatic hydrolysis of buckwheat protein on antigenicity and allergenicity. Nutr. Res. Pract. 2014;8:278–283. doi: 10.4162/nrp.2014.8.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drago SR, Gonzalez RJ, Anon MC. Application of surface response methodology to optimize hydrolysis of wheat gluten and characterization of selected hydrolysate fractions. J. Sci. Food Agric. 2008;88:1415–1422. doi: 10.1002/jsfa.3233. [DOI] [Google Scholar]
- 7.Wang J-S, Zhao M-M, Bao Y, Hong T, Rosella CM. Preparation and characterization of modified wheat gluten by enzymatic hydrolysis-ultrafiltration. J. Food Biochem. 2008;32:316–334. doi: 10.1111/j.1745-4514.2008.00157.x. [DOI] [Google Scholar]
- 8.Saeki K. High pressure enzyme reactor (abstract no. D8-4). In: Abstracts: 2011 Annual Meeting of the Korean Society of Food Science and Technology. June 8–10, EXCO, Daegu, Korea. The Korean Society of Food Science and Technology, Seoul, Korea (2011)
- 9.Kim N, Kim C-T. Reduced thermal inactivation of trypsin and Marugoto E by high-pressure treatment. Food Sci. Technol. Res. 2012;18:911–917. doi: 10.3136/fstr.18.911. [DOI] [Google Scholar]
- 10.Borda D, Indrawati, Smout C, Van Loey A, Hendrickx M. High pressure thermal inactivation of a plasmin system. J. Dairy Sci. 2004;87:2351–2358. doi: 10.3168/jds.S0022-0302(04)73357-3. [DOI] [PubMed] [Google Scholar]
- 11.Rupley JA, Gratton E, Careri G. Water and globular proteins. Trends Biochem. Sci. 1983;8:18–22. doi: 10.1016/0968-0004(83)90063-4. [DOI] [Google Scholar]
- 12.Mozhaev VV, Lange R, Kudryashova EV, Balny C. Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol. Bioeng. 1996;52:320–331. doi: 10.1002/(SICI)1097-0290(19961020)52:2<320::AID-BIT12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 13.Kim N, Maeng J-S, Kim C-T. Effects of medium high pressure treatments on protease activity. Food Sci. Biotechnol. 2013;22:289–294. doi: 10.1007/s10068-013-0079-8. [DOI] [Google Scholar]
- 14.Kim N, Son S-H, Maeng J-S, Cho Y-J, Kim C-T. Enzymatic hydrolysis of anchovy fine powder at high and ambient pressure, and characterization of the hydrolyzates. J. Sci. Food Agric. 2016;96:970–978. doi: 10.1002/jsfa.7173. [DOI] [PubMed] [Google Scholar]
- 15.Ovissipour M, Abedian A, Motamedzadegan A, Rasco B, Safari R, Shahiri H. The effect of enzymatic hydrolysis time and temperature on the properties of protein hydrolysates from Persian sturgeon (Acipenser persicus) viscera. Food Chem. 2009;115:238–242. doi: 10.1016/j.foodchem.2008.12.013. [DOI] [Google Scholar]
- 16.Sun XD. Enzymatic hydrolysis of soy proteins and the hydrolysates utilization. Int. J. Food Sci. Technol. 2011;46:2447–2459. doi: 10.1111/j.1365-2621.2011.02785.x. [DOI] [Google Scholar]
- 17.Beuchat LR, Cherry JP, Quinn MR. Physicochemical properties of peanut flour as affected by proteolysis. J. Agric. Food Chem. 1975;23:616–620. doi: 10.1021/jf60200a045. [DOI] [PubMed] [Google Scholar]
- 18.Payne RE, Hill CG, Jr, Amundson CH. Enzymatic solubilization of leaf protein concentrate in membrane reactors. J. Food Sci. 1978;43:385–389. doi: 10.1111/j.1365-2621.1978.tb02310.x. [DOI] [Google Scholar]
- 19.Sekul AA, Vinnett CH, Ory RL. Some functional properties of peanut proteins partially hydrolyzed with papain. J. Agric. Food Chem. 1978;26:855–858. doi: 10.1021/jf60218a035. [DOI] [Google Scholar]
- 20.Yu LJ, Rupasinghe HPV. Improvement of cloud stability, yield and beta-carotene content of carrot juice by process modification. Food Sci. Technol. Int. 2013;19:399–406. doi: 10.1177/1082013212455342. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Mora P, Penas E, Frias J, Zielinski H, Wiczkowski W, Zielinska D, Martinez-Villaluenga C. High-pressure-assisted enzymatic release of peptides and phenolics increases angiotensin converting enzyme I inhibitory and antioxidant activities of pinto bean hydrolysates. J. Agric. Food Chem. 2016;64:1730–1740. doi: 10.1021/acs.jafc.5b06080. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosi V, Polenta G, Gonzalez C, Ferrari G, Maresca P. High hydrostatic pressure assisted enzymatic hydrolysis of whey proteins. Innov. Food Sci. Emerg. Technol. 2016;38:294–301. doi: 10.1016/j.ifset.2016.05.009. [DOI] [Google Scholar]
- 23.Adler-Nissen J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979;27:1256–1262. doi: 10.1021/jf60226a042. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen PM, Petersen S, Dambmann C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001;66:642–646. doi: 10.1111/j.1365-2621.2001.tb04614.x. [DOI] [Google Scholar]
- 25.Ohmori T, Shigehisa T, Taji S, Hayashi R. Effect of high pressure on the protease activities in meat. Agric. Biol. Chem. 1991;55:357–361. [Google Scholar]


