Abstract
This study was designed to isolate lactic acid bacteria (LAB) with β-glucosidase activity and probiotic properties from Korean fermented foods. Among nine isolates, four LAB strains had excellent survival rates at pH 2.5 with 0.3% (w/v) pepsin for 3 h and 0.3% (w/v) oxgall for 24 h. Four LAB strains did not produce β-glucuronidase and showed adhesion ability to HT-29 cells that was superior to that shown by the reference strain Lactobacillus rhamnosus GG. All four strains were sensitive to ampicillin, tetracycline, chloramphenicol, and doxycycline. These strains were identified as Leuconostoc mesenteroides H40, Lactobacillus plantarum FI10604, L. brevis FI10700, and L. perolens FI10842 by 16S rRNA gene sequence, respectively. It was found that L. perolens FI10842 produced the highest β-glucosidase activity (49.10 mU/mL). These results indicate that the four LAB strains could be used as potential probiotic. Especially L. perolens FI10842 could be used as a starter culture for fermentation.
Keywords: Korean fermented food, Lactic acid bacteria, Screening, Probiotic, β-Glucosidase
Introduction
Probiotics are defined by the World Health Organization (WHO) as “living microorganisms which, when administered in adequate amounts, confer a health benefits to the host” [1]. Lactic acid bacteria (LAB) together with bifidobacteria are classified as the generally recognized as safe (GRAS) and have become widely used worldwide as probiotics to improve health. LAB and bifidobacteria strains have to meet several criteria to be used as probiotics including tolerance to gastric acid and bile salt, adherence ability in the intestinal tract, antibiotic susceptibility, and non-haemolytic activity [2, 3]. The health-promoting effects of probiotics include alleviation of inflammatory symptom [4], anti-cancer activity [4], allergy lowering activity [5], anti-oxidant activity [6], cholesterol lowering activity [7], and alleviation of lactose intolerance [8].
Consumers are interested in the possible health promoting effects of various traditional fermented foods. Traditional fermented foods contain LAB strains that produce valuable biomolecules and play an important role during fermentation [9]. Many researchers have identified promising LAB strains in traditional fermented foods such as kimchi [5], camel milk yogurt [6], and Thai milk kefir [10]. Promising LAB strains from traditional fermented foods should have characteristics such as the ability to survive in the intestinal tract and be safe for human use [11]. Several studies have shown that potential probiotics isolated from Tibetan yak milk reduced serum cholesterol, liver cholesterol, and triglycerides levels in mice [7] and potential probiotics isolated from Marcha of Sikkim produced γ-aminobutyric acid (GABA) and had strong antioxidant activities [12].
Some LAB strains produce enzymes that make use of ingested ingredients to benefit the host. Among the beneficial enzymes produced by LAB strains, β-glucosidase plays a role in the cleavage of glycosidic bonds in disaccharides and oligosaccharides [13]. In addition, β-glucosidase contributes to the removal of bitter flavors through the hydrolysis of bitter compounds and the release of aromatic compounds after conversion of flavorless glucosides [14]. Previous studies have shown that microorganisms producing β-glucosidase produced minor ginsenosides after bioconversion of ginsenosides [15] and LAB strains formed aglycones by bioconversion of isoflavone glucosidases during soymilk fermentation [16]. Therefore, LAB producing β-glucosidase should have applications in various biotechnological processes related to bioconversion of ginsenosides, γ-aminobutyric acid (GABA), isoflavone, and phenolic compound through fermentation.
The objective of this study was to screen LAB strains isolated from Korean traditional fermented foods for probiotic properties and evaluate their β-glucosidase activity.
Materials and methods
Isolation and identification of LAB strains
The eighty LAB strains used in this study were isolated from various Korean fermented foods (cabbage kimchi, chive kimchi, radish kimchi, godeulppaegi kimchi, cheonggukjang, jangajji, pickled clams, gejang, watery radish kimchi, young radish kimchi, soy sauce, vinegar, and radish root kimchi). Samples (1 g) from Korean fermented foods were serially diluted and plated on de Man, Rogosa and Shape (MRS) agar (BD BBL, Franklin Lakes, NJ, USA). After incubation at 37 °C for 24 h, a typical colony was subcultured in MRS broth at 37 °C for 18 h. The isolates were identified as LAB strains using criteria including Gram staining, cell morphology, and catalase reaction. Potential probiotic LAB strains were identified by 16S rRNA gene sequencing and stored at −80 °C in MRS broth with 20% (v/v) glycerol (Sigma-Aldrich, St. Louis, MO, USA). Lactobacillus rhamnosus GG, a reference probiotic strain, was obtained from Korean Collection for Type Cultures (Jeolla-do, Korea).
First screening for acid tolerance
To screen for LAB strains with acid tolerance, 100 μL of overnight culture of each LAB strain was mixed with 100 μL of MRS broth in a 96-well plate, with the pH of the MRS broth adjusted to 2.5 using 1 N HCl, respectively. After incubation at 37 °C for 24 h, optical density (OD) was measured at 630 nm in an ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA).
Acid tolerance (%) was determined based on the OD value of the pH 2.5 culture after 24 h (ODpH2.5) divided by OD value of the control culture after 24 h (ODcontrol). LAB strains that showed acid tolerance (above 70%) were further evaluated for gastric acid and bile salt tolerance.
Gastric acid and bile salt tolerance
The gastric acid and bile salt tolerance of LAB strains were evaluated according to Jeon et al. [17]. To evaluate gastric acid tolerance, overnight cultures of LAB strains were inoculated into pH 2.5 MRS with 0.3% (w/v) pepsin (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37 °C for 3 h. The survival rate (%) was evaluated by plate count on MRS agar, after 0 and 3 h of incubation.
To evaluate bile salt tolerance, overnight cultures of LAB strains were inoculated into MRS broth with 0.3% (w/v) oxgall (BD BBL, Franklin Lakes, NJ, USA) and incubated at 37 °C for 24 h. The survival rate (%) was evaluated by plate count on MRS agar, after 0 and 24 h of incubation.
Survival rate (%) was determined by comparing the number of viable bacterial cells after incubation (N) to the initial number of viable bacterial cells (N0).
Enzyme activity
The enzymatic activities of the LAB strains were evaluated using the API ZYM kit (BioMerieux, Lyon, France) according to Lee et al. [5]. LAB strains were resuspended phosphate-buffered saline (PBS; Gibco Life Technologies, Invitrogen, Carlsbad, CA, USA) at 1 × 106 CFU/mL and inoculated into 20 cupules. After incubation at 37 °C for 4 h, 1 drop each of ZYM A and ZYM B were added to each cupules. The enzymatic activity of each LAB strains was determined by color intensity.
Adhesion ability to intestinal cells
The adhesion ability of the LAB strains was evaluated according to Lee et al. [18] with minor modifications. Monolayers of HT-29 cells (human colon adenocarcinoma cell line, KCLB 30,038) were seeded at a concentration 1 × 105 cells/mL in 24-well tissue culture plates and grown at 37 °C in 5% CO2 for 24 h. Bacterial cell suspensions (1 × 107 CFU/mL) were added to each well. After co-incubation at 37 °C for 2 h, the cells were washed three times with PBS and lysed by the addition of 0.1% (v/v) Triton-X100 (Sigma-Aldrich, St. Louis, MO, USA). Cell lysates were serially diluted, plated on MRS agar, and incubated at 37 °C for 24 h.
The adhesion ability (%) of the LAB strains to the HT-29 cells was calculated as the percentage of adhered bacterial cells after 2 h (N2h) divided by the initial number of bacterial cells (N0h).
Antibiotic susceptibility
The antibiotic susceptibility of the LAB strains was evaluated according to Clinical and Laboratory Standards Institute (CLSI) guidelines [19]. All antibiotics used Sigma-Aldrich (St. Louis, MO, USA). One hundred microliters of each LAB cultures (1 × 107 CFU/mL) was plated on MRS agar and antibiotic discs containing ampicillin (10 μg), gentamicin (10 μg), kanamycin (30 μg), streptomycin (10 μg), tetracycline (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), or doxycycline (30 μg) were placed on the inoculated MRS agar. After incubation at 37 °C for 24 h, the diameter (mm) of the inhibition zone was measured.
Selection for LAB strains with β-glucosidase activity
LAB strains with β-glucosidase activity were identified according to the method reported by Veena et al. [20]. To determine β-glucosidase activity, LAB strains were spotted on MRS agar with both 0.3% (w/v) esculin (Sigma-Aldrich, St. Louis, MO, USA) and 0.02% (w/v) ferric ammonium citrate (Sigma-Aldrich, St. Louis, MO, USA). After incubation at 37 °C for 48 h, colonies with β-glucosidase activity were identified by the browning or blackening discoloration of the medium.
β-Glucosidase activity of LAB strains
The β-glucosidase activity of the LAB strains was evaluated according to Rekha and Vijayalakshmi [21] with minor modifications. An overnight culture of each LAB strain was centrifuged for 10 min at 6000×g at 4 °C, washed twice, and resuspended in PBS. The β-glucosidase activity was determined by mixing 0.2 mL of bacterial suspension (1 × 107 CFU/mL) and 0.4 mL of 5 mM pNPG (para-nitrophenyl β-D-glucopyranoside) (Sigma-Aldrich, St. Louis, MO, USA) and incubating at 37 °C for 30 min. The reaction was stopped by adding 0.8 mL of 1 M sodium carbonate (Junsei Chemical, Nihonbashi, Japan). The amount of p-nitrophenol released in the supernatant was measured at 405 nm using a X-ma 3200 spectrophotometer (Human Corporation, Seoul, Korea). One unit of enzyme activity was defined as the amount of enzyme that released 1 μmol of p-nitrophenol from the substrate per min.
Statistical analysis
All experiments were repeated three times with duplicate samples, and all results are presented as the mean ± standard deviation. SPSS 18.0 was used for all statistical analysis. Significant differences among the means were determined by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests. Values were considered statistically significant for p < 0.05.
Results and discussion
Acid and bile salt tolerance of LAB strains
Probiotic strains should be able to pass through the gastrointestinal tract and survive in the presence of gastric acid and bile salt [22]. Therefore, probiotic strains are generally acid and bile salt tolerant. The acid and bile salt tolerance of LAB strains is shown in Table 1. Among nine LAB strains that were screened for acid tolerance in 1st screening, H40, FI10604, FI10700, and FI10842 had high survival rates exceeding 80% under acidic (pH 2.5 with 0.3% (w/v) pepsin, 3 h) and basic conditions (0.3% (w/v) oxgall, 24 h). The four LAB strains, which had high survival rates in acidic and basic conditions and could pass through the stomach to the intestinal tract, were identified by 16S rRNA gene sequencing as Leuconostoc mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842 having 99% similarity. Previous studies have shown that L. plantarum Lb41 had a 0.06 log reduction in growth at pH 2.5 with 0.3% (w/v) pepsin for 3 h and a 1.36 log reduction in 0.3% (w/v) oxgall for 24 h [17]. L. paracasei FM-LP-4 was found to grow to 7 log CFU/mL at pH 2.5 after 3 h and to 8 log CFU/mL with 0.5% (w/v) bile salt for 4 h [6].
Table 1.
Acid and bile salt tolerance of commercial strain and LAB strains isolated from Korean fermented foods
| LAB strains | Viable counts at pH 2.5 with 0.3% pepsin (log CFU/mL) | Survival rate (%) | Viable counts in 0.3% oxgall (log CFU/mL) | Survival rate (%) | ||
|---|---|---|---|---|---|---|
| 0 h | 3 h | 0 h | 24 h | |||
| Lactobacillus rhamnosus GG | 8.56 ± 0.02a | 8.51 ± 0.05a | 99.47 | 8.56 ± 0.02a | 8.58 ± 0.03b | 100.27 |
| 347 | 8.23 ± 0.06b | 5.63 ± 0.07f | 68.39 | 8.23 ± 0.06b | 7.13 ± 0.02f | 86.63 |
| H40 | 8.18 ± 0.08b | 7.18 ± 0.02e | 87.77 | 8.18 ± 0.08b | 8.26 ± 0.03c | 101.01 |
| FI10016 | 8.03 ± 0.02d | 3.15 ± 0.21i | 39.56 | 8.03 ± 0.02d | 6.96 ± 0.03g | 86.67 |
| FI10018 | 8.05 ± 0.00 cd | 4.66 ± 0.09h | 57.94 | 8.05 ± 0.00cd | 7.86 ± 0.03e | 97.64 |
| FI10166 | 7.35 ± 0.00e | 4.98 ± 0.05g | 67.72 | 7.35 ± 0.00e | 6.61 ± 0.11h | 89.99 |
| FI10562 | 8.58 ± 0.13a | 8.30 ± 0.15b | 96.79 | 8.58 ± 0.13a | 5.69 ± 0.12i | 66.34 |
| FI10604 | 7.99 ± 0.12d | 7.94 ± 0.02d | 99.33 | 7.99 ± 0.12d | 6.69 ± 0.08h | 83.67 |
| FI10700 | 8.16 ± 0.06bc | 8.12 ± 0.01c | 99.50 | 8.16 ± 0.06bc | 8.94 ± 0.06a | 109.56 |
| FI10842 | 8.25 ± 0.01b | 8.14 ± 0.02c | 98.70 | 8.25 ± 0.01b | 8.04 ± 0.03d | 97.45 |
a–iValues with the same letters within a column are not significantly different (p < 0.05) by one-way analysis of variance and Duncan’s multiple range tests
Enzymatic activity of LAB strains
In the selection of probiotic strains, one important criterion is enzymatic activity due to the harmful enzymes such as β-glucuronidase that can be produced by microorganisms [23]. Carcinogens such as a benzo(a)pyrene counteracted in the liver, and then the conjugated products excreted with bile acid. Cleavage by β-glucuronidase can liberate these substances, and make them toxic once again. The enzymatic activity of the isolate LAB strains is shown in Table 2. Four LAB strains (Leu. mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) did not produce carcinogenic enzyme such as β-glucuronidase. Previous studies have shown that the potential probiotic L. lactis KC24 did not produce β-glucuronidase [24]. The potential probiotic Leu. mesenteroides F27 and L. plantarum C182 did not produce β-glucuronidase [18].
Table 2.
Enzymatic activities of commercial and isolated LAB strains using by the API ZYM kit
| Enzymes | L. rhamnosus GG | Leu. mesenteroides H40 | L. plantarum FI10604 | L. brevis FI10700 | L. perolens FI10842 |
|---|---|---|---|---|---|
| Control | 0a | 0 | 0 | 0 | 0 |
| Alkaline phosphatase | 0 | 0 | 0 | 0 | 0 |
| Esterase | 2 | 0 | 0 | 0 | 0 |
| Esterase Lipase | 1 | 0 | 0 | 0 | 0 |
| Lipase | 0 | 0 | 0 | 0 | 0 |
| Leucine arylamidase | 3 | 0 | 2 | 4 | 3 |
| Valine arylamidase | 3 | 0 | 1 | 3 | 1 |
| Cystine arylamidase | 0 | 0 | 0 | 0 | 1 |
| Trypsin | 0 | 0 | 0 | 0 | 0 |
| α-Chymotrypsin | 0 | 0 | 0 | 0 | 0 |
| Acid phophatase | 1 | 1 | 1 | 1 | 1 |
| Naphtol-AS-BI-phosphohydrolase | 2 | 2 | 1 | 1 | 2 |
| α-Galactosidase | 0 | 0 | 0 | 0 | 0 |
| β-Galactosidase | 1 | 0 | 2 | 4 | 1 |
| β-Glucuronidase | 0 | 0 | 0 | 0 | 0 |
| α-Glucosidase | 0 | 3 | 0 | 1 | 3 |
| β-Glucosidase | 1 | 4 | 4 | 5 | 4 |
| N-Acetyl-β-glucosaminidase | 0 | 0 | 3 | 0 | 3 |
| α-Mannosidase | 0 | 0 | 0 | 0 | 0 |
| α-Fucosidase | 0 | 0 | 0 | 0 | 1 |
aEnzymatic activity measured as the approximate amount of substrate hydrolysis: 0, 0 nmol; 1, 5 nmol; 2, 10 nmol; 3, 20 nmol; 4, 30 nmol; 5, ≥40 nmol
Adhesion ability of LAB strains to intestinal cell
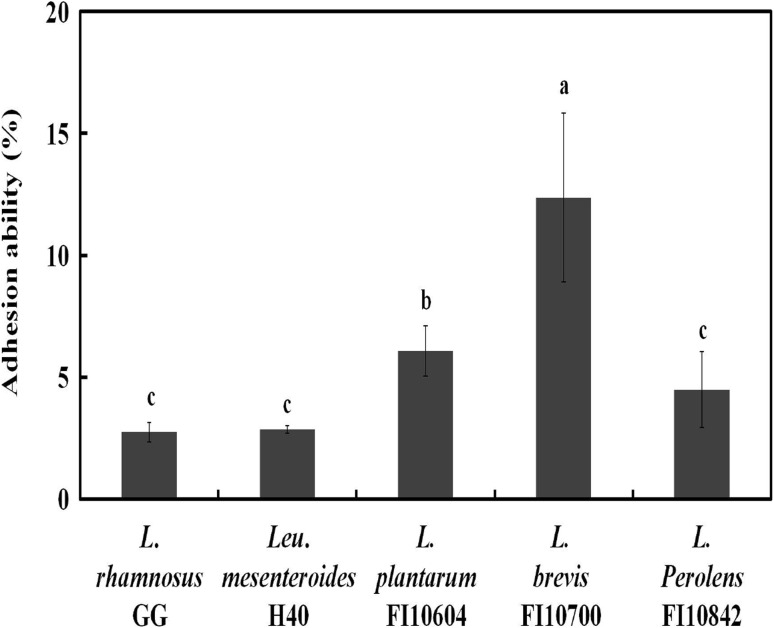
The adhesion ability to intestinal cells was considered for the selection of probiotic strains in regards to colonization of the human gastrointestinal tract and exclusion of pathogenic bacteria [25]. The adhesion ability of the isolated LAB strains is shown in Fig. 1. Four LAB strains (Leu. mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) showed high adhesion ability (2.86–12.37%) compared to the reference strain L. rhamnosus GG (2.74%). Previous studies have shown that seven LAB strains isolated from Inner Mongolia “Hurood” cheese had various adhesion abilities (1–7%) with Caco-2 cells [11]. L. plantarum SY11 and SY12 isolated from kimchi had high adhesion ability (7.2 and 5.3%, respectively) to HT-29 cells [5]. Therefore, the adhesion ability of the four isolated LAB strains to intestinal cell was superior to that of the commercial strain and could play a role in their function as probiotics.
Fig. 1.
Adhesion ability of commercial and isolated LAB strains to human intestinal HT-29 cells. Error bars indicate the standard deviations from three independent experiments. The letters at the base of the bars denote statistical significance (p < 0.05), as determined by one-way analysis of variance and Duncan’s multiple range tests
Antibiotic susceptibility of LAB strains
Probiotic strains have important requirements in regards to antibiotic susceptibility because any antibiotic resistant genes present in probiotic strains may transfer to pathogenic bacterial strains [26]. The antibiotic susceptibility of the isolated LAB strains is shown in Table 3. The four isolated LAB strains (Leu. mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) were found to be sensitive to ampicillin (10 μg), tetracycline (30 μg), chloramphenicol (30 μg), and doxycycline (30 μg) similar to the results for L. rhamnosus GG. A previous study have shown that L. plantarum C182 is susceptible to ampicillin, chloramphenicol, cycloheximide, erythromycin, and tetracycline [18]. L. plantarum Lb41 was shown to be sensitive to commercial antibiotics such as ampicillin, tetracycline, chloramphenicol, and doxycycline [17]. As a result of the previous studies yielding similar patterns of resistance, the four isolated LAB strains meet the CLSI guidelines and are confirmed to be safe.
Table 3.
Antibiotic susceptibility of commercial and isolated LAB strains to commercial antibiotics
| Antibiotics | L. rhamnosus GG | Leu. mesenteroides H40 | L. plantarum FI10604 | L. brevis FI10700 | L. perolens FI10842 |
|---|---|---|---|---|---|
| Ampicillin (10 μg) | S | S | S | S | S |
| Gentamycin (10 μg) | R | R | R | R | I |
| Kanamycin (30 μg) | R | R | R | R | R |
| Streptomycin (10 μg) | R | R | R | R | I |
| Tetracycline (30 μg) | S | S | S | S | S |
| Ciprofloxacin (5 μg) | R | R | R | R | R |
| Chloramphenicol (30 μg) | S | S | S | S | S |
| Doxycycline (30 μg) | S | S | S | S | S |
Resistance was defined according to the CLSI breakpoints (CLSI 2012)
S susceptible, I intermediate, R resistant
Screening for potential probiotic strains with β-glucosidase activity
The potential probiotic strains (Leu. mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) were screened for β-glucosidase production using esculin agar (0.3% (w/v) esculin and 0.02% (w/v) ferric ammonium citrate). Among the four potential probiotic strains and the reference strains, three potential probiotic strains (L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) showed a positive reaction, while one potential probiotic strain (Leu. mesenteroides H40) and the reference strain (L. rhamnosus GG) showed a negative reaction (data not shown). Three potential probiotic strains with positive reactions were used for evaluation of β-glucosidase activity.
β-Glucosidase activity of potential probiotic strains
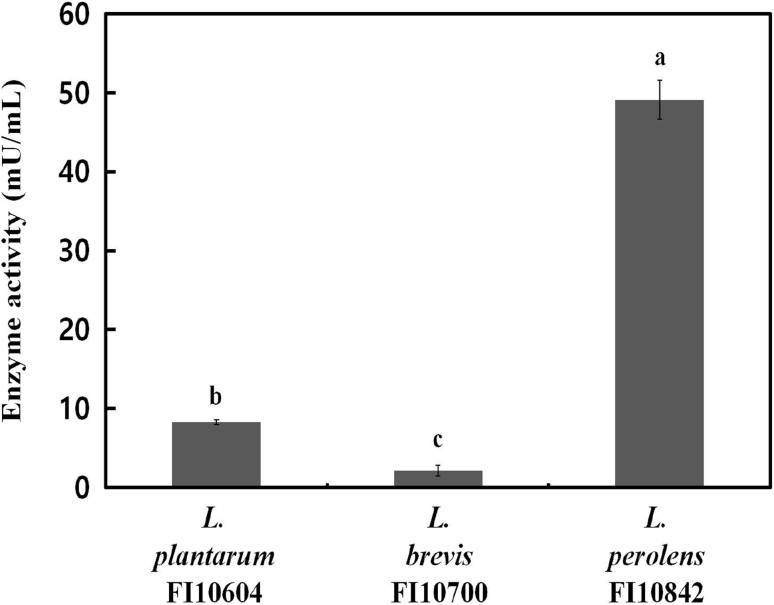
β-Glucosidase plays an important role in various biological processes in industry and improves the bioavailability of isoflavones in soy milk [21]. The β-glucosidase activity of the potential probiotic strains shown in Fig. 2. Among the potential probiotic strains with β-glucosidase activity, L. perolens FI10842 had the highest β-glucosidase activity (49.10 mU/mL), whereas L. brevis FI10700 had the lowest (2.13 mU/mL). Previous studies have shown that among nine tested strains, L. plantarum B4495 produced the highest β-glucosidase activity (35 mU/mL) [22]. Weissella confusa 31 had the highest β-glucosidase activity (115.12 U/mg protein) [27]. These finding suggested that the four LAB strains (Leu. mesenteroides H40, L. plantarum FI10604, L. brevis FI10700, and L. perolens FI10842) could potentially be used as probiotics in a view of high survival in intestinal conditions and safety. In addition, L. perolens FI10842, which had the highest β-glucosidase activity, could be used as a starter culture for plant-derived food bioconversion through fermentation.
Fig. 2.
β-Glucosidase activity of potential probiotic strains at 37 °C for 24 h. Error bars indicate the standard deviations from three independent experiments. The letters at the base of the bars denote statistical significance (p < 0.05), as determined by one-way analysis of variance and Duncan’s multiple range tests
Acknowledgements
This research was supported by the High Value-added Food Technology Development Program of the Ministry of Agriculture, Food, and Rural Affairs (Grant Number 314073-03) and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093824).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.FAO/WHO. Joint FAO/WHO (Food and Agriculture Organization/World Health Organization) working group report on drafting guidelines for the evaluation of probiotics in food. Ontario: London (2002)
- 2.Saad M, Delattre C, Urdaci M, Schmitter JM, Bressollier P. An overview of the last advances in probiotic and prebiotic field. LWT-Food Sci. Technol. 2013;50:1–16. doi: 10.1016/j.lwt.2012.05.014. [DOI] [Google Scholar]
- 3.Kumar BV, Vijayendra SVN, Reddy OVS. Trends in dairy and non-dairy probiotic products-a review. J. Food Sci. Technol. 2015;52:6112–6124. doi: 10.1007/s13197-015-1795-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee NK, Son SH, Jeon EB, Jung GH, Lee JY, Paik HD. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods. 2015;14:513–518. doi: 10.1016/j.jff.2015.02.019. [DOI] [Google Scholar]
- 5.Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starter. LWT-Food Sci. Technol. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- 6.Wang Y, Zhou J, Xia X, Zhao Y, Shao W. Probiotic potential of Lactobacillus paracasei FM-LP-4 isolated from Xinjiang camel milk yogurt. Int. Dairy J. 2016;62:28–34. doi: 10.1016/j.idairyj.2016.07.001. [DOI] [Google Scholar]
- 7.Ding W, Shi C, Chen M, Zhou J, Long R, Guo X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J. Funct. Foods. 2017;32:324–332. doi: 10.1016/j.jff.2017.03.021. [DOI] [Google Scholar]
- 8.Guamer F, Perdiqan G, Corthier G, Salminen S, Koletzko B, Morelli L. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005;93:783–786. doi: 10.1079/BJN20051428. [DOI] [PubMed] [Google Scholar]
- 9.Franz CMAP, Huch M, Mathara JM, Abriouel H, Benomar N, Reid G, Galvez A, Holzapfel WH. African fermented foods and probiotics. Int. J. Food Microbiol. 2014;190:84–96. doi: 10.1016/j.ijfoodmicro.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Luang-In V, Deeseenthum S. Exopolysaccharide-producing isolates from Thai milk kefir and their antioxidant activities. LWT-Food Sci. Technol. 2016;73:592–601. doi: 10.1016/j.lwt.2016.06.068. [DOI] [Google Scholar]
- 11.Zhang J, Zhang X, Zhang L, Zhao Y, Niu C, Yang Z, Li S. Potential probiotic characterization of Lactobacillus plantarum strains isolated from inner mongoli “Hurood” cheese. J. Microbiol. Biotechnol. 2014;24:225–235. doi: 10.4014/jmb.1308.08075. [DOI] [PubMed] [Google Scholar]
- 12.Das D, Goyal A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015;61:263–268. doi: 10.1016/j.lwt.2014.11.013. [DOI] [Google Scholar]
- 13.Wallecha A, Mishra S. Purification and characterization of two β-glucosidases from a thermo-tolerant yeast Pichia etchellsii. Biochim. Biophys. Acta. 2003;1649:74–84. doi: 10.1016/S1570-9639(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 14.Riou C, Salmon JM, Vallier MJ, Gunata Z, Barre P. Purification, characterization, and substrate specificity of novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl. Environ. Microbiol. 1998;64:3607–3614. doi: 10.1128/aem.64.10.3607-3614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SH, Lee YH, Park JM, Bai DH, Jang JK, Park YS. Bioconversion of Ginsenosides from red ginseng extract using Candida allociferrii JNO301 isolated from meju. Mycobiology. 2014;42:368–375. doi: 10.5941/MYCO.2014.42.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun J, Kim GM, Lee KW, Choi ID, Kwon GH, Park JY, Jeong SJ, Kim JS, Kim JH. Conversion of isoflavone glucosidase to aglycones in soy milk by fermentation with lactic acid bacteria. J. Food Sci. 2007;72:M39–44. doi: 10.1111/j.1750-3841.2007.00276.x. [DOI] [PubMed] [Google Scholar]
- 17.Jeon EB, Son SH, Jeewanthi RKC, Lee NK, Paik HD. Characterization of Lactobacillus plantarum Lb41, an isolate from kimchi and its application as a probiotic cottage cheese. Food Sci. Biotechnol. 2016;25:1129–1133. doi: 10.1007/s10068-016-0181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KW, Shim JM, Park SK, Heo HJ, Kim HJ, Ham KS, Kim JH. Isolation of lactic acid bacteria with probiotic potentials from kimchi, traditional Korean fermented vegetable. LWT-Food Sci. Technol. 2016;71:130–137. doi: 10.1016/j.lwt.2016.03.029. [DOI] [Google Scholar]
- 19.CLSI. Performance standards for antimicrobial susceptibility testing; twenty-second informational supplement. Clinical and Laboratory Standards Institute 32: 1–184 (2012)
- 20.Veena V, Poornima P, Parvatham R. Sivapriyadharsini and Kalaiselvi K. Isolation and characterization of β-glucosidase producing bacteria from different sources. Afr. J. Biotechnol. 2011;10:14907–14912. doi: 10.5897/AJB09.314. [DOI] [Google Scholar]
- 21.Rekha CR, Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010;109:1198–1208. doi: 10.1111/j.1365-2672.2010.04745.x. [DOI] [PubMed] [Google Scholar]
- 22.Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J. Biotechnol. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 23.Cole CB, Fuller R, Carter SM. Effect of probiotic supplements of Lactobacillus acidophilus and Bifidobacterium adolescentis 2204 on β-glucosidase and β-glucuronidase activity in the lower gut of rats associated with a human faecal flora. Microb. Ecol. Health Dis. 1989;2:223–225. doi: 10.3109/08910608909140223. [DOI] [Google Scholar]
- 24.Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- 25.Collado MC, Meriluoto J, Salminen S. In vitro analysis of probiotic strain combination to inhibit pathogen adhesion to human intestinal mucus. Food Res. Int. 2007;40:629–636. doi: 10.1016/j.foodres.2006.11.007. [DOI] [Google Scholar]
- 26.Hoque MZ, Akter F, Hossain KM, Rahman MSM, Billah MM, Islam KMD. Isolation, identification and analysis of probiotic properties of Lactobacillus spp. from selective regional yoghurts. World J. Dairy & Food Sci. 5: 39–46 (2010)
- 27.Lee KW, Park JY, Jeong HR, Heo HJ, Han NS, Kim JH. Probiotic properties of Weissella strains isolated from human faeces. Anaerobe. 2012;18:96–102. doi: 10.1016/j.anaerobe.2011.12.015. [DOI] [PubMed] [Google Scholar]