Abstract
Whole grain comprises starchy endosperm, germ, and bran tissues, which contain fibers, minerals, vitamins, and several phytochemicals. Whole grain cereal (WGC)-based food products supply beneficial nutrients (essential for health care) and macronutrients (essential for body maintenance and support). The present study investigated the inhibitory effect of WGC on obesity-induced muscle atrophy in obese C57BL/6N mice. WGC attenuated the body weight gain, fat pad mass, adipocyte size, food efficiency ratio, serum lipid profile, and non-alcoholic fatty liver. Furthermore, WGC increased muscle mass and muscle strength by activating the phosphatidylinositol 3-kinase/protein kinase B pathway. Accordingly, WGC up-regulated the expression of factors that regulate muscle hypertrophy and myogenesis, whereas it down-regulated the atrophy-related factors. Overall, these results demonstrate that WGC effectively attenuates obesity-induced muscle atrophy as well as overall obesity, suggesting that WGC can be used as a functional food.
Keywords: Whole grain cereal, Muscle atrophy, Skeletal muscle mass, Muscle strength, Anti-obesity
Introduction
The increasing incidence of obesity and obesity-related metabolic diseases constitutes considerable health problems [1]. Obesity is caused by an imbalance between energy intake and expenditure, mediated through lifestyle, genetic factors, and physical inactivity [2]. The condition is associated with various metabolic diseases, including muscle atrophy, hypertension, diabetes, and non-alcoholic fatty liver [3].
Skeletal muscle is the largest organ, accounting for about 40% of the body weight, and performs various physiological functions [4]. Muscle atrophy is defined as a reduction in the mass and strength of muscle, caused by a decrease in the size and number of muscle fibers [5]. The onset of muscle atrophy is related to several factors, such as oxidative stress, denervation, disuse, and aging [6]. Previous studies have revealed that skeletal muscle loss and dysfunction are associated with obesity, defined as sarcopenic obesity [4].
Obesity is associated with inflammatory cytokines that suppress the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, which is an anabolic pathway for protein synthesis [7]. Activation of the PI3K/Akt pathway stimulates mammalian target of rapamycin (mTOR) signaling cascades, modulating two master molecules associated with the initiation of mRNA translation, namely, 70-kDa ribosomal protein S6 kinase (p70S6K) and eukaryotic initiation factor 4E binding protein 1 (4EBP1) [8]. Akt activation results in the activation of the master myogenic regulatory factors MyoD and myogenin, which regulate the differentiation of muscle and the formation of myoblasts [9]. In addition, forkhead box O3a (FoxO3a) phosphorylation inhibits muscle protein degradation through the ubiquitin–proteasome system, including atrogin-1 and muscle ring finger 1 (MuRF1), by activating Akt [7].
There is an increasing global demand for functional foods that can benefit the body composition and be effective against metabolic diseases [10]. Whole grain cereal (WGC)-based food products are a source of beneficial minerals, vitamins, and other phytochemicals, which are required for to maintain health, as well as of macronutrients required for body maintenance and support [11]. Previous studies have shown the functional effects of WGC on various health problems, including the metabolic syndrome, diabetes, cardiovascular disease, stroke, hypertension, and cancer [11, 12]. The present study demonstrates that WGC attenuates obesity-related skeletal muscle atrophy by stimulating PI3K/Akt pathway signaling.
Materials and methods
Materials
Supplied from Mother’s Love (Seoul, Republic of Korea), the WGC contained 51% of whole grains consisting of barley (30%), brown rice (11%), black rice (4%), corn (5%), and sorghum (1%). The other 49% of the WGC comprised fructooligosaccharide (15%), isolate soybean protein (8%), gelatinized rice flour (7.8%), whey protein concentrate (6%), fruit sugar (5%), non-digestible maltodextrin (3%), vitamin and mineral mixture (1.8%), almond (1%), black sesame (0.5%), superfood mixture (0.5%), garcinia cambogia peel extract (0.2%), stevia (0.1%), and berry powder (0.1%). The ingredients and nutrient contents of the WGC are shown in Table 1.
Table 1.
Nutrient content of whole grain cereal
| Nutrient | Content |
|---|---|
| Carbohydrate (%) | 65.71 |
| Crude protein (%) | 17.14 |
| Crude fat (%) | 2.86 |
| Ash (%) | 2.86 |
| Dietary fiber (%) | 11.43 |
| Na (mg/100 g) | 91.43 |
| Ca (mg/100 g) | 200.00 |
| Fe (mg/100 g) | 3.43 |
| Zn (mg/100 g) | 2.43 |
| Vitamin B1 (mg/100 g) | 0.86 |
| Vitamin B2 (mg/100 g) | 1.00 |
| Vitamin B6 (mg/100 g) | 1.07 |
| Vitamin C (mg/100 g) | 71.43 |
| Vitamin E (mg/100 g) | 7.86 |
| Niacin (NE/100 g) | 10.71 |
Chemical reagents
Antibodies against p-PI3K, PI3K, p-Akt, Akt, p-mTOR, mTOR, p-p70S6K, p70S6K, p-4EBP1, 4EBP1, p-FoxO3a, FoxO3a, and α-tubulin were purchased from Cell Signaling Technology (Beverly, MA, USA). Horseradish peroxidase-conjugated secondary antibodies were supplied from Bethyl Laboratories, Inc. (Montgomery, TX, USA). Enhanced chemiluminescence (ECL) solution was purchased from Amersham Biosciences (Little Chalfont, UK). NP-40 buffer was supplied from ELPIS-Biotech (Daejeon, Korea).
Animal experiment
Four-week-old male C57BL/6N mice (DBL, Umsung, Korea) were housed under the following conditions: 55 ± 5% humidity, 12 h day/night cycles, and 25 ± 2 °C at the Yonsei Laboratory Animal Research Center (Seoul, Korea). During the entire experimental period, the mice were provided with food and water ad libitum. The mice were randomly divided into three dietary groups (n = 7). Group 1 mice were fed a normal chow diet (ND) (Modified Rodent Diet D12450B; Unifaith Inc., Seoul, Korea); Group 2 mice were fed a high-fat diet (HFD) (Modified Rodent diet D12451; Unifaith Inc.); Group 3 mice were fed a HFD for 8 weeks to induce obesity and then received a HFD supplemented with WGC (HFD + WGC) for 4 weeks (Unifaith Inc.). The compositions of the experimental diets are shown in Table 2. The calories and nutrient contents of the experimental diets were adjusted to resolve any imbalance of nutrients. All diets were prepared to have an equivalent energy density. Based on Table 1, 59.5% WGC in HFD + WGC in Table 2 was calculated to contain 39.1% crude carbohydrate, 10.2% crude protein, 1.7% crude fat, and 8.5% dietary fiber and ash. Thus, total corn starch, maltodextrin 10, and sucrose in HFD were replaced with 39.1% crude carbohydrate in 59.5% WGC, 10.2% casein with 10.2% crude protein, 1.7% soybean oil with 1.7% crude fat, and total cellulose with 8.5% dietary fiber and ash (Table 2). The body weight gain and feed intake were estimated twice in a week during the entire experimental period. The gastrocnemius (GN), soleus (SOL), tibialis anterior (TA), and extensor digitorum longus (EDL) muscles, the liver, and the epididymal, subcutaneous, and perirenal fat tissues isolated from mice were measured and stored at − 70 °C. This study was approved by the Institutional Animal Care and Use Committee of Yonsei University (Permit No.: 201602-144-01).
Table 2.
Composition of the experimental diets (%)
| Ingredients | Normal diet | High-fat diet | High-fat diet with whole grain cereal |
|---|---|---|---|
| Casein | 18.17 | 22.63 | 12.43 |
| l-Cystine | 0.27 | 0.34 | 0.34 |
| Corn starch | 28.63 | 8.24 | 0 |
| Maltodextrin 10 | 3.19 | 11.32 | 0 |
| Sucrose | 31.81 | 19.55 | 0 |
| Cellulose | 8.55 | 8.55 | 0 |
| Soybean oil | 2.37 | 2.83 | 1.13 |
| Lard | 1.82 | 20.08 | 20.08 |
| Mineral mixture | 0.91 | 1.13 | 1.13 |
| Dicalcium phosphate | 1.18 | 1.47 | 1.47 |
| Calcium carbonate | 0.50 | 0.63 | 0.63 |
| Potassium citrate | 1.49 | 1.87 | 1.87 |
| Vitamin mix | 0.91 | 1.13 | 1.13 |
| Choline bitartrate | 0.19 | 0.23 | 0.23 |
| Dye | 0.005 | 0.005 | 0.005 |
| Whole grain cereal | 0 | 0 | 59.50 |
| Fat (% calories) | 10 | 45 | 45 |
| Total (%) | 100 | 100 | 100 |
| Total kcal | 370 | 459 | 458 |
| Total energy (kcal/g) | 3.7 | 4.6 | 4.6 |
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNAs extracted from homogenized skeletal muscle tissues were analyzed by conducting the reverse transcription-polymerase chain reaction according to a previous study [13]. The following primer pairs (Bioneer, Daejeon, Korea) were used: MyoD forward 5′-CAA GAC CAC CAA CGC TGA T-3′ and MyoD reverse 5′-CAT CTG AGT CGC CAC TGT AG-3′; myogenin forward 5′-ACC TTC CTG TCC ACC TTC AG-3′ and myogenin reverse 5′-CAG ACT TCC TCT TAC ACA CC-3′; atrogin-1 forward 5′-CAG TGA TCC ATT CTG TTC ATC CTT G-3′ and atrogin-1 reverse 5′-TTA TTT CCA GCC AAA TGG AGA GAG A-3′; MuRF1 forward 5′-TCT GGA CTT AGA ACA CAT AGC AGA G-3′ and MuRF1 reverse 5′-TCT CCT TCT TCA TTG GTG TTC TTC T-3′; and β-actin forward 5′-CAG CTC AGT AAC AGT CCG CC-3′ and β-actin reverse 5′-TCA CTA TTG GCA ACG AGC GG-3′. β-Actin was used as the housekeeping gene.
Western blot assay
Total homogenized skeletal muscle tissues were analyzed by western blot assay according to a previous study [13]. The primary antibodies against p-PI3K, PI3K, p-Akt, Akt, p-mTOR, mTOR, p-p70S6K, p70S6K, p-4EBP1, 4EBP1, p-FoxO3a, FoxO3a, and α-tubulin were used in a 1:1000 dilution. Horseradish peroxidase-conjugated secondary antibodies in a 1:5000 dilution were used for visualizing the proteins on the blotted membrane. The proteins were detected using an ECL solution and visualized with the G:BOX image analysis system (Syngene, Cambridge, UK).
Analysis of blood biochemical parameters
Blood samples were collected from all mice by heart puncture and incubated at room temperature. Serum was prepared by centrifugation of the blood at 4000 rpm for 15 min, and stored at − 70 °C until analysis. Serum lipid profiles and serum levels of hepatotoxicity markers were measured according to the International Federation of Clinical Chemistry method using an automated biochemical analyzer (Mindray; Nanshan, Shenzhen, China) as per the manufacturer’s protocol.
Measurement of liver triglyceride content
To determine the hepatic triglyceride (TG) content, liver tissue was homogenized in NP-40 buffer (ELPIS-Biotech). The TG content was measured with a TG quantification assay kit (Biovision, Mountain View, CA, USA).
Histological analysis
GN muscle tissues, epididymal adipose tissues, and liver tissues fixed with 10% formalin solution were embedded in paraffin to produce paraffin block slides. To observe the fiber cross-sectional area of GN muscle, adipocyte size of epididymal adipose tissues, and liver tissues, the paraffin slides were stained with haematoxylin and eosin. The images from 60 random areas of the stained tissues per group were captured under an Eclipse TE2000U Inverted Microscope with twin charge-coupled device cameras (Nikon, Tokyo, Japan). The adipocyte size of epididymal tissues and the fiber cross-sectional area of GN muscle from the captured images were quantified using ImageJ software (version 1.47; National Institutes of Health, Bethesda, MD, USA). The representative images were displayed according to the quantification of adipocyte size, fiber cross-sectional area of GN muscle, and TG contents.
Grip strength test
The muscle strength of mice was evaluated using a Chatillon force measurement system (Columbus Instrument, Columbus, OH, USA) equipped with a pull bar. Fore/hind-limb and fore-limb grip strengths were measured at the end of the experimental feeding period. This system has an electronic digital force gauge that determines the peak force. The mouse was held by the tail until it released the pull bar. Five consecutive tests were performed on each mouse to obtain the peak value.
Statistical analysis
All experiments were performed in triplicates. Results are presented as the mean ± standard deviation. Statistical analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). Group differences were assessed with the one-way analysis of variance, followed by Duncan test. A p value of less than 0.05 was considered statistically significant.
Results and discussion
WGC reduces HFD-induced body weight gain
According to the American Association of Cereal Chemists International, whole grain comprises the following principal anatomical components: starchy endosperm, germ, and bran [11]. Whole grains, including barley, brown rice, corn, sorghum, and millet, contain several functional components. The outer layers of the grain are rich in beneficial nutrients such as dietary fiber, inulin, glucans, resistant starch, phenolics, carotenoids, vitamin E, and phytochemicals [14]. In this study, the inhibitory effect of WGC on obesity was observed by feeding it to obese C57BL/6N mice for 4 weeks. The body weights of the obese HFD group were much higher than those of the ND group (Table 3). WGC diminished the body weight gain by 11.9% in the HFD + WGC group, compared with the HFD group. The feed intake was significantly decreased in the HFD + WGC mice relative to that in the HFD group. The food efficiency ratio (FER) was the highest in the HFD group. The reduction in body weight gain of the HFD-fed obese mice supplemented with WGC was accompanied by a decrease in the FER.
Table 3.
Effect of whole grain cereal on metabolic parameters in high-fat diet-fed obese mice
| Parameter | Normal diet | High-fat diet | High-fat diet with whole grain cereal |
|---|---|---|---|
| Initial weight (g) | 18.72 ± 1.25 | 17.94 ± 2.06 | 18.77 ± 0.67 |
| Final weight (g) | 34.69 ± 1.91a,1 | 42.87 ± 2.46b | 37.78 ± 1.31c |
| Weight gain (g/day) | 0.190 ± 0.012a | 0.297 ± 0.023c | 0.226 ± 0.011b |
| Feed intake (g day−1 mouse−1) | 2.62 ± 0.27 | 2.77 ± 0.35 | 2.51 ± 0.23 |
| Food efficiency ratio2 | 0.07 ± 0.004a | 0.11 ± 0.009c | 0.09 ± 0.005b |
| Adipose tissue weight (g) | |||
| Epididymal fat | 1.64 ± 0.18a | 2.22 ± 0.18b | 1.76 ± 0.17a |
| Subcutaneous fat | 1.96 ± 0.29a | 4.61 ± 0.37c | 3.06 ± 0.27b |
| Perirenal fat | 0.84 ± 0.07a | 1.30 ± 0.03c | 1.06 ± 0.06b |
| Serum lipid levels (mg/dL) | |||
| Triglyceride | 40.40 ± 4.28a | 53.60 ± 4.51b | 45.20 ± 2.59a |
| Total chol. | 189.57 ± 8.26a | 250.86 ± 12.65c | 205.00 ± 7.21b |
| HDL chol. | 172.86 ± 12.60 | 189.43 ± 22.47 | 175.86 ± 8.13 |
| LDL chol. | 31.71 ± 4.61a | 62.43 ± 9.03c | 40.43 ± 6.32b |
| HDL chol./total chol. (%) | 88.2 ± 1.76a | 79.4 ± 4.50b | 84.89 ± 2.42a |
| Liver tissue weight (g) | 1.42 ± 0.13a | 1.83 ± 0.16b | 1.54 ± 0.10a |
1 a–cvalues are significantly different at p < 0.05 by Duncan’s test
2 Food efficiency ratio: body weight gain/feed intake. Data are presented as the mean ± SD of 7 animals per group
WGC-based food products are a good source of healthy nutrients as well as macronutrients for body maintenance and support [11]. The US Food and Drug Administration has approved a whole-grain health claim for any food product containing ≥ 51% whole grain ingredients by weight to provide more standardized nomenclature and promote consumer recognition of foods that are high in whole grains [15, 16]. In this study, the WGC contains 51% of whole grains comprising barley, brown rice, black rice, corn, and sorghum, which reduces body weight gain of the HFD-fed obese mice by decreasing the FER.
WGC attenuates HFD-induced fat accumulation by decreasing white adipocyte size
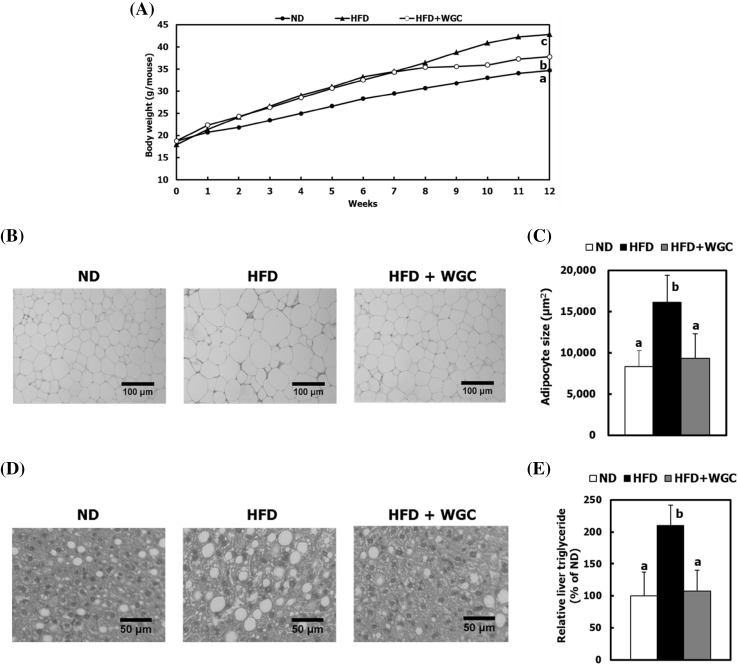
In this study, we supplied HFD, including 59% of WGC, for 4 weeks to mice that were taken HFD for 8 weeks to induce obesity. Compared with HFD group, HFD + WGC group showed gradual reduction of body weight [Fig. 1(A)]. The HFD group exhibited increased fat volume, whereas the HFD + WGC group showed significantly lower fat accumulation. The epididymal, subcutaneous, and perirenal fat pad masses were suppressed by 20.7, 33.6, and 18.4%, respectively, in the HFD + WGC group relative to the HFD group (Table 3). Furthermore, histological analysis of the epididymal adipose tissue showed that the suppression of fat pad mass in the HFD + WGC group was due to an effective reduction in white adipocyte size [Fig. 1(B, C)]. The characteristic of obesity is the increase of body weight resulting from the increase of adipocyte size and adipose tissue [17]. Thus, these results indicate that WGC attenuates HFD-induced body weight by suppressing fat pad masses and adipocyte enlargement, suggesting that WGC improves HFD-induced obesity condition in mice.
Fig. 1.
Effect of whole grain cereal (WGC) on obesity in high-fat diet (HFD)-fed mice. (A) Changes in the body weights. Images from 60 random areas of the stained epididymal adipose tissue. and liver tissue per group were captured. The adipocyte size from the captured images was measured using Image J software. (B) Representative images of epididymal adipose tissue (magnification × 100). (C) Quantitation of the epididymal adipocyte size. (D) Histological analysis of liver tissue (magnification × 100). (E) Triglyceride content in liver tissue. Data are presented as the mean ± SD of 7 animals per group. a–cvalues are significantly different at p < 0.05 by Duncan’s test
WGC prevents HFD-induced hyperlipidaemia and non-alcoholic fatty liver
Prolonged excessive energy intake leads to hyperlipidaemia, non-alcoholic fatty liver, and abnormal fat accumulation, which are related to obesity conditions [18]. The serum total cholesterol, low-density lipoprotein (LDL) cholesterol, and TG levels were significantly increased in the HFD group as compared with the ND group (Table 3). In contrast, the serum levels of these lipids were lower in the HGD + WGC group than in the HFD group. However, the ratio of high-density lipoprotein (HDL) cholesterol/total cholesterol was improved in the HFD + WGC group as compared with the obese HFD group. WGC prevents obesity-induced hyperlipidaemia by attenuating hypertriacylglycerolemia and hypercholesterolemia.
Moreover, the increases of liver weight (Table 3) and abnormal lipid accumulation in the liver were shown in the obese HFD group [Fig. 1(D, E)]. WGC decreased the HFD-induced liver weight by 15.8% in the HFD + WGC group relative to the HFD group. The lipid droplet size in liver tissue was decreased in the HFD + WGC group, resulting in a significant reduction in hepatic TG level by 46.1% compared with the HFD group. Since WGC reduced HFD-induced non-alcoholic fatty liver by decreasing the lipid droplet size and hepatic TG level, it may regulate imbalances in hepatic lipid metabolism of HFD-induced obesity in mice. Thus, these results suggest that WGC alleviates HFD-induced obesity, hyperlipidaemia, and non-alcoholic fatty liver.
It was reported that whole grain, cereal fiber, and bran were associated with a reduction in the risk of obesity, type 2 diabetes, and cardiovascular disease in humans [19]. Barley, a main ingredient in WGC, containing coumaric acid and ferulic acid, prevented adipogenic differentiation [20]. Beta-glucan, found in the cell walls of barley, improved fatty liver [21]. Brown rice and its major component, γ-oryzanol, decreased the preference for HFD in mice given the choice of a normal chow diet or HFD [22] and reduced obesity and diabetes in humans [23]. Moreover, a germinated brown rice extract suppressed body weight gain in HFD-fed mice by controlling lipogenic genes [24] Collectively, these results indicate that the contents of WGC, as a mixture of multiple whole grain ingredients, have a synergistic effect on lipid metabolism.
WGC improves skeletal muscle masses and enhances muscle strength in HFD-fed obese mice
Previous studies have shown the beneficial effects of WGC on various health problems, including the metabolic syndrome, diabetes, cardiovascular disease, stroke, hypertension, and cancer [11, 12]. A recent study observed for the first time that γ-oryzanol, a major component of brown rice, influenced the proliferation of muscle satellite cells [25]. However, there are not many studies showing the effect of WGC on muscle atrophy in obese mice. In this study, using a mouse model of HFD-induced obesity, we demonstrated that WGC improves obesity-induced muscle atrophy.
The GN, SOL, TA, and EDL muscle weights were increased by 8.1, 19.9, 8.0, and 33.2%, respectively, in the HFD + WGC group in comparison with the HFD group [Fig. 2(A)]. The ratio of skeletal muscle mass/body mass was diminished in the HFD group but increased in the HFD + WGC group [Fig. 2(B)]. The decrease in muscle mass ratio seen in the HFD group relative to the ND group was recovered in the HFD + WGC group. Histological analysis of GN muscle tissue showed that the increase of muscle mass in the HFD + WGC group was due to an effective increase in muscle size [Fig. 2(C, D)]. These results demonstrate that WGC improves muscle mass in HFD-fed obese mice by elevating the muscle fiber diameter.
Fig. 2.
Effect of whole grain cereal (WGC) on muscle mass. (A) Gastrocnemius (GN), soleus (SOL), tibialis anterior (TA), extensor digitorum longus (EDL), and total muscle mass. (B) Skeletal muscle mass/body weight ratio. The images from 60 random areas of the stained GN muscle per group were captured. The fiber cross-sectional area of GN muscle from the captured images was measured using the Image J software. (C) Representative images of GN muscle tissue (magnification × 100). (D) Quantitation of the muscle fiber cross-sectional area of GN muscle. (E) Grip strength test of fore/hind-limb and fore-limb. Data are presented as the mean ± SD of 7 animals per group. a–cvalues are significantly different at p < 0.05 by Duncan’s test
The muscle strength of the HFD group was decreased relative to that of the ND group [Fig. 2(E)]. In contrast, the muscle strength of the HFD + WGC group was significantly better than that of the HFD group. Fore/hind-limb and fore-limb muscle strengths were recovered by 14.3 and 17.6%, respectively, in the HFD + WGC group as compared with the HFD group. The results confirm that WGC improves muscle strength through increase of the muscle mass.
Loss of skeletal muscle is a debilitating complication resulting from inactivity, aging, denervation, cancer, and several other chronic illnesses [7]. In particular, obesity is associated with inflammatory cytokines, wherein tumor necrosis factor-alpha accelerates the suppression of muscle protein synthesis [7]. The characteristics of muscle atrophy is the loss of muscle mass and muscle strength by decreased protein synthesis and increased protein degradation [26, 27]. The results study showed that WGC inhibited obesity-induced muscle atrophy by improving the muscle mass, muscle strength, and muscle fiber size in HFD-fed obese mice.
WGC up-regulates muscle protein synthesis and down-regulates muscle protein degradation
On the molecular level, mTOR plays important roles in protein synthesis and leads to muscle hypertrophy by regulating p70S6K and 4EBP1 [9, 28]. MyoD and myogenin are the master myogenic regulatory factors, modulating the differentiation of muscle cells and the formation of myoblasts [9, 29]. In this study, WGC up-regulated the phosphorylation of PI3K, Akt, mTOR, p70S6K, and 4EBP1 and also activated the mRNA expression of MyoD and myogenin [Fig. 3(A–C)]. These results suggest that WGC improves both the skeletal muscle mass by activating muscle hypertrophy and the synthesis of muscle fibers by stimulating muscle myogenesis.
Fig. 3.
Effect of whole grain cereal (WGC) on the expression of skeletal muscle hypertrophy- and atrophy-related genes in the gastrocnemius muscle. (A) Western blot analysis of the expression levels of p-PI3K, t-PI3K, p-Akt, and t-Akt. (B) Western blot analysis of the expression levels of p-mTOR, t-mTOR, p-p70S6K, t-p70S6K, p-4EBP1, and t-4EBP1. (C) RT-PCR analysis of the expression levels of MyoD and myogenin. (D) Western blot analysis of the expression levels of p-FoxO3a and t-FoxO3a. (E) RT-PCR analysis of the expression levels of atrogin-1 and MuRF1. α-Tubulin and β-actin were used as the housekeeping genes
In contrast, the dephosphorylation of FoxO3a leads to nuclear entry and induces a reduction of muscle mass by activating transcriptional activity that modulates muscle development negatively [7, 29]. Subsequently, E3 ubiquitin ligase-related genes, such as atrogin-1 and MuRF1, promote the degradation of muscle protein, leading to muscle atrophy [4, 7]. Cytoplasmic FoxO3a protein expression was up-regulated, whereas E3 ubiquitin ligase-related gene mRNA expression was down-regulated in the HFD + WGC group [Fig. 3(D, E)], indicating that the positive effects of WGC on muscle mass and muscle strength could be through hypertrophy activation and atrophy inhibition. Taken together, the results imply that WGC attenuates HFD-induced muscle atrophy by up-regulating protein synthesis and down-regulating protein degradation.
In conclusion, WGC formulation attenuated the symptoms of obesity, including body weight gain, hyperlipidaemia, non-alcoholic fatty liver, fat pad mass, and adipocyte size. Concomitantly, WGC significantly improved skeletal muscle mass and muscle strength through up-regulation of muscle hypertrophy and muscle differentiation factors, with concomitant down-regulation of muscle atrophic factors. The results suggest that WGC could be used as a functional food for the treatment of obesity-induced muscle atrophy. Further extensive studies are required to draw a conclusion about the inhibitory effect of WGC on muscle atrophy through animal and clinical experiments, applying a variety of WGC formulations.
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (315071-03).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ho JN, Son ME, Lim WC, Lim ST, Cho HY. Germinated brown rice extract inhibits adipogenesis through the down-regulation of adipogenic genes in 3T3-L1 adipocytes. Plant Foods Hum. Nutr. 2013;68:274–278. doi: 10.1007/s11130-013-0366-9. [DOI] [PubMed] [Google Scholar]
- 2.Ho JN, Choi JW, Lim WC, Kim MK, Lee IY, Cho HY. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2013;93:485–490. doi: 10.1002/jsfa.5792. [DOI] [PubMed] [Google Scholar]
- 3.Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/β-catenin signaling. Am. J. Physiol. Cell Physiol. 2010;298:C1510–C1516. doi: 10.1152/ajpcell.00369.2009. [DOI] [PubMed] [Google Scholar]
- 4.Le NH, Kim CS, Park T, Park JH, Sung MK, Lee DG, Hong SM, Choe SY, Goto T, Kawada T, Yu R. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014;2014:834294. doi: 10.1155/2014/834294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pompeani N, Rybalka E, Latchman H, Murphy RM, Croft K, Hayes A. Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J. Negat. Results Biomed. 2014;13:19. doi: 10.1186/s12952-014-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015;74:405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 7.Sishi B, Loos B, Ellis B, Smith W, du Toit EF, Engelbrecht AM. Diet-induced obesity alters signalling pathways and induces atrophy and apoptosis in skeletal muscle in a prediabetic rat model. Exp. Physiol. 2011;96:179–193. doi: 10.1113/expphysiol.2010.054189. [DOI] [PubMed] [Google Scholar]
- 8.Wang DT, Lu L, Shi Y, Geng ZB, Yin Y, Wang M, Wei LB. Supplementation of ketoacids contributes to the up-regulation of the Wnt7a/Akt/p70S6K pathway and the down-regulation of apoptotic and ubiquitin-proteasome systems in the muscle of 5/6 nephrectomised rats. Br. J. Nutr. 2014;111:1536–1548. doi: 10.1017/S0007114513004091. [DOI] [PubMed] [Google Scholar]
- 9.Ge Y, Chen J. Mammalian target of rapamycin (mTOR) signaling network in skeletal myogenesis. J. Biol. Chem. 2012;287:43928–43935. doi: 10.1074/jbc.R112.406942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung SI, Kim TH, Rico CW, Kang MY. Effect of instant cooked giant embryonic rice on body fat weight and plasma lipid profile in high fat-fed mice. Nutrients. 2014;6:2266–2278. doi: 10.3390/nu6062266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borneo R, Leon AE. Whole grain cereals: functional components and health benefits. Food Funct. 2012;3:110–119. doi: 10.1039/C1FO10165J. [DOI] [PubMed] [Google Scholar]
- 12.Shin SH, Song JL, Park MG, Park MH, Hwang SJ, Park KY. Effects of natural raw meal (NRM) on high-fat diet and dextran sulfate sodium (DSS)-induced ulcerative colitis in C57BL/6 J mice. Nutr. Res. Pract. 2015;9:619–627. doi: 10.4162/nrp.2015.9.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim MB, Song Y, Kim C, Hwang JK. Kirenol inhibits adipogenesis through activation of the Wnt/β-catenin signaling pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2014;445:433–438. doi: 10.1016/j.bbrc.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer-Lequart C, Lehmann U, Ross AB, Roger O, Eldridge AL, Ananta E, Bietry MF, King LR, Moroni A, Srichuwong S. Whole grain in foods: current use, challenges and the way forward. Crit. Rev. Food Sci. Nut. 2017;57:1562–1568. doi: 10.1080/10408398.2013.781012. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Ross AB, Åman P, Kamal-Eldin A. Alkylresorcinols as markers of whole grain wheat and rye in cereal products. J. Agric. Food Chem. 2004;52:8242–8246. doi: 10.1021/jf049726v. [DOI] [PubMed] [Google Scholar]
- 16.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR, Spiegelman D, Willett W, Rimm E. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. Am. J. Clin. Nutr. 2004;80:1237–1245. doi: 10.1093/ajcn/80.5.1237. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari U, Chaudhari HS, Bisnoi AN, Kumar V, Khanna G, Javed K. Anti-obesity effect of standardized ethanol extract of Embelia ribes in murine model of high fat diet-induced obesity. PharmaNutrition. 2013;1:50–57. doi: 10.1016/j.phanu.2013.01.001. [DOI] [Google Scholar]
- 18.Davaatseren M, Hur HJ, Yang HJ, Hwang JT, Park JH, Kim H-J, Kim MJ, Kwon DY, Sung MJ. Taraxacum official (dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013;58:30–36. doi: 10.1016/j.fct.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Cho SS, Qi L, Fahey GC, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013;98:594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 20.Seo CR, Yi B, Oh S, Kwon SM, Kim S, Song NJ, Cho JY, Park KM, Ahn JY, Hong JW. Aqueous extracts of hulled barley containing coumaric acid and ferulic acid inhibit adipogenesis in vitro and obesity in vivo. J. Funct. Foods. 2015;12:208–218. doi: 10.1016/j.jff.2014.11.022. [DOI] [Google Scholar]
- 21.Choi JS, Kim H, Jung MH, Hong S, Song J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- 22.Kozuka C, Yabiku K, Sunagawa S, Ueda R, Taira S, Ohshiro H, Ikema T, Yamakawa K, Higa M, Tanaka H, Takayama C, Matsushita M, Oyadomari S, Shimabukuro M, Masuzaki H. Brown rice and its component, gamma-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes. 2012;61:3084–3093. doi: 10.2337/db11-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozuka C, Yabiku K, Takayama C, Matsushita M, Shimabukuro M, Masuzaki H. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ-oryzanol. Obes. Res. Clin. Pract. 2013;7:e165–e172. doi: 10.1016/j.orcp.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Ho JN, Son ME, Lim WC, Lim ST, Cho HY. Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012;76:1068–1074. doi: 10.1271/bbb.110666. [DOI] [PubMed] [Google Scholar]
- 25.Szcześniak K, Ciecierska A, Ostaszewski P, Sadkowski T. Transcriptomic profile adaptations following exposure of equine satellite cells to nutriactive phytochemical gamma-oryzanol. Genes Nutr. 2016;11:5. doi: 10.1186/s12263-016-0523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 27.Jeong JW, Shim JJ, Choi ID, Kim SH, Ra J, Ku HK, Lee DE, Kim TY, Jeung W, Lee JH. Apple pomace extract improves endurance in exercise performance by increasing strength and weight of skeletal muscle. J. Med. Food. 2015;18:1380–1386. doi: 10.1089/jmf.2014.3401. [DOI] [PubMed] [Google Scholar]
- 28.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int. J. Biochem. Cell Biol. 2011;43:1267–1276. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanou N, Gailly P. Skeletal muscle hypertrophy and regeneration: interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol. Life Sci. 2013;70:4117–4130. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]