Abstract
The effects of slightly acidic electrolyzed water ice (SAEW-ice) and grapefruit seed extract ice (GSE-ice) on changes in brown sole quality during storage were determined using microbial, chemical, and sensory analyses to prolong the shelf life of brown sole. Microbiological analyses showed that GSE-ice storage was more effective for inhibiting the growth of total plate count, Pseudomonas, and H2S-producing bacteria than SAEW-ice storage. Chemical indexes of brown sole showed that SAEW-ice and GSE-ice storage extended the shelf life of fish compared with TW-ice. Sensory scores following GSE-ice storage were higher than those following storage under the other conditions. Taken together, the present study indicated that the quality of brown sole was maintained for 9–10 days in TW-ice, and 11–12 days in SAEW-ice and 12–13 days in GSE-ice. Therefore, ice storage using SAEW-ice and GSE-ice effectively extended the shelf life of brown sole.
Keywords: Pleuronectes herzensteini, Ice storage, Slightly acidic electrolyzed water, Grapefruit seed extract, Shelf life
Introduction
Brown sole Pleuronectes herzensteini is a marine fish that lives in the sand of tropical and temperate regions, and it is distributed in Korea, Japan, the Yellow Sea, and the East China Sea. It is mainly caught using set nets, gill nets, and Danish seines. This species not only is an important coastal fishery resource but also is ecologically and morphologically unique [1, 2].
Fish is a highly perishable food that spoils faster than other muscle foods [3]. Spoilage can occur due to bacterial growth, chemical reactions such as autolytic reactions, and physical damage [4]. Ice storage effectively prevents corruption and extends the shelf life of fish [5]. Storage and transport of fish inland depends mainly on ice storage. However, unsanitary management of fisheries and contamination of ice water causes fish deterioration [6]. Recently, the effects of ice containing antimicrobial agent, like plant extract ice or ozonized slurry ice, on biochemical and microbiological properties related to fish spoilage have been reported [7].
Electrolyzed water is an effective disinfectant that facilitates preservation of freshness and safety of fish [8–10] and is used widely in medicine and to reduce numbers of surface microorganisms on fruit and vegetables [11, 12]. Electrolyzed water is produced by electrolysis of water containing sodium chloride (NaCl) or hydrochloric acid (HCl), leading to production of sodium hypochlorite (NaClO) or hypochlorous acid (HClO) [13]. Slightly acidic electrolyzed water (SAEW) has been produced by electrolysis of 2–6% HCl in an electrolytic seawater tank and has high sterilization effects at low effective chlorine concentrations [14]. Previous studies have demonstrated that SAEW has strong bactericidal activities against many foodborne pathogens, including Vibrio parahaemolyticus, Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enteritidis, Staphylococcus aureus, and Pseudomonas aeruginosa [ 15 – 17 ].
Recently, consumers preferences have moved toward natural antimicrobials rather than chemical antimicrobials [18]. Grapefruit seed extract (GSE) is a natural antimicrobial agent having antibacterial, antiviral, antifungal, and anti-parasite activities [19, 20]. The components of GSE such as ascorbyl palmitate, ascorbic acid, tocopherol have been reported to disrupt cell wall and cell membrane functions of pathogenic microorganisms, inhibit enzyme activity, and inhibit cell proliferation [21].
Although there are several studies that have examined the effect of SAEW-ice on fishery products, there is no report on ice made with natural antimicrobials such as GSE. In this study, the possibility of shelf life extension by ice containing antimicrobial agents, especially GSE, was identified and the effects of SAEW-ice and GSE-ice on improving shelf life of brown sole were explored using microbiological, chemical, and sensory analyses.
Materials and methods
Fish samples
Brown sole were purchased at the Noryangjin Fisheries Market in Seoul and were transported to the laboratory in an ice box. The weight and length of fish were 243.68 ± 37.53 g and 29.45 ± 2.26 cm, respectively.
Preparation of ice and storage conditions
Slightly acidic electrolyzed water was produced by electrolysis of 6% HCl solution in a chamber without a membrane using a SAEW generator. Grapefruit seed extract solution was made by 5% w/v using distilled water. Tap water (Available chlorine 0.03 ppm, pH 6.54) as a control, slightly acidic electrolyzed water (Available chlorine 45 ppm, pH 5.07) and 0.5% w/v grapefruit seed extract solution were frozen using the ice trays and used to preserve whole fish. Whole fish were placed in polystyrene boxes with a 2:1 ratio of ice: sample. The ice boxes were then stored at 0–1 °C for 30 days. Melted ice water was drained through provided holes and was replaced with new fresh ice periodically. Three fish samples were randomly taken from each batch held in ice every 5 days for microbiological, chemical, and sensory analyses.
Microbiological analyses
Ten grams of fish flesh were placed in sterile bag (Whirl–pak, 19 × 30 cm; Nasco, Fort Atkinson, WI, USA) and were homogenized in 90 mL of sterile saline (0.85% NaCl) using stomacher (Laboratory Blender Stomacher 400; Seward, MO, USA) for 2 min. Then, the homogenized samples were serially diluted using sterile saline, and 0.1 mL aliquots of dilutions were plated on appropriate media. Total plate counts were determined using plate count agar (PCA, Oxoid code CM325), and the inoculated plates were incubated at 20 °C for 4 days. The presence of Pseudomonas spp. was determined using Cetrimide fusidin cephaloridine agar (CFC, Oxoid) and incubated at 20 °C for 4 days. Iron agar (IA, Conda) was plated and incubated at 20 °C for 4 days in order to determine the presence of H2S-producing bacteria.
Chemical analyses (pH, TVB-N, K value)
The pH of fish was measured using a digital pH meter (Orion 2 STAR, Thermo Scientific, MA, USA) after homogenizing 5 g of fish flesh with 10 mL of distilled water.
TVB-N (Total volatile basic nitrogen) concentrations were estimated by the microdiffusion method. Extracts were prepared by homogenizing 10 g samples with 50 mL of 6% trichloroacetic acid (TCA) for 2 min. Then extracts were centrifuged at 3000g for 10 min and were filtered, and TVB-N values were determined according to the methods of Conway and Byrne [22].
K value were defined as the ratio of the sum of hypoxanthine (Hx) and inosine (HxR) to total concentrations of other nucleotides. Concentrations of ATP-related compounds were determined using HPLC (high performance liquid chromatography, Jasco) according to the procedure described by Ryder [23]. Chemical reagents used for nucleotide standard solutions were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standard curves for each of the nucleotides were prepared using solutions of 10, 20, 50, 100, and 200 mg/L. Five grams of the fish flesh were blended with 25 mL of chilled 0.6 M perchloric acid solution in the ice box, and centrifuged at 6000g for 10 min. Subsequently, pH values of the 10 mL supernatants were adjusted to 6.8–7.0 using 1 M-KOH solution. After standing in the ice box for 30 min, supernatants were made up to 20 mL with HPLC grade water and were filtered through a 0.45 μm syringe filter, then stored at −80 °C until analysis. The mobile phase was comprised of 0.06 M dipotassium hydrogen phosphate and 0.04 M potassium dihydrogen phosphate in HPLC grade water and adjusted to pH 7. The mobile phase solutions were prepared daily and filtered using 0.45 μm filter. A 5 μL of samples were injected and nucleotides were separated using Phenomenex Luna 5 μm C18 [2] 100 Å (250 × 4.6 mm) column. The flow rate was 1.5 mL/min, and the wavelength for monitoring peak was set as 254 nm. K value was calculated by the following equation [24].
Sensory analyses
Sensory analyses of brown sole were performed by 10 trained panelists using a 9-point hedonic scale (9 in like extremely and 1 is dislike extremely) as described by Kamalakanth [3]. The panelists scored for appearance, colour, odour, and overall acceptability of the given whole fish samples. The scores above 4 were considered acceptable.
Statistical analyses
All experiments were performed in triplicate and data were expressed as means ± standard deviations. Statistical analyses were performed using SPSS statistic software (ver. 23.0, IBM Corp., Armonk, NY, USA). Differences were identified using ANOVA and Duncan’s multiple range tests and were considered significant when p < 0.05.
Results and discussion
Changes in total plate counts, Pseudomonas spp. and H2S-producing bacteria
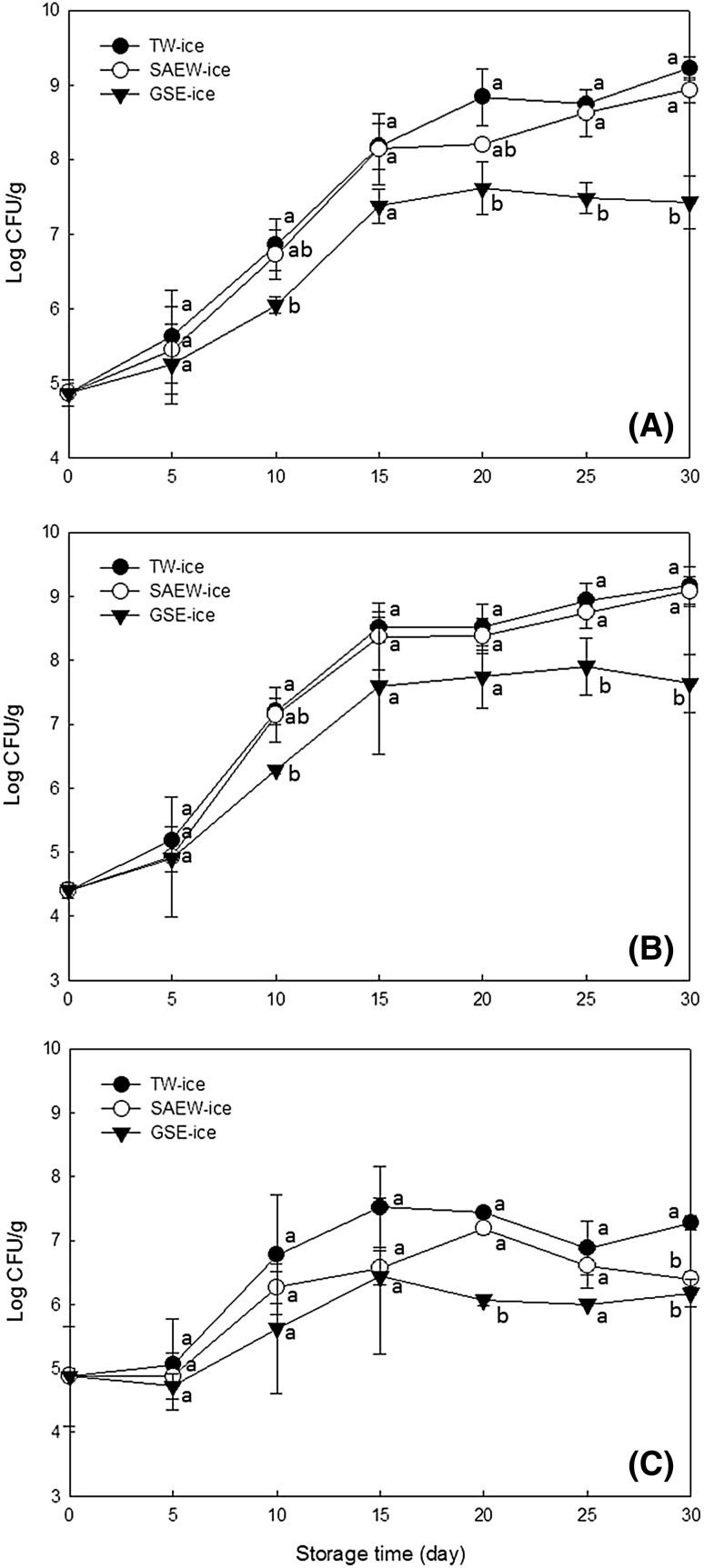
The changes in microbiological counts of brown sole stored in TW-ice, SAEW-ice, and GSE-ice are presented in Fig. 1. Total plate counts of brown sole are shown in Fig. 1A. Total plate counts were correlated with storage times, and increased significantly after 10 days under all ice storage conditions (p < 0.05). Samples that were stored in SAEW-ice tended to have lower total plate counts than those stored in TW-ice, although no significant differences were identified. In contrast, total plate counts in samples stored in GSE-ice tended to be significantly lower than those stored in TW-ice or SAEW-ice after day 10 of storage (p < 0.05). The maximum allowable total plate counts of freshwater or saltwater fish are 7 log CFU/g [4], and those in brown sole stored in TW-ice and SAEW-ice exceeded this value on day 11, whereas those stored in GSE-ice reached this threshold on day 13 of storage.
Fig. 1.
Changes in microbial count of brown sole during ice storage under tap water ice (TW-ice), slightly acidic electrolyzed water ice (SAEW-ice), grapefruit seed extract ice (GSE-ice). (A) Total plate count; (B) Pseudomonas spp.; (C) H2S-producing bacteria
The changes of Pseudomonas spp. counts are shown in Fig. 1B. The number of Pseudomonas spp. increased with increasing storage times in all treatment groups, and increased significantly from day 5 of storage in TW-ice (p < 0.05) and from 10 days of storage in SAEW-ice and GSE-ice (p < 0.05). Growth rates of Pseudomonas were the slowest in GSE-ice treatment, and the results showed that there was a significant difference between TW-ice treatment and GSE-ice treatment after 10 days of storage (p < 0.05). Storage in SAEW-ice led to slightly lower Pseudomonas growth rates than storage in TW-ice but no significant differences were identified (p > 0.05).
Pseudomonas spp. has been widely associated with aquatic product degradation [25]. In this study, Pseudomonas counts were similar to total plate counts, indicating that Pseudomonas was the dominant spoilage species in the present storage conditions. The initial numbers of Pseudomonas (day 0) were 4.40 log CFU/g and reached 7 log CFU/g after 10, 10 and 13 days of storage in TW-ice, SAEW-ice, and GSE-ice, respectively.
H2S-producing bacteria changes can be seen in Fig. 1C. The numbers of H2S-producing bacteria showed an increasing trend during the ice storage, but increased relatively slowly compared with other microorganisms. Whereas significant increases in counts were detected from day 10 of storage in TW-ice and SAEW-ice (p < 0.05), significant increases were detected after 15 days of storage in GSE-ice (p < 0.05). Accordingly, significant differences (p < 0.05) in H2S-producing bacteria were observed between TW-ice samples and GSE-ice samples from 20th day of storage, and H2S-producing bacterial counts of samples treated with TW-ice and SAEW-ice reached 7 log CFU/g on days 12 and 18, respectively. The maximum H2S-producing bacterial count in fish samples stored in GSE-ice (6.44 log CFU/g) did not reach 7 log CFU/g.
Similar to Pseudomonas, H2S-producing bacteria are spoilage bacteria [25]. Shewanella putrefaciens is a representative strain of H2S-producing bacteria and its number increases as spoilage progresses [26]. However, in the present study, H2S-producing bacteria tended to grow more slowly than Pseudomonas, likely reflecting inhibition of growth by dominant Pseudomonas. The reason for this was assumed that Pseudomonas has grown so fast that shewanella could be diluted out [27] because H2S-producing bacteria and Pseudomonas have been shown to be highly competitive psychrotrophic bacteria [28].
It is known that SAEW has effective disinfection so that many previous studies have demonstrated about its bactericidal effects against many pathogens such as Vibrio parahaemolyticus, Staphylococcus aureus, Escherichia coli O157:H7, Listeria monocytogenes. However, antibacterial activities of SAEW are lost rapidly, and its antimicrobial efficacy deteriorates in the presence of organic materials [29]. Accordingly, in this study, the antimicrobial effects of SAEW-ice were weaker than those of GSE-ice.
In this study, GSE-ice effectively inhibited the growth of microorganisms, especially Pseudomonas, whereas SAEW-ice had no significant effect on microbial counts. These data indicate that GSE-ice prolongs the shelf life of brown sole more effectively than conventional ice storage, with more stable and stronger antimicrobial activity in the presence of organic materials such as fish skin. Song et al. [30] referred that grapefruit seed extract has antimicrobial activity by inhibiting bacterial enzymes and weakening bacterial cell membranes and walls. The antimicrobial activities of grapefruit seed extract have demonstrated in other several studies [30, 31], however, there is no study to prolong the shelf life of aquatic products using grapefruit seed extract ice. Therefore, this study is considered to be meaningful for applying to the seafood industry.
Change in pH, TVB-N and K value
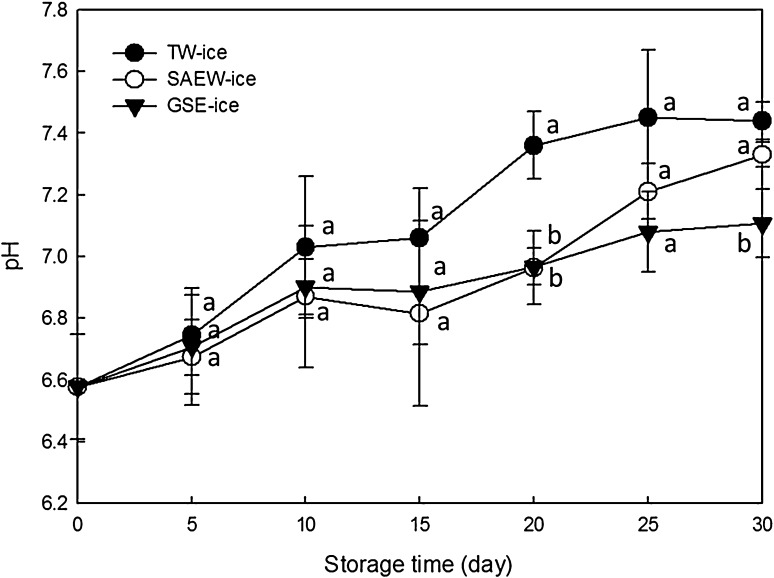
Chemical analyses of pH, TVB-N, and K values showed lower spoilage values and longer maintenance of freshness in SAEW-ice and GSE-ice storage conditions compared with TW-ice storage. Figure 2 shows changes in pH of the brown sole samples stored in TW-ice, SAEW-ice, and GSE-ice.
Fig. 2.
Changes in pH of brown sole during ice storage under various conditions; tap water ice (TW-ice), slightly acidic electrolyzed water ice (SAEW-ice), grapefruit seed extract ice (GSE-ice). Bars indicate means ± standard deviations of three replicates
The pH of fresh fish is close to neutral, but increases due to decomposition of nitrogen compounds. Thus, an increase in pH can be an indicator of aquatic products corruption [32]. In the present study, pH values tended to increase from the initial value of 6.58 with storage time in all treated samples. However, significant increases of pH in brown sole stored in TW-ice, SAEW-ice, and GSE-ice were identified after 10, 20, and 10 days of storage, respectively (p < 0.05). Moreover, significant differences were noticed between SAEW-ice, GSE-ice and TW-ice samples on day 20 of storage (p < 0.05), and the pH of samples placed in TW-ice, SAEW-ice and GSE-ice were 7.36, 6.96, and 6.97 on day 20, respectively.
TVB-N levels in brown sole samples during ice storage are shown in Fig. 3. TVB-N steadily increased with the storage time under all conditions, and these increases were significant from days 10, 15, and 10 in samples stored in TW-ice, SAEW-ice, and GSE-ice, respectively (p < 0.05). On the 20th day of storage, TVB-N of samples stored in TW-ice, SAEW-ice, and GSE-ice were 39.08, 26.45, and 33.93 mg%, respectively, and there was a significant difference between TW-ice and SAEW-ice samples (p < 0.05).
Fig. 3.
Changes in TVB-N of brown sole during ice storage under various conditions: tap water ice (TW-ice), slightly acidic electrolyzed water ice (SAEW-ice), grapefruit seed extract ice (GSE-ice). Bars indicate means ± standard deviations of three replicates
TVB-N is produced by endogenous enzymes during the progression of bacterial spoilage and is widely used as an indicator for freshness and quality of fish [7]. Previous studies report a maximum allowable upper TVB-N limit of 30 mg% for consumable aquatic products [7, 33]. In the present study, TVB-N values reached 30 mg% after 14, 23, and 18 days in TW-ice, SAEW-ice, and GSE-ice storage, respectively.
TVB-N values of brown sole on days 11 and 13 day did not reach 30 mg%, whereas total plate counts reached 7 log/CFU in these samples, indicating that TVB-N values alone are insufficient to determine the freshness of aquatic products. Therefore, in agreement with Kyrana et al. [34] and Tejada and Huidobro [32], we suggest the need for multiple indices of freshness, including microbiological changes and sensory analyses.
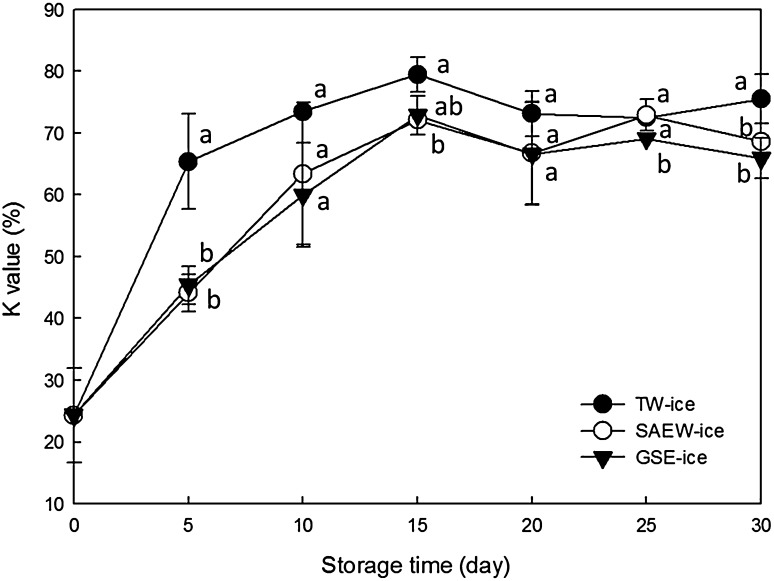
Changes in K values of brown sole stored in TW-ice, SAEW-ice, and GSE-ice are illustrated in Fig. 4. In these experiments, K values n brown sole stored in TW-ice, SAEW-ice, and GSE-ice were significantly increased from day 5 of storage (p < 0.05). K values of samples stored in GSE-ice and SAEW-ice tended to be significantly lower than those stored in TW-ice after day 5 of storage (p < 0.05).
Fig. 4.
Changes in K-value of brown sole during ice storage under various conditions: tap water ice (TW-ice), slightly acidic electrolyzed water ice (SAEW-ice), grapefruit seed extract ice (GSE-ice). Bars indicate means ± standard deviations of three replicates
Increasing K values reflect degradation of nucleic acid-related substances in muscles and are used as an effective indicator of the freshness of fish and shellfish. In general, K values of less than 5% are observed in fresh fish, and K values of up to 20% have been observed in raw fish. Moreover, K values of more than 50% are known to lead to early spoilage [35]. Ehira [35] and Ehira and Uchiyama [35] set the upper K value limit of acceptable fish at 60%. In this study, K values of brown sole approached 60% on days 4, 9, and 10 of storage in TW-ice, SAEW-ice, and GSE-ice, respectively, indicating that K values are a more sensitive indicator of the freshness of fish than microbiological and other physicochemical analyses such as TVB-N.
Sensory evaluation
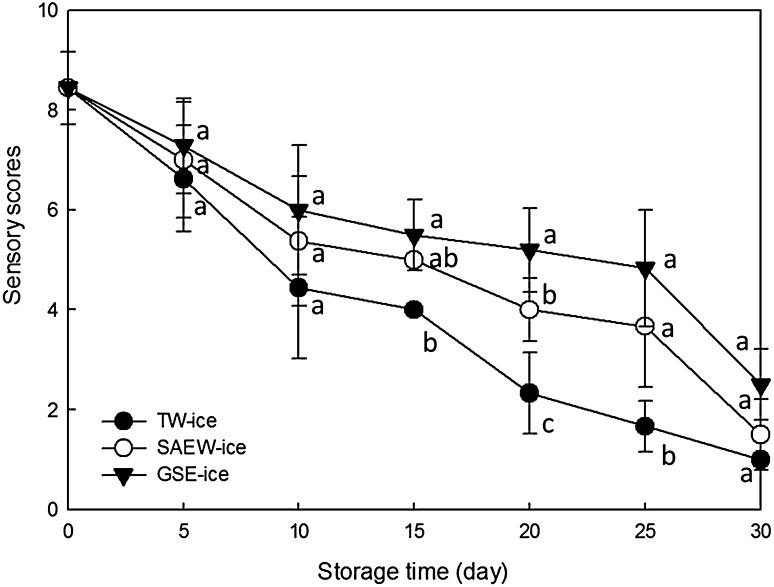
The sensory analyses of appearance, color, odor, and overall acceptability were performed by panelists on each sampling day, and average scores of overall acceptability were calculated (Fig. 5). These results indicate declines in sensory scores with increasing ice storage periods in all cases. Specifically, sensory scores of fish from TW-ice and SAEW-ice storage were significantly decreased from 5 day (p < 0.05) and were significantly decreased from day 10 in the GSE-ice group (p < 0.05). On day 10 of storage, sensory scores of brown sole stored in TW-ice, SAEW-ice, and GSE-ice were 4.44, 5.38, and 6.00, respectively, and there were significantly differences (p < 0.05) between TW-ice samples and GSE-ice samples from 10 day of storage. On day 20, sensory scores of samples stored in TW-ice, SAEW-ice, and GSE-ice were 2.33, 4.00, and 5.20, respectively, and there were significant differences among all treatments (p < 0.05). Previously, Kamalakanth et al. [3] considered the score of 4 as the margin of acceptance, and in this study, samples stored in TW-ice, SAEW-ice, and GSE-ice declined to 4 points on days 15, 20, and 26, respectively.
Fig. 5.
Changes in sensory scores of brown sole during ice storage under various conditions: tap water ice (TW-ice), slightly acidic electrolyzed water ice (SAEW-ice), grapefruit seed extract ice (GSE-ice). Mean values are presented from all acceptability scores provided by 10 panelists
In conclusion, the microbial, chemical, and sensory properties were highly correlated with the freshness of brown sole. According to comprehensive analyses of all the present results, the required quality standard of the brown sole stored in TW-ice, SAEW-ice, and GSE-ice was maintained up to 9–10, 11–12, and 12–13 days, respectively. Taken together, the results of this study indicate that SAEW-ice and GSE-ice can extend the shelf life of brown sole. In particular, the GSE-ice can be used as preservation method to improve quality and extend shelf life of fish products. However, only a single concentration of GSE was used in the present study, and further studies are needed to compare the effects of various concentrations of GSE or other natural antimicrobials.
Acknowledgements
This research was a part of the project titled “Development of Freshness Maintenance System Using Slightly Acidic Hypochlorous Seawater Ice,” funded by the Ministry of Oceans and Fisheries, Korea.
References
- 1.Cha H, Park K, Lee S, Park H, Kwon H, Choi S. Maturity and spawning of brown sole, Pleuronectes herzensteini (Jordan et Snyder) in the East Sea of Korea. Korean. J. Ichthyol. 2006;18:363–367. [Google Scholar]
- 2.Takahashi T, Hayakawa Y, Kamiharako T, Nakatani T, Takatsu T. Age and growth of brown sole Pleuronectes herzensteini in the coastal waters of western Aomori Prefecture. Japan. Fish. Sci. 1995;61:893–897. doi: 10.2331/fishsci.61.893. [DOI] [Google Scholar]
- 3.Kamalakanth C, Ginson J, Bindu J, Venkateswarlu R, Das S, Chauhan O, Gopal T. Effect of high pressure on K-value, microbial and sensory characteristics of yellowfin tuna (Thunnus albacares) chunks in EVOH films during chill storage. Innov. Food Sci. Emerg. Technol. 2011;12:451–455. doi: 10.1016/j.ifset.2011.06.001. [DOI] [Google Scholar]
- 4.Mohan C, Ravishankar C, Gopal TS, Lalitha K, Kumar KA. Effect of reduced oxygen atmosphere and sodium acetate treatment on the microbial quality changes of seer fish (Scomberomorus commerson) steaks stored in ice. Food microbiol. 2010;27:526–534. doi: 10.1016/j.fm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Fan W, Chi Y, Zhang S. The use of a tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food chem. 2008;108:148–153. doi: 10.1016/j.foodchem.2007.10.057. [DOI] [Google Scholar]
- 6.Lee N-G. Water properties of electrolytic machine by stainless diaphragm and effects of electrolytic ice water storage for keeping freshness of squid, Todarodes pacificus. J. Fish. Marine Sci. Edu. 18 (2006)
- 7.Bensid A, Ucar Y, Bendeddouche B, Özogul F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food chem. 2014;145:681–686. doi: 10.1016/j.foodchem.2013.08.106. [DOI] [PubMed] [Google Scholar]
- 8.Quan Y, Choi K-D, Chung D, Shin I-S. Evaluation of bactericidal activity of weakly acidic electrolyzed water (WAEW) against Vibrio vulnificus and Vibrio parahaemolyticus. Int. J. Food Microbiol. 2010;136:255–260. doi: 10.1016/j.ijfoodmicro.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B. Ma L-k, Deng S-g, Xie C, Qiu X-h. Shelf-life of Pacific white shrimp (Litopenaeus vannamei) as affected by weakly acidic electrolyzed water ice-glazing and modified atmosphere packaging. Food Control. 2015;51:114–121. doi: 10.1016/j.foodcont.2014.11.016. [DOI] [Google Scholar]
- 10.Xuan X-T, Fan Y-F, Ling J-G, Hu Y-Q, Liu D-H, Chen S-G, Ye X-Q, Ding T. Preservation of squid by slightly acidic electrolyzed water ice. Food Control. 2017;73:1483–1489. doi: 10.1016/j.foodcont.2016.11.013. [DOI] [Google Scholar]
- 11.Xie J, Sun XH, Pan YJ, Zhao Y. Physicochemical properties and bactericidal activities of acidic electrolyzed water used or stored at different temperatures on shrimp. Food Res. Int. 2012;47:331–336. doi: 10.1016/j.foodres.2011.07.041. [DOI] [Google Scholar]
- 12.Mansur AR, Oh DH. Combined effect of thermosonication and slightly acidic electrolyzed water to reduce foodborne pathogens and spoilage microorganisms on fresh‐cut kale. J. Food Sci. 80 (2015) [DOI] [PubMed]
- 13.Yoo J-Y, Jang K-I. Changes in quality of soybean sprouts washed with electrolyzed water during storage. J. Korean Soc. Food Sci. Nutrition. 2011;40:586–592. doi: 10.3746/jkfn.2011.40.4.586. [DOI] [Google Scholar]
- 14.Kim H-Y, Choi J-K, Shin I-S. Bactericidal effects of hypochlorous acid water against Vibrio parahaemolyticus contaminated on raw fish and shellfish. Korean J. Food Sci. Technol. 2015;47:719–724. doi: 10.9721/KJFST.2015.47.6.719. [DOI] [Google Scholar]
- 15.Cao W, Zhu ZW, Shi ZX, Wang CY, Li BM. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int. J. Food Microbiol. 2009;130:88–93. doi: 10.1016/j.ijfoodmicro.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Issa-Zacharia A, Kamitani Y, Morita K, Iwasaki K. Sanitization potency of slightly acidic electrolyzed water against pure cultures of Escherichia coli and Staphylococcus aureus, in comparison with that of other food sanitizers. Food Control. 2010;21:740–745. doi: 10.1016/j.foodcont.2009.11.002. [DOI] [Google Scholar]
- 17.Deza M, Araujo M, Garrido M. Efficacy of neutral electrolyzed water to inactivate Escherichia coli, Listeria monocytogenes, Pseudomonas aeruginosa, and Staphylococcus aureus on plastic and wooden kitchen cutting boards. J. Food Prot. 2007;70:102–108. doi: 10.4315/0362-028X-70.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Yang S-K, Kim J-J, Kim S-J, Oh S-W. Synergistic effect of grapefruit seed extract, EDTA and heat on inactivation of Bacillus cereus spore. J. Korean Soc. Food Sci. Nutr. 2011;40:1469–1473. doi: 10.3746/jkfn.2011.40.10.1469. [DOI] [Google Scholar]
- 19.Heggers JP, Cottingham J, Gusman J, Reagor L, McCoy L, Carino E, Cox R, Zhao J-G. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: II. Mechanism of action and in vitro toxicity. J. Altern. Complement. Med. 8: 333–340 (2002) [DOI] [PubMed]
- 20.Reagor L, Gusman J, McCoy L, Carino E, Heggers JP. The effectiveness of processed grapefruit-seed extract as an antibacterial agent: I. An in vitro agar assay. J. Altern. Complement. Med. 8: 325–332 (2002) [DOI] [PubMed]
- 21.Park H-K, Kim S-B. Antimicrobial activity of grapefruit seed extract. Korean J. Food Nutr. 2006;19:526–531. [Google Scholar]
- 22.Conway EJ, Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem. J. 1933;27:419. [PMC free article] [PubMed] [Google Scholar]
- 23.Ryder JM. Determination of adenosine triphosphate and its breakdown products in fish muscle by high-performance liquid chromatography. J. Agric. Food Chem. 1985;33:678–680. doi: 10.1021/jf00064a027. [DOI] [Google Scholar]
- 24.Saito T. A new method for estimating freshness of fish. Nippon Suisan Gakkaishi. 1958;24:749–750. doi: 10.2331/suisan.24.749. [DOI] [Google Scholar]
- 25.Chytiri S, Chouliara I, Savvaidis I, Kontominas M. Microbiological, chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol. 2004;21:157–165. doi: 10.1016/S0740-0020(03)00059-5. [DOI] [Google Scholar]
- 26.Kyrana VR, Lougovois VP. Sensory, chemical and microbiological assessment of farm-raised European sea bass (Dicentrarchus labrax) stored in melting ice. Int. J. Food Sci. Technol. 2002;37:319–328. doi: 10.1046/j.1365-2621.2002.00572.x. [DOI] [Google Scholar]
- 27.Gram L, Melchiorsen J. Interaction between fish spoilage bacteria Pseudomonas sp. and Shewanella putrefaciens in fish extracts and on fish tissue. J. Appl. Microbiol. 1996;80:589–595. doi: 10.1111/j.1365-2672.1996.tb03262.x. [DOI] [PubMed] [Google Scholar]
- 28.Koutsoumanis K, Lampropoulou K. Nychas G-JE. Biogenic amines and sensory changes associated with the microbial flora of Mediterranean gilt-head sea bream (Sparus aurata) stored aerobically at 0, 8, and 15 C. J. Food Prot. 1999;62:398–402. doi: 10.4315/0362-028X-62.4.398. [DOI] [PubMed] [Google Scholar]
- 29.Oomori T, Oka T, Inuta T. ARATA Y. The efficiency of disinfection of acidic electrolyzed water in the presence of organic materials. Anal. Sci. 2000;16:365–369. doi: 10.2116/analsci.16.365. [DOI] [Google Scholar]
- 30.Song HY, Shin YJ, Song KB. Preparation of a barley bran protein–gelatin composite film containing grapefruit seed extract and its application in salmon packaging. J. Food Eng. 2012;113:541–547. doi: 10.1016/j.jfoodeng.2012.07.010. [DOI] [Google Scholar]
- 31.Xu W, Qu W, Huang K, Guo F, Yang J, Zhao H, Luo Y. Antibacterial effect of grapefruit seed extract on food-borne pathogens and its application in the preservation of minimally processed vegetables. Postharvest Biol. Technol. 2007;45:126–133. doi: 10.1016/j.postharvbio.2006.11.019. [DOI] [Google Scholar]
- 32.Tejada M, Huidobro A. Quality of farmed gilthead seabream (Sparus aurata) during ice storage related to the slaughter method and gutting. Eur. Food Res. Technol. 2002;215:1–7. doi: 10.1007/s00217-002-0494-1. [DOI] [Google Scholar]
- 33.Harpaz S, Glatman L, Drabkin V, Gelman A. Effects of herbal essential oils used to extend the shelf life of freshwater-reared Asian sea bass fish (Lates calcarifer) J. Food Prot. 2003;66:410–417. doi: 10.4315/0362-028X-66.3.410. [DOI] [PubMed] [Google Scholar]
- 34.Kyrana VR, Lougovois VP, Valsamis DS. Assessment of shelf-life of maricultured gilthead sea bream (Sparus aurata) stored in ice. Int. J. Food Sci. Technol. 1997;32:339–347. doi: 10.1046/j.1365-2621.1997.00408.x. [DOI] [Google Scholar]
- 35.Ehira S. A biochemical study on the freshness of fish. Lab: Bull. Tokai Reg. Fish. Res; 1976. [Google Scholar]