Abstract
This study evaluated the antimicrobial activities [diffusion inhibition zone, minimum inhibitory concentration (MIC), and minimum bactericidal concentration], of heated ginseng extracts (ethanol and methanol). The extract yields, ginsenoside compositions, growth inhibitory effects against Bacillus cereus and Staphylococcus aureus and bacterial cell membrane potential changes, were also investigated. Methanol extracts of heated ginseng, showed higher antimicrobial activity than ethanol extracts. B. cereus was more easily inhibited than S. aureus. Ginseng heated at 100 °C for 2 and 16 h, showed maximum antimicrobial activity against B. cereus and S. aureus, respectively. In the growth inhibitory test, S. aureus and B. cereus were completely inhibited after 2 and 8 h culture at the MIC. The cell membrane potential decreased with increasing concentration of extract, indicating cell metabolism disruption. Ginsenosides Rg3, a potent antibacterial substance, which were absent in non-heated ginseng, were produced by heating ginseng at 100 °C for 4 and 8 h, respectively.
Keywords: Ginseng, Antimicrobial activity, Methanol, Ethanol, Heat treatment
Introduction
The outbreaks of food poisoning have been recently increasing owing to environmental changes such as climate change, and foodborne bacteria are considered to be an important issue in the food industry [1]. The incidence of Bacillus cereus and Staphylococcus aureus in food has been widely reported because they are ubiquitously found in the environment and thus easily contaminate food [2, 3]. B. cereus and S. aureus produce thermostable enterotoxins that cause food poisoning, which is accompanied by symptoms such as vomiting, diarrhea, and stomach cramps [2, 4]. B. cereus is a gram-positive motile, spore-forming bacterium, which can germinate and grow even after heat treatments [5, 6]. B. cereus is present in the soil and can cause food poisoning in fresh foods, as well as in cooked foods, such as cooked rice, a common food in Korea [7, 8]. S. aureus is a gram-positive bacterium that is present on the skin and mucosal surfaces of humans and animals and has been isolated from kimbap and sushi in Korea [9, 10]. S. aureus can easily contaminate foods such as meat and dairy products because it is resistant to various environmental factors and antibiotics [11].
In the food industry, synthetic additives or chemicals are widely used to inhibit the growth of microorganisms to prevent spoilage and extend the shelf life of food. However, safety and health concerns regarding synthetic additives have increased, and thus, there is an increasing demand for safe and effective natural antimicrobials [12–15]. Therefore, the search for natural antimicrobial agents has increased to discover new agents with food applications [16].
Ginseng (Panax ginseng C.A. Meyer) is a widely known pharmacologically effective plant in East Asian countries, mainly in Korea, China, and Japan. Many studies have reported regarding its physiological activities such as antiaging, antiobesity, anticancer, antioxidant, antiinflammatory, analgesic, and antipyretic activities [17–20].
Ginseng contains an abundance of ginseng saponins called ginsenosides, which are the pharmacological components of ginseng. More than 25 types of ginsenosides have been identified [21]. Other non-saponin components such as polysaccharides, nitrogenous compounds, lipid-soluble components, organic acids, vitamins, minerals, and phenolic compounds, which have physiological activities, have also been reported [22–25]. The total amount of ginsenosides in ginseng increases with heat treatment, heating produces new ginsenoside types such as Rg3 and Rh2 [26]. In addition, heat treatment increases the free amino acid content of ginseng, and increased anticancer and antioxidant activities have been observed [24].
The antimicrobial activity of ginseng has been investigated in many studies. Ginseng extracts do not inhibit the growth of Pseudomonas aeruginosa, the causative organism of cystic fibrosis, but only 0.25% of a ginseng extract inhibits the swarming motility and biofilm formation of P. aeruginosa [27]. The less polar ginsenosides, produced via the heat treatment of ginseng saponins, have antimicrobial activities against Fusobacterium nucleatum, Clostridium perfringens, and Porphyromonas gingivalis, which cause oral malodor [28]. Another study showed that white, red, and extruded ginseng have antimicrobial activities against some gram-positive bacteria and yeast. The red ginseng extract has a higher antimicrobial activity than the white ginseng and extruded ginseng extracts, which is considered to occur because of heat treatment [29].
In this study, ginseng was heated to increase its antimicrobial activity against B. cereus and S. aureus. Ginseng was heated at various temperatures and for various time periods, and the antimicrobial activity was measured by the disc diffusion, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) assays. The growth inhibitory effects of heated ginseng on B. cereus and S. aureus, as well as the effect on the membrane potential, was also determined according to the concentration of ginseng extract. This study aimed to test the possibility of using heated ginseng as a natural antimicrobial agent.
Materials and methods
Materials
Four-year-old cultivated ginseng was purchased from Kyungdong market, Seoul, Korea. The water content of ginseng was measured as 76.83 ± 0.47%. All chemicals and solvents used in this study were at least of analytical grade.
Bacterial strains and culture conditions
Bacillus cereus (ATCC 14579) and S. aureus (ATCC 6538) were maintained in stock culture containing 50% glycerol at −80 °C. Each culture was transferred to tryptic soy agar (TSA; Oxoid, Hampshire, England) for activation and incubated at 37 °C for 24 h. One colony of each strain on TSA was inoculated into tryptic soy broth (TSB; Oxoid) and incubated at 37 °C for 24 h.
Heat treatment of ginseng
The antimicrobial activity of ginseng was tested after heat treatment. The ginseng was washed under running tap water and heated at 60, 80, 100, or 120 °C for 1 h using an autoclave. The ginseng was also heated at 100 °C for 1, 2, 4, 8, or 16 h. The heated ginseng was then extracted using 60% ethanol or methanol. The antimicrobial activities of all the extracts were tested.
Extract preparation and determination of the extract yield
Heated ginseng was freeze-dried for 3 days and ground to a fine powder. The powder (15 g) was mixed with 150 mL of 60% ethanol or methanol and then shaken at 200 rpm and 35 °C for 24 h [29]. The extracts were filtered through a Buchner funnel containing a Whatman no. 2 filter paper and the solvent was removed by vacuum rotary evaporation (Eyela, Tokyo, Japan) at 45 °C. The concentrated extracts were freeze-dried and stored at 4 °C. The extract yield was measured as the ratio of the weight of dried ginseng extract (g) relative to the weight of ginseng powder (g).
Disc diffusion assay
To compare the antimicrobial activities of the different extracts, the inhibitory effects on B. cereus and S. aureus were determined by the disc diffusion assay. Sterile TSA was cooled to 50 °C, and the bacterial strains (diluted to 6 log CFU/mL) were transferred to the medium. TSA was poured into Petri dishes and solidified. Next, 6-mm diameter paper discs were impregnated with 15 μL of the ginseng extract dissolved in dimethyl sulfoxide (DMSO) (400 mg/mL) and placed onto TSA, before incubating at 37 °C for 24 h. The antimicrobial activities were evaluated by measuring the zone of inhibition including the diameter of the disc.
MIC and MBC
To determine MIC and MBC of the ginseng extracts, the microdilution method was performed. Serial twofold dilutions of the extracts were added directly to a 96-well plate in the range of 0.346–200 mg/mL with TSB, and 20 μL of each diluted bacterial strain was inoculated into each well. The final concentration of each strain was approximately 6 log CFU/mL. The inoculated TSB without any extract was used as the control. All of the treatment and control tests included 5% (v/v) DMSO. The 96-well plate containing samples was incubated at 37 °C for 24 h. The absorbance was measured at 595 nm using an ELISA reader (BioTek Instrument Inc., Winooski, VT, USA) to confirm the MIC that did not increase the absorbance. A non-turbid MIC well was inoculated with 10 μL of the bacterial suspension onto TSA medium and cultured at 37 °C for 24 h to determine MBC at which the bacteria did not grow.
Measurement of bacterial inhibitory effects
To determine the antimicrobial effects of the ginseng extracts at various concentrations (1/4, 1/2, and 1 × MIC), 100 mL of TSB containing a final inoculum with 2–3 log CFU/mL of bacteria and the extract was incubated at 150 rpm and 37 °C for 48 h. The viable cells were serially diluted with 0.85% saline, plated on TSA every 4 h, and the plates were then incubated at 37 °C for 24 h. Countable colonies were expressed as log CFU/mL. A control experiment without ginseng extracts was also conducted. Ethanol extracts heated at 100 °C for 2 and 16 h were used for B. cereus (1/4 MIC, 0.39 mg/mL; 1/2 MIC, 0.78 mg/mL; MIC, 1.56 mg/mL) and S. aureus (1/4 MIC, 1.56 mg/mL; 1/2 MIC, 3.125 mg/mL; MIC, 6.25 mg/mL), respectively.
Measurement of membrane potential changes
The rhodamine 123 fluorescence method was used to determine the effects of ginseng extracts on the metabolic activity of bacterial cells [30]. B. cereus and S. aureus were cultivated in TSB at 37 °C for 24 h. The ethanol extract of ginseng heated at 100 °C for 16 h was added to B. cereus and the ethanol extract of ginseng heated at 100 °C for 2 h was added to S. aureus at 0, 1/4 MIC, 1/2 MIC, and MIC, respectively, and then cultured for 8 h. The bacterial suspension was washed with phosphate-buffered saline (PBS; 0.1 M, pH 7.4) and rhodamine 123 was added at a final concentration of 2 μg/mL. The mixture was left in the dark for 30 min. Next, the suspension was washed thoroughly and resuspended in PBS. A 100-mL aliquot of the suspension was dispensed into a 96-well plate and the fluorescence intensity was measured using a spectrophotometer (BioTek Instrument Inc.). Rhodamine 123 fluorescence was expressed as the mean fluorescence intensity (MFI) at excitation and emission wavelengths of 480 and 530 nm, respectively.
High-performance liquid chromatography (HPLC) analysis of ginsenosides
Each ginseng extract was analyzed using HPLC to investigate the changes in the ginsenoside contents after heat treatment. Each ginseng extract (100 mg) was dissolved in 5 mL of distilled water and ginsenosides Rg1, Re, Rf, Rh1 (S, R), Rb1, Rc, Rf1, Rb2, Rb3, Rd, Rf2, Rg3 (S, R), PPT (S, R), compound K, and Rh2 (S, R) were used as analytical standard materials. The ginsenoside contents were determined using a Waters SunFire C18 column (4.6 × 250 mm, 5 μm) with a mobile phase gradient system comprising water (A) and acetonitrile (B). The flow rate was 1.6 mL/min. The gradient elution conditions were 80% (0 min), 80% (10 min), 68% (40 min), 50% (55 min), 35% (72 min), 10% (82 min), 80% (84 min), and 80% (90 min). The absorbance was recorded at 203 nm using an ultraviolet detector (Jasco, Tokyo, Japan).
Statistical analysis
Experimental data were expressed as the mean ± SD. The results were analyzed using SPSS statistics software (ver. 23.0, IBM Corp., Armonk, NY, USA). One-way analysis of variance and Duncan’s multiple range test were used to detect significant differences between means (p < 0.05).
Results and discussion
Comparison of the extract yields
Ginseng samples were extracted using methanol or ethanol and then treated for 1 h at various temperatures (60, 80, 100, or 120 °C), and for 1, 2, 4, 8, or 16 h at 100 °C. The extract yield was calculated based on the weight of the ginseng powder (Table 1). The extract yield from the methanol extract increased from 28.11% for untreated ginseng to 30.06, 33.64, 34.28, and 31.96% as the heat treatment temperature increased from 60 to 80, 100, and 120 °C, respectively. When ethanol was used as the extraction solvent, there were no remarkable differences between the ginseng extract yields irrespective of the heating temperature. In general, the extract yields increased with the heat treatment period for both the methanol and ethanol extracts.
Table 1.
Extraction yield (%) of methanol and ethanol extracts of heated ginseng
| Yield (%) | ||
|---|---|---|
| Methanol | Ethanol | |
| Controla | 28.11 | 31.96 |
| Temperatureb (°C) | ||
| 60 | 30.06 | 32.48 |
| 80 | 33.64 | 32.44 |
| 100 | 34.28 | 32.20 |
| 120 | 31.96 | 30.04 |
| Timec (h) | ||
| 1 | 34.28 | 32.20 |
| 2 | 35.33 | 36.36 |
| 4 | 37.08 | 36.73 |
| 8 | 32.02 | 42.17 |
| 16 | 37.93 | 37.34 |
aNon-heat-treated ginseng
bHeat-treated for 1 h
cHeat treated at 100 °C
Similarly, a previous study obtained extract yields between 30 and 40% when ginseng was extracted using 60% methanol or ethanol [29], although the extract yields differed according to the extraction solvent employed, possibly owing to differences in the solvent extraction affinity for the target analytes [31].
Antimicrobial activities determined using the disc diffusion method
The antimicrobial activities of the heat-treated ginseng extracts were tested using the disc diffusion method (Table 2). All of the extracts had antimicrobial activities against B. cereus and S. aureus, but B. cereus was more sensitive to the ginseng extracts than S. aureus. When methanol was used as the extraction solvent, the antimicrobial activity against B. cereus did not differ among the ginseng extracts treated at temperatures of 60, 80, 100, and 120 °C. In contrast, the diameter of the zone of inhibition decreased as the heat treatment temperature increased with S. aureus. The ethanol extracts had the maximum antimicrobial activity against B. cereus when the ginseng was heated to 100 °C. Compared with B. cereus, the zones of inhibition obtained with S. aureus increased slightly when the ethanol extract was heat treated at all temperatures. In particular, the ethanol extract treated at 100 °C had the highest antimicrobial activities against B. cereus and S. aureus. The antimicrobial activities were also determined for the ginseng extracts treated at 100 °C for 1, 2, 4, 8, and 16 h, which showed that the antimicrobial activity against B. cereus gradually increased with the heat treatment time. The methanol and ethanol extracts treated for 16 h had the greatest antimicrobial activity against B. cereus (13.5 mm). For S. aureus, only the methanol extract treated at 100 °C for 16 h had a higher antimicrobial activity than the non-heated ginseng extract. The ethanol extracts heated for ≥4 h had slightly increased antimicrobial activities compared with the non-heated sample. The antimicrobial activities of the methanol extracts were generally higher than those of the ethanol extracts. The maximum antimicrobial activity against B. cereus was obtained using the ginseng extract (methanol and ethanol) heated at 100 °C for 16 h. For S. aureus, the results were less clear, where the maximum antimicrobial effects were obtained using the ethanol and methanol extracts heated for 8 and 16 h, respectively.
Table 2.
Antimicrobial activity determined by diameter of inhibition zone, minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) of ginseng methanol and ethanol extracts
| Bacillus cereus | Staphylococcus aureus | |||||
|---|---|---|---|---|---|---|
| Paper disc(1) (mm) | MIC (mg/mL) | MBC (mg/mL) | Paper disc (mm) | MIC (mg/mL) | MBC (mg/mL) | |
| Methanol | ||||||
| Control(2) | 8.88 ± 1.36Aa(3) | 6.25 | 12.50 | 10.19 ± 0.92Bbc | 50.00 | 100.00 |
| Temperature(4) (°C) | ||||||
| 60 | 9.17 ± 1.47A | 3.13 | 6.25 | 10.60 ± 1.08B | 12.50 | 25.00 |
| 80 | 9.83 ± 1.13A | 3.13 | 6.25 | 10.50 ± 1.12B | 25.00 | 50.00 |
| 100 | 9.58 ± 1.11A | 3.13 | 6.25 | 9.80 ± 1.64AB | 12.50 | 25.00 |
| 120 | 8.58 ± 2.01A | 3.13 | 6.25 | 8.80 ± 2.25A | 100.00 | 200.00 |
| Time(5) (h) | ||||||
| 1 | 8.06 ± 1.64a | 6.25 | 12.50 | 9.31 ± 0.80abc | 12.50 | 25.00 |
| 2 | 8.56 ± 1.76a | 3.13 | 6.25 | 9.31 ± 1.44ab | 6.25 | 12.50 |
| 4 | 9.50 ± 1.41a | 3.13 | 6.25 | 8.50 ± 1.31a | 12.50 | 100.00 |
| 8 | 13.00 ± 1.41b | 1.56 | 3.13 | 10.50 ± 0.00cd | 50.00 | 100.00 |
| 16 | 13.50 ± 2.12b | 1.56 | 3.13 | 11.25 ± 1.06d | 50.00 | 100.00 |
| Ethanol | ||||||
| Control | 8.06 ± 1.50Aa | 12.50 | 25.00 | 7.88 ± 0.79Aa | 50.00 | 100.00 |
| Temperature (°C) | ||||||
| 60 | 8.67 ± 1.25AB | 12.50 | 25.00 | 9.00 ± 0.71B | 50.00 | 100.00 |
| 80 | 8.33 ± 0.88A | 12.50 | 25.00 | 9.10 ± 0.55B | 50.00 | 100.00 |
| 100 | 9.50 ± 1.05B | 6.25 | 12.50 | 9.40 ± 0.89B | 50.00 | 100.00 |
| 120 | 9.17 ± 1.29AB | 12.50 | 25.00 | 8.70 ± 2.22AB | 200.00 | >200.00 |
| Time (h) | ||||||
| 1 | 7.88 ± 1.41ab | 12.50 | 25.00 | 8.44 ± 0.73bc | 25.00 | 50.00 |
| 2 | 8.88 ± 1.48a | 3.13 | 6.25 | 9.06 ± 1.57b | 6.25 | 12.50 |
| 4 | 10.94 ± 1.08bc | 3.13 | 6.25 | 10.00 ± 1.10cd | 25.00 | >200.00 |
| 8 | 12.25 ± 1.77cd | 3.13 | 6.25 | 11.50 ± 0.71e | 100.00 | >200.00 |
| 16 | 13.50 ± 2.12d | 1.56 | 3.13 | 11.00 ± 0.00de | 50.00 | 100.00 |
(1)Diameter of inhibition zone. Values represent the mean of triplicates ± SD, including the diameter of the paper disc (6 mm)
(2)Non-heat-treated ginseng
(3)Means with different uppercase and lowercase letters in the same column are significant differences (p < 0.05)
(4)Heat-treated for 1 h
(5)Heat-treated at 100 °C
Antimicrobial activities determined based on MIC and MBC
Table 2 shows MICs against B. cereus and S. aureus determined for the methanol and ethanol ginseng extracts heated at various temperatures, and at 100 °C for 1 to 16 h. The antimicrobial activities against B. cereus did not differ among the ginseng extracts in methanol treated at various temperatures. The ginseng extracts in ethanol had the highest activity after heating at 100 °C, where the MIC value was 6.25 mg/mL. For S. aureus, the MIC values of the ginseng extracts in methanol heated at 60, 80, 100, and 120 °C were 12.5, 25, 12.5, and 100 mg/mL, respectively. The ethanol extracts did not differ in terms of their antimicrobial activities against S, aureus irrespective of the temperature, except MIC decreased for the ginseng extract heated at 120 °C.
The antimicrobial activity against B. cereus increased with the heat treatment period. The antimicrobial activities of the ginseng extract heated at 100 °C for 16 h were four and eight times higher for the methanol and ethanol extracts, respectively, compared with the corresponding unheated ginseng extracts, where both had a maximum MIC of 1.56 mg/mL. For S. aureus, the antimicrobial activities of the methanol and ethanol extracts heated at 100 °C for 2 h both had the lowest MIC of 6.25 mg/mL, which was eight times higher than that determined for the corresponding unheated ginseng extracts.
In a previous study, white, red, and extruded white ginseng were extracted with water, methanol, or ethanol and their antibacterial activities were compared [29], and the lowest MIC determined for the ethanol and methanol extracts of red ginseng was 6.25 mg/mL, where the methanol extracts had the highest antimicrobial activities, thereby agreeing with our findings.
Growth inhibitory effects of ginseng extracts
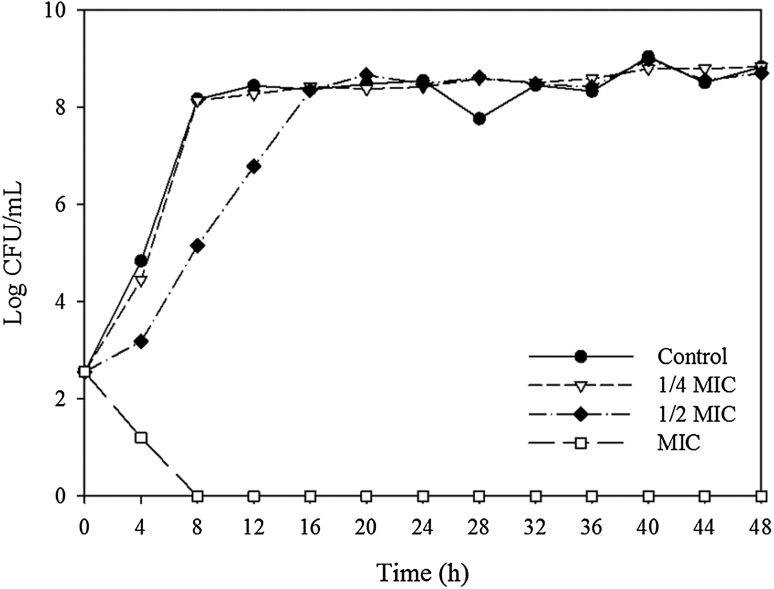
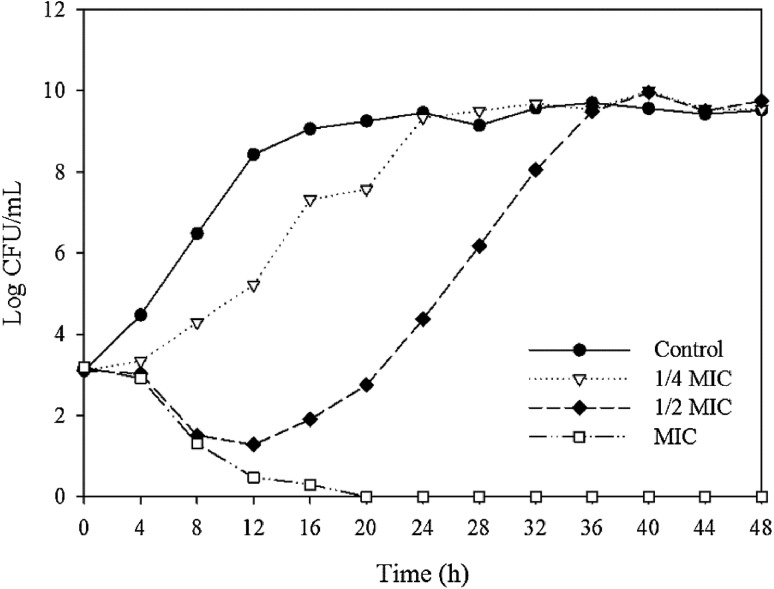
The growth inhibitory effects on B. cereus and S. aureus were analyzed using various concentrations of the ethanol extract, and the results are shown in Figs. 1 and 2. For B. cereus, the addition of 1/4 of the MIC value of the ethanol extract heated at 100 °C for 16 h did not inhibit growth compared with the control. When added at 1/2 of the MIC value, the increase in the bacterial count was slower than that in the control. However, at the MIC value, the number of bacteria decreased gradually and the bacteria were eliminated completely at 8 h. For S. aureus, the addition of 1/4 of the MIC value for the ethanol extract heated at 100 °C for 2 h inhibited the growth rate of the bacteria, and the time required to reach the maximum growth concentration was only 8 h. When 1/2 of the MIC value was added, the number of bacteria decreased from 3.10 to 1.27 log CFU/mL up to 12 h, which was followed by a continual increase until the maximum growth rate at 36 h. At the MIC value, the number of bacteria decreased gradually and complete inhibition was achieved at 20 h.
Fig. 1.
Growth inhibitory effect of ginseng ethanol extract at various concentrations on B. cereus. Ginseng was heat-treated at 100 °C for 16 h. MIC is the minimum inhibitory concentration
Fig. 2.
Growth inhibitory effect of ginseng ethanol extract at various concentrations on S. aureus. Ginseng was heat-treated at 100 °C for 2 h. MIC is the minimum inhibitory concentration
The factors that might influence the activity of an antimicrobial include the number of bacteria inoculated, the source of the bacteria, and the persistence and sensitivity of the antimicrobial agent [32]. The complete inhibition of B. cereus and S. aureus even when the ginseng extracts were added at only the MIC value appears to be attributable to the different initial loads of bacteria. The growth inhibitory test at 2–3 log CFU/mL was more sensitive than the MIC and MBC tests at 7 log CFU/mL. Similar results were obtained in a previous study, which showed that the antimicrobial activity of a ginseng extract decreased as the number of bacteria increased according to the disc diffusion method [33].
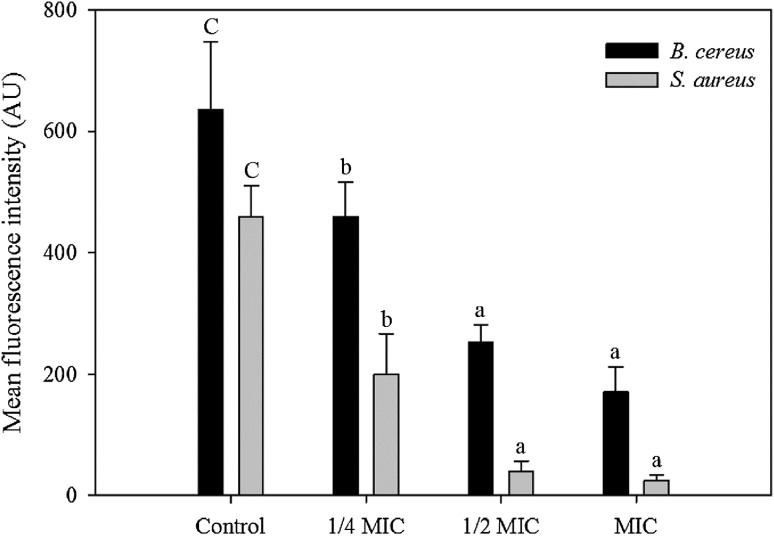
Changes in the membrane potential with ginseng extracts
The membrane potential for bacteria was expressed as the MFI of rhodamine 123 (Fig. 3). The MFI values decreased rapidly compared with the control by 27.74, 60.25, and 73.20% for B. cereus, and by 56.61, 91.21, and 94.48% for S. aureus when treated with the ethanol extract heated at 100 °C for 16 h at 1/4 MIC, 1/2 MIC, and MIC, respectively. The membrane potential affects the production of ATP via internal and external differences in the membrane potential of bacterial cells. The fluorescence intensity is correlated with the bacterial membrane potential. The loss of fluorescence is caused by depolarization of the cell membrane due to a reduction in the metabolic activity of the cell and death of the microorganism [34]. Therefore, the ethanol extract of ginseng reduced the membrane potential of the microbial cells and affected their metabolic activity. The membrane potential decreased further and the antimicrobial activity increased as the amount of the ginseng extract increased.
Fig. 3.
Changes in the membrane potential of B. cereus and S. aureus by ginseng ethanol extract. Ginseng was heat-treated at 100 °C for 16 h for B. cereus and 2 h for S. aureus, respectively. MIC is the minimum inhibitory concentration. Different letters indicate significant differences (p < 0.05)
Changes in ginsenoside compositions and contents by heat treatment
The ginsenoside contents of the ginseng extracts after different heating periods are shown in Table 3. Ginseng was heated at 100 °C for 1, 2, 4, 8, or 16 h, and the heated ginseng was extracted using methanol and ethanol. The total ginsenoside contents of the methanol extracts were higher than those of the ethanol extracts during 0–2 h, but the total ginsenoside contents of the ethanol extracts were higher as the heating time increased further. In the methanol extracts, the amounts of Rg1, Re, and Rf did not change, but those of Rg2, Rh1, Rb1, Rc, Rb2, Rb3, Rf2, and Rh2 increased significantly. The amount of Rd decreased, but those of Rg3 (S, R) and compound K, which were not present in the untreated ginseng, increased as the heating duration increased. In the ethanol extracts, the ginsenoside contents increased with the heating time. The amounts of Rf2, Rg3 (S, R), compound K, and Rh2, which were not detected in the untreated ginseng before heat treatment, increased with the heating duration.
Table 3.
Changes in ginsenoside content (mg/g) of ginseng extracts by heating at 100 °C as a function of heating time
| Methanol | Ethanol | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controla | 1 h | 2 h | 4 h | 8 h | 16 h | Control | 1 h | 2 h | 4 h | 8 h | 16 h | |
| Rg1 | 0.698 | 0.869 | 0.688 | 0.7 | 0.882 | 0.77 | 0.111 | 0.823 | 0.548 | 0.692 | 0.914 | 0.854 |
| Re | 1.415 | 1.296 | 1.1 | 1.279 | 1.049 | 0.958 | 0.237 | 1.27 | 0.929 | 1.348 | 1.082 | 1.096 |
| Rf | 0.327 | 0.337 | 0.287 | 0.272 | 0.359 | 0.441 | 0.051 | 0.455 | 0.253 | 0.293 | 0.377 | 0.541 |
| Rg2 + Rh1 | 0.071 | 0.056 | 0.089 | 0.072 | 0.139 | 0.338 | 0.012 | 0.057 | 0.042 | 0.088 | 0.135 | 0.342 |
| Rb1 | 0.457 | 0.449 | 0.457 | 0.706 | 0.975 | 1.047 | 0.061 | 0.429 | 0.36 | 0.723 | 0.981 | 1.294 |
| Rc | 0.635 | 0.681 | 0.665 | 1.04 | 1.425 | 1.405 | 0.087 | 0.64 | 0.528 | 1.058 | 1.431 | 1.711 |
| Rb2 | 0.241 | 0.346 | 0.296 | 0.349 | 0.669 | 0.689 | –b | 0.346 | 0.221 | 0.346 | 0.665 | 0.842 |
| Rb3 | 0.063 | 0.082 | 0.066 | 0.082 | 0.156 | 0.167 | 0.012 | 0.083 | 0.054 | 0.092 | 0.157 | 0.207 |
| Rd | 0.101 | 0.139 | 0.134 | 0.273 | 0.385 | 0.035 | 0.014 | 0.129 | 0.097 | 0.268 | 0.401 | 0.548 |
| Rf2 | 0.112 | –b | – | 0.035 | 0.16 | 0.301 | – | – | – | – | 0.087 | – |
| Rg3 (S, R) | – | – | – | 0.063 | 0.321 | 0.691 | – | – | – | 0.084 | 0.29 | 0.83 |
| CK | – | – | – | – | 0.126 | 0.255 | – | – | – | – | 0.127 | 0.4 |
| Rh2 | 0.11 | 0.025 | 0.052 | 0.075 | 0.094 | 0.111 | – | 0.034 | 0.059 | 0.028 | 0.101 | 0.174 |
| Total | 4.231 | 4.281 | 3.832 | 4.983 | 6.814 | 7.402 | 0.585 | 4.264 | 3.091 | 5.02 | 6.824 | 8.973 |
aNon-heat-treated ginseng
bNot detected
Similarly, a previous study showed that malonyl-ginsenosides (mRb1, mRb2, mRc, and mRd) possess malonyl groups that are readily demalonylated by heat treatment (36). Thus, the observed increases in the major ginsenosides (Rb1, Rb2, and Rc) could be attributable to heat-induced demalonylation. Increased conversion to minor ginsenosides such as Rg3 with increasing temperature has also been reported [35]. Another study found that less polar ginsenosides such as Rg3 and Rh2 are also produced by heat treatment, and they can penetrate the bacterial cell membrane more easily than polar ginsenosides, thereby affecting bacterial growth [28]. Therefore, it seems that minor ginsenosides, i.e., less polar ginsenosides, are produced by the heat treatment of ginseng, which increases the antimicrobial activity of the extract. However, the ginsenoside contents determined in this study differed from those reported by Norajit et al. [29], and this differences may be associated with the environmental conditions, such as the ginseng cultivation parameters, as well as the specific heat treatment and extraction methods employed [21].
This study aimed to enhance the antimicrobial activity of ginseng. Ginseng was heat treated at various temperatures and for various time periods, and we compared the antimicrobial activities of the heat-treated ginseng extracts against B. cereus and S. aureus. The antimicrobial activities of the methanol and ethanol extracts were increased by heat treatment. For B. cereus, the ginseng extract heated at 100 °C for 16 h had the highest antimicrobial activity, whereas that against S. aureus was highest when the extract was heated at 100 °C for 2 h. According to HPLC analysis, minor ginsenosides were formed as the heating period increased and it is considered that they increased the antimicrobial activity. Therefore, an ethanol extract of ginseng with enhanced antimicrobial activity after heat treatment could be used effectively as a natural antimicrobial in grain-based foods that are readily contaminated by B. cereus and S. aureus.
Acknowledgements
This study was supported by the Main Research Program of the Korea Food Research Institute funded by the Ministry of Science, ICT, and Future Planning.
References
- 1.Tirado M, Clarke R, Jaykus L, McQuatters-Gollop A, Frank J. Climate change and food safety: A review. Food Res Int. 2010;43:1745–1765. doi: 10.1016/j.foodres.2010.07.003. [DOI] [Google Scholar]
- 2.Arnesen LPS, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev. 2008;32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- 3.Hennekinne JA, De Buyser ML, Dragacci S. Staphylococcus aureus and its food poisoning toxins: characterization and outbreak investigation. FEMS Microbiol Rev. 2012;36:815–836. doi: 10.1111/j.1574-6976.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 4.Alarcon B, Vicedo B, Aznar R. PCR-based procedures for detection and quantification of Staphylococcus aureus and their application in food. J Appl Microbiol. 2006;100:352–364. doi: 10.1111/j.1365-2672.2005.02768.x. [DOI] [PubMed] [Google Scholar]
- 5.Hornstra L, De Leeuw P, Moezelaar R, Wolbert E, De Vries Y, De Vos W, Abee T. Germination of Bacillus cereus spores adhered to stainless steel. Int J Food Microbiol. 2007;116:367–371. doi: 10.1016/j.ijfoodmicro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Yang SK, Kim JJ, Kim SJ, Oh SW. Synergistic effect of grapefruit seed extract, EDTA and heat on inactivation of Bacillus cereus spore. J Korean Soc Food Sci Nutr. 2011;40:1469–1473. doi: 10.3746/jkfn.2011.40.10.1469. [DOI] [Google Scholar]
- 7.Ultee A, Slump R, Steging G, Smid E. Antimicrobial activity of carvacrol toward Bacillus cereus on rice. J Food Prot. 2000;63:620–624. doi: 10.4315/0362-028X-63.5.620. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK, Kim K-P, Jang SS, Shin EM, Kim M-J, Oh S, Ryu S. Prevalence and toxigenic profiles of Bacillus cereus isolated from dried red peppers, rice, and Sunsik in Korea. J Food Prot. 2009;72:578–582. doi: 10.4315/0362-028X-72.3.578. [DOI] [PubMed] [Google Scholar]
- 9.Baptista I, Rocha SM, Cunha Â, Saraiva JA, Almeida A. Inactivation of Staphylococcus aureus by high pressure processing: An overview. Innov Food Sci Emerg Technol. 2016;36:128–149. doi: 10.1016/j.ifset.2016.06.008. [DOI] [Google Scholar]
- 10.Oh SK, Lee N, Cho YS, Shin D-B, Choi SY, Koo M. Occurrence of toxigenic Staphylococcus aureus in ready-to-eat food in Korea. J Food Prot. 2007;70:1153–1158. doi: 10.4315/0362-028X-70.5.1153. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Li J, Yang L, Shi F, Yang L, Ye M. Antibacterial activity and a membrane damage mechanism of Lachnum YM30 melanin against Vibrio parahaemolyticus and Staphylococcus aureus. Food Control. 2017;73:1445–1451. doi: 10.1016/j.foodcont.2016.10.048. [DOI] [Google Scholar]
- 12.Xu JG, Liu T, Hu QP, Cao XM. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules. 2016;21:1194. doi: 10.3390/molecules21091194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diao WR, Hu QP, Zhang H, Xu JG. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.) Food Control. 2014;35:109–116. doi: 10.1016/j.foodcont.2013.06.056. [DOI] [Google Scholar]
- 14.Wu VCH, Qiu X, Bushway A, Harper L. Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT-Food Science and Technology. 2008;41:1834–1841. doi: 10.1016/j.lwt.2008.01.001. [DOI] [Google Scholar]
- 15.Gould GW. Industry perspectives on the use of natural antimicrobials and inhibitors for food applications. J Food Prot. 1996;59:82–86. doi: 10.4315/0362-028X-59.13.82. [DOI] [PubMed] [Google Scholar]
- 16.Rios J, Recio M. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol Sin. 2008;29:1109. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 19.Abe H, Arichi S, Hayashi T, Odashima S. Ultrastructural studies of Morris hepatoma cells reversely transformed by ginsenosides. Cell Mol Life Sci. 1979;35:1647–1649. doi: 10.1007/BF01953246. [DOI] [PubMed] [Google Scholar]
- 20.Hu C, Kitts DD. Free radical scavenging capacity as related to antioxidant activity and ginsenoside composition of Asian and North American ginseng extracts. J Am Oil Chem Soc. 2001;78:249–255. doi: 10.1007/s11746-001-0253-8. [DOI] [Google Scholar]
- 21.Chung IM, Kim JW, Seguin P, Jun YM, Kim SH. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng CA Meyer): Effects of cultivation location, year, and storage period. Food Chem. 2012;130:73–83. doi: 10.1016/j.foodchem.2011.06.056. [DOI] [Google Scholar]
- 22.Wan JY, Fan Y, Yu QT, Ge YZ, Yan CP, Alolga RN, Li P, Ma ZH, Qi LW. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J Pharm Biomed Anal. 2015;107:89–97. doi: 10.1016/j.jpba.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng CA Meyer: An overview. Carbohydr Polym. 2011;85:490–499. doi: 10.1016/j.carbpol.2011.03.033. [DOI] [Google Scholar]
- 24.Cho EJ, Piao XL, Jang MH, Baek SH, Kim HY, Kang KS, Kwon SW, Park JH. The effect of steaming on the free amino acid contents and antioxidant activity of Panax ginseng. Food Chem. 2008;107:876–882. doi: 10.1016/j.foodchem.2007.09.007. [DOI] [Google Scholar]
- 25.Jung MY, Jeon BS, Bock JY. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng CA Meyer) Food Chem. 2002;79:105–111. doi: 10.1016/S0308-8146(02)00185-1. [DOI] [Google Scholar]
- 26.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Lee B, Yang L, Wang H, Givskov M, Molin S, Høiby N, Song Z. Effects of ginseng on Pseudomonas aeruginosa motility and biofilm formation. FEMS Immunol Med Microbiol. 2011;62:49–56. doi: 10.1111/j.1574-695X.2011.00787.x. [DOI] [PubMed] [Google Scholar]
- 28.Xue P, Yao Y, Yang XS, Feng J, Ren GX. Improved antimicrobial effect of ginseng extract by heat transformation. J Ginseng Res. 2017;41:180–187. doi: 10.1016/j.jgr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norajit K, Park MJ, Ryu GH. Antimicrobial activities of white, red, and extruded ginsengs with different extraction conditions. Food Sci Biotechnol. 2008;17:850–856. [Google Scholar]
- 30.Comas J, Vives Rego J. Assessment of the effects of gramicidin, formaldehyde, and surfactants on Escherichia coli by flow cytometry using nucleic acid and membrane potential dyes. Cytometry. 1997;29:58–64. doi: 10.1002/(SICI)1097-0320(19970901)29:1<58::AID-CYTO6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- 32.Gomes B, Ferraz C, Vianna ME, Berber V, Teixeira F, Souza-Filho F. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–428. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 33.Son HJ, Han MS, Ryu GH. Antibacterial Activities of Et-OH Extract from Extruded White Ginseng on Tooth Decay Bacteria. J Korean Soc Food Sci Nutr. 2009;38:951–957. doi: 10.3746/jkfn.2009.38.7.951. [DOI] [Google Scholar]
- 34.Zhang Y, Liu X, Wang Y, Jiang P, Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. doi: 10.1016/j.foodcont.2015.05.032. [DOI] [Google Scholar]
- 35.Hwang IG, Kim HY, Joung EM, Woo KS, Jeong JH, Yu KW, Lee J, Jeong HS. Changes in ginsenosides and antioxidant activity of Korean ginseng (Panax ginseng CA Meyer) with heating temperature and pressure. Food Sci Biotechnol. 2010;19:941–949. doi: 10.1007/s10068-010-0132-9. [DOI] [Google Scholar]