Abstract
Resistant starches (RS) were prepared from purple yam by dual autoclaving-retrogradation (DAS), and pullulanase debranching treatment (PDS). DAS and PDS were then hydrolyzed by α-amylase and amyloglucosidase to obtain DAS.H and PDS.H. Differences in structural characteristics and in vitro digestibility among the four samples were investigated. The results showed that granules of RS had a rough surface and irregular shape. DAS had the lowest amylose content (29.52%), whereas PDS.H had the highest amylose content (41.96%). The order of crystallinity of the RS was: PDS.H (31.23%) > DAS.H (30.16%) > PDS (21.23%) > DAS (15.30%). Analysis by in vitro digestibility indicated a decreased hydrolysis index and glycemic index due to lower swelling power and water-binding capacity, and a well-ordered double helix structure and more crystallization in PDS.H than in the other RS samples. These results suggest that pullulanase debranching combined with α-amylase and amyloglucosidase hydrolysis may produce better RS with improved crystalline structure and higher digestion resistibility.
Keywords: Purple yam, Resistant starch, Hydrolysis, Structure, Digestibility
Introduction
Starch is the main biological macromolecule in green plants, and plays a critical role in supplying the essential metabolic energy that enables body functions to perform normally. However, diabetes, cardiovascular diseases and obesity have positive correlations with long-term consumption of food with a high glycemic index (GI), which have a high content of rapidly digestible starch [1]. Therefore, an interest in the development and utilization of starch with high digestive resistibility is growing due to its functional properties.
Resistant starch (RS), a product of starch digestion, is poorly absorbed by the small intestine and has a beneficial effect on human health [2]. As a native starch, in most cases RS1 (physically trapped starch) and RS2 (resistant starch granules) will lose their resistance during food processing. RS3 (retrograded starch), which is formed by gelatinization and subsequent recrystallization processes, is stable when heated above 100 °C. Furthermore, RS can be prepared by chemical, physical, enzymatic, and genetic methods; however, different methods can affect the RS content and physicochemical properties [3]. Zhao and Lin [4] reported that RS yield (32.4%) increased significantly when retrograded maize starch was subjected to one autoclaving-cooling cycle coupled with pullulanase debranching and then followed by two autoclaving-cooling cycles. Miao et al. [5] attempted the production of RS based on the hydrolysis of waxy maize starch by pancreatin and amyloglucosidase. However, the preparation process causes the rearrangement of amylose and amylopectin chains in the starch, and may modify its X-ray pattern, double helix structure, swelling power and water-binding capacity, as well as its morphology. According to Zhang et al. [6], α-amylase and amyloglucosidase hydrolysis followed by autoclaving-cooling have a significant impact on the structural and physicochemical properties of lotus seed starch. Intriguingly, byproducts of α-amylase and amyloglucosidase hydrolysis such as oligosaccharides are reported to have significant research value [7]. Therefore, the foregoing hydrolysis has a number of practical applications.
Purple yam (Dioscorea alata L.), is widely planted in tropical and subtropical regions, and is used as a medicinal and edible plant [8, 9]. Previous research has mainly focused on its nutritional and functional properties [10, 11], and there are few reports on purple yam RS. The content of starch in purple yam is approximately 16–20% of the total biomass, and the starch contains a high level of amylose [12], which may contribute to the formation of RS. Rosida et al. [13] reported that the autoclaving-cooling process affected purple yam starch structure leading to an increase in the content of RS. Furthermore, purple yam starch has round and oval granules, a B-type pattern with low relative crystallinity, and a high proportion of large granules, resulting in resistance to digestive enzymes [12]. Nevertheless, limited information is available on the preparation of purple yam starch with a high content of RS and the relationship between digestion resistibility and structure.
Based on previous work, in the present study, we prepared RS from purple yam starch by applying autoclaving-retrogradation cycles, pullulanase debranching and hydrolysis by α-amylase and amyloglucosidase. Differences in the structural and digestive characteristics of RS were evaluated. The aims of this study were to obtain a more appropriate treatment to increase the RS content and demonstrate the relationship between digestion resistibility and structure.
Materials and methods
Materials
Purple yam (D. alata L.) tubers of the common variety were purchased from small producers in Fuzhou, China, and were used within 24 h of harvesting. Porcine pancreas α-amylase (EC 3.2.1.1, 16 U/mg) type-B and amyloglucosidase (EC 3.2.1.3, 300 U/mL) from Aspergillus niger were bought from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Thermostable α-amylase (8000 U/mL) from Bacillus licheniformis and amyloglucosidase from A. niger (100,000 U/mL) were obtained from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Pullulanase (EC 3.2.1.41, 2800 ASPU/g) from B. licheniformis was obtained from Guangzhou Yulibao Biotechnology Co., Ltd. (Guangzhou, China). The d-glucose assay kit was obtained from Megazyme International Ireland Ltd. (Wicklow, Ireland). Other chemicals and solvents were of analytical grade.
Starch extraction and gelatinization
Starch was extracted from purple yam according to the modified method of Jiang et al. [14]. The cleaned purple yam tuber was cut into slices, and immediately suspended in 0.35% (w/v) sodium erythorbate solution. The sample was then homogenized in a blender and washed repeatedly through a sieve (200 mesh) with distilled water. The slurry containing the starch was then centrifuged at 3000g for 15 min. After discarding the upper nonwhite layer, the white layer was resuspended in 95% ethanol, and recentrifuged 4–5 times. The starch layer was collected and then dried at 40 °C. Finally, the dried starch was mashed and passed through a 100 mesh sieve to obtain native purple yam starch (NS). NS (10 g, dry basis) slurry (25% w/v in distilled water) was boiled with continuous stirring for 30 min, then dried and mashed to obtain gelatinized starch (GS).
Preparation of purple yam resistant starch
Dual autoclaving-retrogradation of purple yam starch
Purple yam starch (50 g, dry basis) was suspended in 200 mL of distilled water, and then pressure-cooked in an autoclave at 120 °C for 30 min. The paste was then cooled to room temperature and then stored at 4 °C for 24 h. The autoclaving-retrogradation cycle was repeated twice, and then the paste was oven-dried at 40 °C for 24 h. The paste was mashed and passed through a 100 mesh sieve to obtain resistant starch (DAS).
Pullulanase debranching of gelatinized purple yam starch
Purple yam starch (50 g, dry basis) slurry (25% w/v in diluted pH 5.0 buffer solution containing 0.2 M sodium acetate and 0.2 M acetic acid) was boiled with continuous stirring for 30 min. The temperature of the cooked starch was adjusted to 60 °C and debranched by pullulanase at 8 ASPU/g of starch for 12 h under continuous agitation. The starch gel was exposed to deactivated enzyme during the reaction at 100 °C for 30 min and then cooled to room temperature. The pH was adjusted to 7.0 with diluted water and treated with dual autoclaving-retrogradation cycles (conditions as previously described). The retrograded starch was oven-dried at 40 °C for 24 h and mashed to pass through a 100 mesh sieve to obtain resistant starch (PDS).
Double enzyme hydrolysis of DAS
Double enzyme hydrolysis was performed according to Wang et al. [15] with slight modifications as follows: 20 g of DAS (dry basis) (preparation as previously described) was suspended in a bioreactor containing 100 mL of pH 6.0 sodium acetate buffer solution. When the temperature of the mixture in a water bath shaker reached 90 °C, 0.3 mL of thermostable α-amylase (8000 U/mL) was added and incubated for 1 h, the reaction mixture was cooled to 60 °C and the pH was adjusted to 4.5 with diluted citric acid, and then amyloglucosidase (100,000 U/mL, 0.3 mL) was added and the mixture incubated for 1 h. The mixture was centrifuged at 3000g for 15 min, the insoluble residue was washed three times with distilled water and three times with 95% (v/v) ethanol, dried at 40 °C for 24 h, and then mashed to pass through a 100 mesh sieve to obtain resistant starch (DAS.H).
Double enzyme hydrolysis of PDS
20 g of PDS (dry basis) (preparation as previously described) was hydrolyzed by double enzyme (conditions as previously described) to obtain resistant starch (PDS.H).
Chemical composition of starch
The moisture content of starch was recorded in terms of weight loss after heating at 105 ± 2 °C for 2 h. The amount of protein, fat, ash and fiber were analyzed according to American Association of Cereal Chemists (AACC) methods 08-01, 46-13, 30-25 and 32-23, respectively [16]. Amylose content was determined according to the method described by Srichuwong et al. [17]. The ratio of the weight of production to the weight of raw material (or native starch) was calculated to estimate the yield of starch samples.
Swelling power (SP)
Starch (0.5 g) was mixed with 25 mL of distilled water and heated at 50, 60, 70, 80 and 90 °C in a water bath for 30 min and shaken every 5 min. The samples were cooled to room temperature using a water bath, and the suspension was centrifuged at 3000g for 20 min. The supernatant and the sediment samples were separated, an aliquot of supernatant was evaporated at 110 °C for 4 h, and the swollen starch sediment was weighed. SP was calculated as follows:
where D is the weight of wet sediment, g; W is dried starch weight, g; A is the weight of dried supernatant, g.
Water-binding capacity (WBC)
A suspension of 0.5 g starch (dry basis) in 10 mL distilled water was heated in a shaking water bath at 50, 60, 70, 80 and 90 °C, respectively. The suspension was centrifuged at 3000g for 15 min. The free water was removed. The test tube with wet starch was tilted at 45° for 10 min to remove water and then weighed. WBC was calculated as follows:
where M 0, M 1, and M 2 are the weight of starch, centrifuge tube, and the weight of starch and centrifuge tube after removal of water, respectively.
Morphology
A small amount of the starch sample was suspended in a glycerol solution (glycerol/water, 1:1, v/v). The morphology was photographed using a BX-43 microscope (Olympus Corporation, Tokyo, Japan) equipped with a camera.
Morphological properties of the starch sample were studied using a scanning electron microscope (JSM-6380LV; JEOL, Tokyo, Japan). Finely ground samples were mounted on an aluminum stub using double-sided sticky tape and coated with a thin film of gold, then examined at an accelerating voltage of 15 kV.
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectra of starch samples were obtained with an FT-IR spectrometer (VERTEX 70; Bruker, Ettlingen, Germany) using an attenuated total reflectance (ATR) mode. Each spectrum was recorded at a resolution of 4 cm−1 and at room temperature with 16 scans. Spectra were corrected and then deconvoluted in the range of 1200–800 cm−1. The ratio of height at 1047 cm−1 to the height at 1022 cm−1 was obtained from the deconvoluted spectra.
X-ray diffraction (XRD)
XRD analysis was performed with an X-ray diffractometer (TTR-III; Rigaku, Tokyo, Japan) operating at 40 kV and 200 mA. The starch powders were scanned at a rate of 8°/min from 3° to 35° at room temperature. Relative crystallinity was calculated according to the method of Nara and Komiya [18] using MDI Jade 5.0 software (Material Date, Inc., Livermore, CA, USA).
In vitro starch digestion
Starch digestibility was analyzed according to the method described by Englyst et al. [19] with minor modifications. Starch (200 mg) was mixed with 15 mL of buffer solution at pH 5.2 containing 0.2 M sodium acetate and 0.2 M acetic acid. The suspension was incubated at 37 °C with 290 units of porcine pancreas α-amylase (16 U/mg) and 15 units of amyloglucosidase (300 U/mL) (approximately 10 mL) in a shaking water bath. Aliquots of 0.5 mL were removed at 20 and 120 min, respectively, and each aliquot was mixed with 4 mL of absolute ethanol to eliminate enzyme activity. The contents of rapidly digestible starch (RDS), slowly digestible starch (SDS) and RS were calculated based on the glucose concentration in the supernatant obtained after centrifugation at 3000g for 10 min using the Glucose oxidase peroxidase aminoantipyrine (GOPOD) method (Megayme International, Wicklow, Ireland). Analyses were performed in triplicate.
Kinetics of in vitro enzymatic hydrolysis of starch
The glucose content was measured by the GOPOD method at 10, 20, 30, 40, 60, 90, 120 and 180 min, respectively. Hydrolysis rate (HR) was calculated as follows [20]:
where W is the weight of starch; G is the content of glucose released from starches at different times.
Hydrolysis index (HI) and estimation of glycemic index (GI)
The human intestinal system was simulated according to the kinetics of in vitro digestion described by Goñi et al. [21]. The area of white bread under the hydrolysis curve (0–180 min) was used as the standard, and the HI (%) was calculated as the percentage of integral area of the hydrolysis curve released from the sample compared to that of white bread. The GI (%) of the samples was estimated as follows:
Statistical analysis
Mean values and standard deviations were determined and compared using the Tukey test. Statistical significance was set at p < 0.05. All the statistical analyzes were conducted using the DPS 9.05 system (Science Press, Beijing, China).
Results and discussion
Chemical compositions
The compositional analysis of NS, GS, and RS samples (PDS.H, DAS.H, PDS, DAS) showed a moisture content of 8.75–8.96%, fat content of 0.07–0.12%, ash content of 0.08–0.17%, and fiber content of 0.110.20% (Table 1). These parameters were not significantly different between the starch samples, indicating that they were pure enough for use. Similar chemical composition values were also reported in the study by Rosida et al. [13].
Table 1.
Chemical composition and yield of native, gelatinized and resistant starches prepared from purple yam (%)
| Sample | Moisture | Protein | Fat | Ash | Fiber | Amylose | Yield2) |
|---|---|---|---|---|---|---|---|
| NS | 8.87 ± 0.08a,1) | 0.54 ± 0.04d | 0.12 ± 0.02a | 0.11 ± 0.01a | 0.20 ± 0.07a | 28.37 ± 0.08f | 13.46 ± 1.23 |
| GS | 8.91 ± 0.05a | 0.52 ± 0.07d | 0.09 ± 0.03a | 0.10 ± 0.04a | 0.19 ± 0.03a | 28.89 ± 0.06e | 99.87 ± 0.12a |
| DAS | 8.96 ± 0.13a | 0.50 ± 0.03d | 0.07 ± 0.01a | 0.08 ± 0.02a | 0.17 ± 0.05a | 29.52 ± 0.14d | 97.63 ± 0.47a |
| PDS | 8.92 ± 0.10a | 1.27 ± 0.12c | 0.09 ± 0.04a | 0.12 ± 0.01a | 0.15 ± 0.04a | 32.79 ± 0.17c | 45.34 ± 3.61b |
| DAS.H | 8.79 ± 0.07a | 1.79 ± 0.05b | 0.10 ± 0.08a | 0.13 ± 0.04a | 0.12 ± 0.02a | 40.57 ± 0.30b | 10.36 ± 1.04c |
| PDS.H | 8.75 ± 0.11a | 2.34 ± 0.07a | 0.08 ± 0.01a | 0.17 ± 0.06a | 0.11 ± 0.03a | 41.96 ± 0.23a | 8.79 ± 0.65c |
1)Results are mean ± SD. Means with different letters in a column are significantly different (p < 0.05). Values are the means of triplicates
2)The yield of NS was calculated based on raw material. The yields of GS, DAS, PDS, DAS.H and PDS.H were calculated based on NS
PDS, DAS.H and PDS.H had a higher protein content ranging from 1.27 to 2.34% compared with NS (0.54%), GS (0.52%) and DAS (0.50%) due to the amylase residue in starch particles during the enzymolysis process. Residual enzyme after starch hydrolysis can lead to a significant increase in the content of protein [22]. Correspondingly, the amylose content differed significantly (p < 0.05) and followed the order: PDS.H (41.96%) > DAS.H (40.57%) > PDS (32.79%) > DAS (29.52%) > GS (28.89%) > NS (28.37%). It has been reported that the amylose content in starches is influenced by multiple factors, such as plant variety, cultivar, growing zone, starch isolation and processing method [23]. The debranching of amylopectin by pullulanase provides an increased opportunity to form amylose molecules. Some amylopectin molecules and long amylose molecular chains were further broken into small linear polysaccharides like amylose molecules by double enzymatic hydrolysis [17], resulting in the highest amylose content in PDS.H. The amylose content in NS was 28.37%, similar to that in other starches, such as Indian purple yam starch (26.99%) [12], potato starch (27.9%) and corn starch (27.3%) [24]. Based on NS (13.46%), the yields of PDS.H, DAS.H, PDS, DAS and GS were 8.79, 10.36, 45.34, 97.63 and 99.87%, respectively. Although the yields of PDS.H and DAS.H were low (around 10%), those treatments are still beneficial in functional food development with high amylose content.
SP
The SP (Table 2) of the starches was positively correlated with the temperature range of 50–90 °C. Interestingly, PDS.H, DAS.H, PDS, DAS and GS exhibited higher swelling power at low temperature (50–60 °C) with poorer values at higher temperatures (80–90 °C) compared with NS. These results are in accordance with observations on maca starch [25] and elephant foot yam starch [16]. The reduced SP was due to increased amylose content, as starches with higher amylose content were more rigid and better reinforced, and resulted in less swelling when heated.
Table 2.
The swelling power and water-binding capacity of native, gelatinized and resistant starches prepared from purple yam
| Sample | 50 °C | 60 °C | 70 °C | 80 °C | 90 °C |
|---|---|---|---|---|---|
| Swelling power (%) | |||||
| NS | 1.87 ± 0.01e,1) | 2.01 ± 0.05f | 6.88 ± 0.02d | 14.22 ± 0.08a | 15.92 ± 0.06a |
| GS | 6.74 ± 0.09a | 8.42 ± 0.13a | 11.63 ± 0.18a | 12.58 ± 0.07b | 14.37 ± 0.12b |
| DAS | 5.49 ± 0.04b | 7.85 ± 0.12b | 10.75 ± 0.10b | 11.90 ± 0.13c | 12.04 ± 0.07c |
| PDS | 5.33 ± 0.03b | 6.78 ± 0.02c | 7.58 ± 0.03c | 8.84 ± 0.06d | 9.46 ± 0.11d |
| DAS.H | 5.07 ± 0.06c | 5.89 ± 0.07d | 6.67 ± 0.01d | 6.81 ± 0.04e | 7.30 ± 0.09e |
| PDS.H | 4.58 ± 0.12d | 5.08 ± 0.02e | 6.24 ± 0.05e | 6.73 ± 0.17e | 7.28 ± 0.23e |
| Water-binding capacity (%) | |||||
| NS | 70.98 ± 1.67e | 92.48 ± 6.15e | 96.17 ± 8.13f | 717.22 ± 15.23a | 896.23 ± 17.41a |
| GS | 425.41 ± 14.39a | 467.89 ± 8.96a | 542.34 ± 10.43a | 695.39 ± 11.35ab | 768.46 ± 16.35b |
| DAS | 361.51 ± 12.56b | 431.46 ± 10.15b | 493.61 ± 9.55b | 660.69 ± 17.73b | 720.65 ± 20.42c |
| PDS | 212.11 ± 5.36c | 239.97 ± 7.14c | 245.77 ± 11.70c | 458.78 ± 19.85c | 498.18 ± 24.37d |
| DAS.H | 107.89 ± 3.89d | 121.55 ± 3.84d | 157.58 ± 6.25d | 229.95 ± 10.57d | 266.90 ± 7.12e |
| PDS.H | 98.95 ± 2.37d | 117.54 ± 5.28d | 121.79 ± 4.67e | 141.96 ± 4.84e | 166.56 ± 6.85f |
1)Results are mean ± SD. Means with different letters in a column are significantly different (p < 0.05). Values are the means of triplicates
WBC
WBC represents the amount of bound water produced by the covalent bond between the hydroxyl group and the starch molecular chain, which is related to the particle morphology and internal complex structure [26]. The WBC of PDS.H, DAS.H, PDS, DAS, GS and NS is shown in Table 2. The WBC of starches increased gradually when the temperature increased from 50 to 90 °C, and particularly increased for NS between 75 and 85 °C. Furthermore, the WBC of GS was found to be significantly (p < 0.05) higher than DAS, PDS, and DAS.H, especially PDS.H which had the lowest value. Starch was gelatinized (GS) or treated by dual autoclaving-retrogradation treatment (DAS), which formed a loose association between amylose and amylopectin molecules in starch granules [27], thereby increased the combination ability of starch with water. Compared with GS, the decreased WBC of DAS may have been due to increased crystallinity [28]. The debranching and recrystallization process of PDS gave molecular chains a greater opportunity to aggregate to form a compact and rigid crystal [4]. After double enzyme hydrolysis treatment, PDS was converted into smaller linear chain polysaccharides like amylose molecules, which were closely arranged and formed a strong gel network via the retrogradation and blocked the combination [29], which resulted in the lowest value for PDS.H.
Morphology
The morphology of NS, GS and RS samples (PDS.H, DAS.H, PDS, DAS) are shown in Fig. 1. NS showed oval or circular shapes with a smooth surface. The granule size, measured by the scale bar of microscopy, was in the range of 10–45 μm (Fig. 1A). Similar observations for Chinese yam starches have been reported by Wang et al. [30]. However, the granular structure of NS was destroyed after gelatinization, retrogradation or enzymolysis, and exhibited a cohesive structure and an irregular shape. GS exhibited small sized, irregular and block shapes. Dual autoclaving-retrogradation resulted in disruption of granules and the formation of an amorphous precision block structure, which is in agreement with the results of rice resistant starch reported by Ashwar et al. [29]. PDS showed an uneven distribution of particle sizes and a rough surface. In contrast, flaky and gully shapes were observed on the rough surfaces of DAS.H and PDS.H, which formed more compact structures. These observations may be attributed to the double enzyme synergistic hydrolysis of the weak tissue structure, leaving abundant gully shapes on the surface. Furthermore, the study of NS, GS and RS samples by normal light microscopy confirmed the morphological results obtained by scanning electron microscope (SEM). Under normal light, significantly different morphologies were found for RS prepared under various conditions (Fig. 1B). DAS.H, and especially PDS.H, showed many holes on the surface, whereas NS exhibited a smooth surface.
Fig. 1.
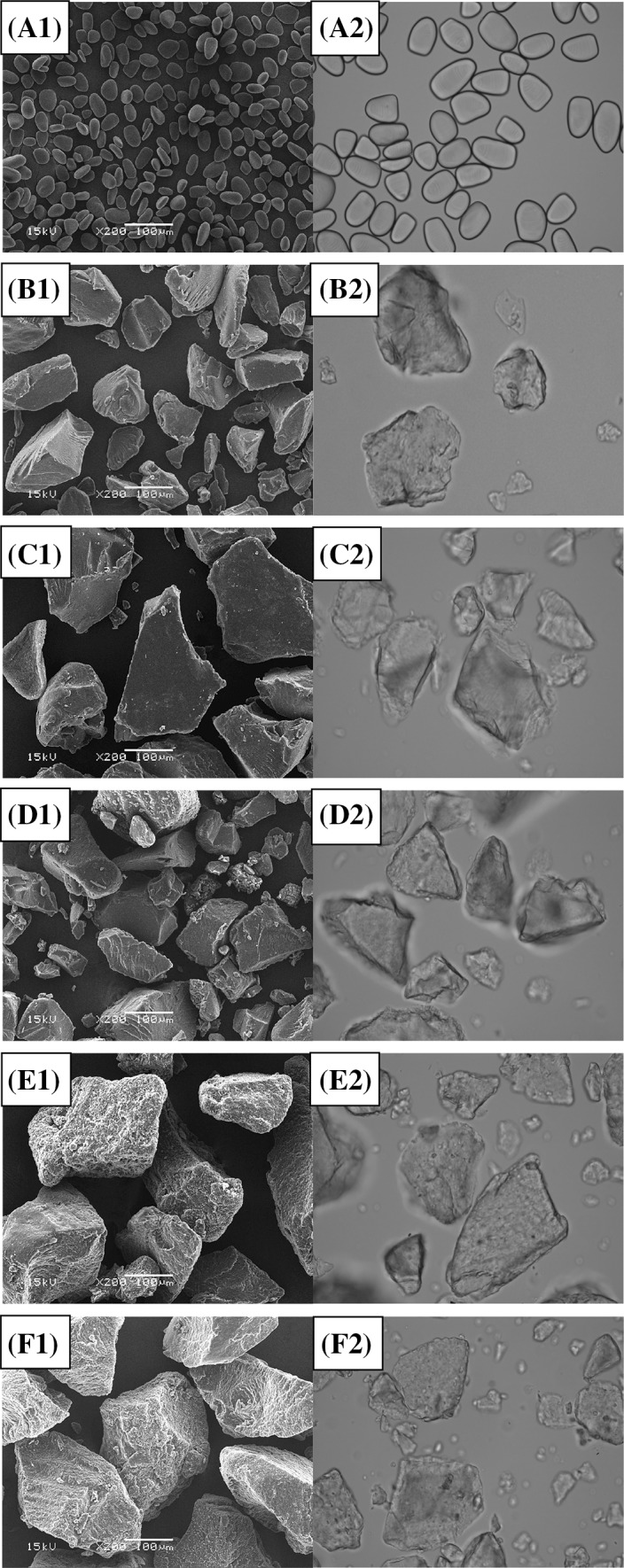
Micrographs of native, gelatinized and resistant starches prepared from purple yam. (A) NS, (B) GS, (C) DAS, (D) PDS, (E) DAS.H, (F) PDS.H. 1-scanning electron micrographs at magnification of ×200; 2-optical micrographs at magnification of ×200 (eyepiece of ×10; objective of ×20)
Short-range order
Figure 2A shows the deconvoluted ATR–FT-IR spectra of starches in the region of 1200–800 cm−1. According to Miao et al. [5], the infrared spectrum of starch has been shown to be the so-called short-range order, defined as the double helical order. Of the starches in this study, the 1047/1022 cm−1 ratio followed the order: PDS.H (0.678) > DAS.H (0.663) > NS (0.628) > PDS (0.561) > DAS (0.422) > GS (0.363). The higher ratio in PDS.H suggests a higher proportion of crystalline regions in the starch granules, resulting in a more perfect crystalline structure. The lower ratio in DAS, and especially GS, may be attributed to dissociation and unraveling of double helices forming the crystalline array, and reflected the disruption of crystals.
Fig. 2.
Structural characteristics of native, gelatinized and resistant starches prepared from purple yam. (A) ATR–FT-IR spectra, (B) XRD patterns. Values in brackets of (A) and (B) are the ratio of 1047/1022 cm−1 and relative crystallinity (%), respectively
XRD
The XRD patterns and relative crystallinity of NS, GS and RS samples (PDS.H, DAS.H, PDS, DAS) are presented in Fig. 2B. NS exhibited a typical B-type crystalline pattern with reflection intensities at 5.6°, 17.1°, 22.1° and 23.9° (2θ), which is in agreement with the results reported by other authors for purple yam starch [13]. After gelatinization or autoclaving-retrogradation treatment, the positions of characteristic peaks did not change for GS or DAS, but the intensity decreased and became a dispersion peak which was similar to a sub-crystal structure. PDS had a B-type crystalline pattern with more intense diffraction peaks at 19.3° (2θ), but the intensity at 5.6° (2θ) decreased more than NS. These results are consistent with the report by Zeng et al. [31] where waxy rice starch showed B- and V-type complex crystalline structure following pullulanase debranching and the subsequent recrystallization process. Interestingly, DAS.H and PDS.H exhibited C + V type with characteristics of the A- and B-type and V-type crystalline patterns, and showed intense peaks at 15.1°, 17.1°, 19.3°, 22.1°, and 23.9°. The appearance of a sharp peak at 19.3° (2θ) was due to the disappearance of amylopectin-forming amorphous regions, and this change in crystal structure may also be due to an increase in amylose content [26].
Compared with NS, the relative crystallinity of GS, DAS and PDS decreased, which was in agreement with the decrease in the ratio of 1047/1022 cm−1 (Fig. 2A) as discussed earlier. This may be due to the breakdown of the molecular double helix structure. However, the significantly increased crystallinity of PDS.H and DAS.H is an indicator of increased perfection of the double-helix structure and crystal size. There were no differences in either the crystalline type or crystallinity between DAS.H and PDS.H, which indicated that these two treatments have a similar influence on the crystal structure.
In vitro digestibility
Kinetics of in vitro enzymatic hydrolysis
The in vitro enzymatic hydrolysis rate of NS, GS and RS samples (PDS.H, DAS.H, PDS, DAS) are shown in Fig. 3. Hydrolysis rate (HR) increased rapidly from 0 to 60 min, but increased slowly from 60 to 120 min and was stable from 120 to 180 min. The HR at 180 min followed the order: GS (75.96%) > DAS (70.35%) > PDS (52.76%) > DAS.H (43.77%) > PDS.H (42.48%) > NS (19.47%). NS had a native resistance to enzyme hydrolysis due to its smooth and dense surface (Fig. 1), ordered double helix structure (Fig. 2A), and high crystallinity (Fig. 2B). However, the inter- and intra-molecular hydrogen bonds between starch chains were disrupted and the crystallites were disrupted after gelatinization and autoclaving treatments, which led to the transformation to a continuous amorphous structure that allowed physical accessibility to digestive enzymes. Zeng et al. [31] suggested that gelatinization substantially increased the RDS level and decreased SDS and RS levels. Thus, DAS and PDS, especially GS, were extensively hydrolyzed to achieve maximum biological digestion, which was responsible for the lower HR of DAS.H and PDS.H.
Fig. 3.
Kinetics of in vitro enzymatic hydrolysis of different starches. (A) curve of hydrolysis rate, (B) fitting curve of hydrolysis rate
The lower values in swelling power and WBC of DAS.H and PDS.H indicated that the order and compact structure could prevent water and digestive enzymes entering the starch granules, and thus decreased digestibility. According to the SEM results (Fig. 1), a gully-like rough surface, even particle size and dense exterior structure of DAS.H, particularly PDS.H, hindered enzymolysis. High relative crystallinity is known to be resistant to enzyme hydrolysis. The ordered crystal restricted the swelling of starch granules and hindered adherence of the enzyme to the matrix, resulting in reduced starch hydrolysis [32]. Furthermore, B- and C-type starches showed more resistance to enzyme hydrolysis than A-type starch. In the present study, the relative crystallinity and crystal pattern were generally correlated with digestibility. DAS.H and PDS.H which were composed of C + V-type were less susceptible to amylase hydrolysis than PDS with B + V-type (B-type polymorph was predominant), PDS, DAS and GS with B-type starches.
HI and estimation of GI
The RDS, SDS and RS contents of different starches are shown in Table 3. The amount of RDS, SDS and RS in the samples differed significantly (p < 0.05). RS content in NS was higher than that in SDS and RDS. However, the sample with the lowest RS value of 28.29% was GS, suggesting that NS was not very stable after gelatinization. The higher RS content in PDS.H and DAS.H was attributed to high-strength hydrolysis of thermostable α-amylase and amyloglucosidase to achieve sufficient digestion, which ultimately resulted in lowering RDS. Furthermore, the SDS contents in PDS.H and DAS.H were higher than those in NS, GS, DAS and PDS.
Table 3.
Content of RDS, SDS, RS, C∞, HI, and GI in starches separated from purple yam by in vitro digestion
| Sample | RDS1) (%) | SDS1) (%) | RS1) (%) | C1)∞ (%) | R2 | HI1) | GI1) |
|---|---|---|---|---|---|---|---|
| NS | 10.19 ± 0.02f,2) | 8.47 ± 0.05f | 81.34 ± 0.03a | 18.36 ± 0.52e | 0.9766 | 17.13 ± 0.47f | 49.11 ± 0.23e |
| GS | 48.50 ± 0.11a | 23.21 ± 0.04e | 28.29 ± 0.12f | 72.28 ± 1.31a | 0.9870 | 73.83 ± 1.23a | 80.24 ± 0.87a |
| DAS | 43.88 ± 0.10b | 25.31 ± 0.08d | 30.81 ± 0.10e | 67.76 ± 1.48b | 0.9903 | 69.54 ± 1.56b | 77.89 ± 1.03b |
| PDS | 24.72 ± 0.06c | 26.43 ± 0.14c | 48.85 ± 0.14d | 50.45 ± 0.86c | 0.9943 | 51.84 ± 0.75c | 68.17 ± 0.38c |
| DAS.H | 13.88 ± 0.12d | 27.22 ± 0.08b | 58.90 ± 0.17c | 40.78 ± 0.54d | 0.9831 | 41.26 ± 0.92d | 62.36 ± 0.56d |
| PDS.H | 11.41 ± 0.10e | 28.37 ± 0.12a | 60.22 ± 0.23b | 38.31 ± 0.36d | 0.9840 | 38.45 ± 0.53e | 60.82 ± 0.21d |
1) RDS rapidly digestible starch, SDS slowly digestible starch, RS resistant starch, C ∞ equilibrium concentration, R 2 fitting degree, HI hydrolysis index, GI glycemic index
2)Results are mean ± SD. Means with different letters in a column are significantly different (p < 0.05). Values are the means of triplicates
Goñi et al. [21] reported that the starch hydrolysis curve followed a first-order reaction. The data (Fig. 3A) in the present study was fitted and the HI and GI of different starches were calculated (Table 3). The equilibrium concentration (C∞) which represented the concentration of glucose at the hydrolytic equilibrium state of the starch samples ranged from 18.47% (NS) to 72.28% (GS), reflecting the amount of easily digested starch. HI and GI of the starch samples were significant different (p < 0.05). The HI and GI of PDS.H were very low compared to those of the other three RS samples. Interestingly, the values of HI and GI were related to the hydrolysis rate as reflected by morphology (Fig. 1), relative crystallinity (Fig. 2B) and the content of RS (Table 3). Jiang et al. [14] suggested that HI and GI were significantly negatively correlated with relative crystallinity.
Acknowledgements
This study was supported by the Key Program of National Natural Science Foundation of China (No. 31271913) and High Level University Construction Project of Fujian Agriculture and Forestry University (No. 612014042).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Zhang C, Shin M. Forming rice starch gels by adding retrograded and cross-linked resistant starch prepared from rice starch. Food Sci. Biotechnol. 2015;24:835–841. doi: 10.1007/s10068-015-0108-x. [DOI] [Google Scholar]
- 3.Nep EI, Ngwuluka NC, Kemas CU, Ochekpe NA. Rheological and structural properties of modified starches from the young shoots of Borassus aethiopium. Food Hydrocolloid. 2016;60:265–270. doi: 10.1016/j.foodhyd.2016.02.037. [DOI] [Google Scholar]
- 4.Zhao XH, Lin Y. The impact of coupled acid or pullulanase debranching on the formation of resistant starch from maize starch with autoclaving-cooling cycles. Eur. Food Res. Technol. 2009;230:179–184. doi: 10.1007/s00217-009-1151-8. [DOI] [Google Scholar]
- 5.Miao M, Zhang T, Mu W, Jiang B. Structural characterizations of waxy maize starch residue following in vitro pancreatin and amyloglucosidase synergistic hydrolysis. Food Hydrocolloid. 2011;25:214–220. doi: 10.1016/j.foodhyd.2009.12.004. [DOI] [Google Scholar]
- 6.Zhang Y, Zeng H, Wang Y, Zeng S, Zheng B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014;155:311–318. doi: 10.1016/j.foodchem.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Zeng S, Zhang Y, Guo Z, Tian Y, Miao S, Zheng B. Effects of water soluble oligosaccharides extracted from lotus (Nelumbo nucifera Gaertn.) seeds on growth ability of Bifidobacterium adolescentis. Eur. Food Res. Technol. 2015;241:459–467. doi: 10.1007/s00217-015-2462-6. [DOI] [Google Scholar]
- 8.Guo XX, Sha XH, Cai SB, Wang O, Ji BP. Antiglycative and antioxidative properties of ethyl acetate fraction of Chinese purple yam (Dioscorea alata L) extracts. Food Sci. Technol. Res. 2015;21:563–571. doi: 10.3136/fstr.21.563. [DOI] [Google Scholar]
- 9.Yin JM, Yan RX, Zhang PT, Han XY, Wang L. Anthocyanin accumulation rate and the biosynthesis related gene expression in Dioscorea alata. Biol. Plantarum. 2015;59:325–330. doi: 10.1007/s10535-015-0502-5. [DOI] [Google Scholar]
- 10.Chiemi M, Takahiro H, Sayuri A, Yasumasa S, Ikuko K, Kazuo S, Terahara N, Kuma-zawa S. New acylated anthocyanins from purple yam and their antioxidant activity. Biosci. Biotechnol. Biochem. 2015;79:1484–1492. doi: 10.1080/09168451.2015.1027652. [DOI] [PubMed] [Google Scholar]
- 11.Harijono H, Estiasih T, Ariestiningsih AD, Wardani NAK. The effect of crude diosgenin extract from purple and yellow greater yams (Dioscorea alata) on the lipid profile of dyslipidemia rats. Emir. J. Food Agr. 2016;28:506–512. doi: 10.9755/ejfa.2016-01-086. [DOI] [Google Scholar]
- 12.Nadia L, Wirakartakusumah MA, Andarwulan N, Purnomo EH, Koaze H, Noda T. Characterization of physicochemical and functional properties of starch from five yam (Dioscorea alata) cultivars in Indonesia. Int. J. Chem. Eng. 2014;5:489–496. [Google Scholar]
- 13.Rosida R. Harijono, Estiasih T, Sriwahyuni E. Physicochemical properties and starch digestibility of autoclaved-cooled water yam (Dioscorea alata L.) flour. Int. J. Food Prop. 2015;19:1659–1670. doi: 10.1080/10942912.2015.1105818. [DOI] [Google Scholar]
- 14.Jiang QQ, Gao WY, Shi YP, Li X, Wang HY, Huang LQ. Physicochemical properties and in vitro digestion of starches from different Dioscorea plants. Food Hydrocolloid. 2013;32:432–439. doi: 10.1016/j.foodhyd.2013.02.001. [DOI] [Google Scholar]
- 15.Wang J, Jin ZY, Yuan XP. Preparation of resistant starch from starch-guar gum extrudates and their properties. Food Chem. 2007;101:20–25. doi: 10.1016/j.foodchem.2006.01.005. [DOI] [Google Scholar]
- 16.Reddy CK, Haripriya S, Mohamed AN, Suriya M. Preparation and characterization of resistant starch III from elephant foot yam (Amorphophallus paeonifolius) starch. Food Chem. 2014;155:38–44. doi: 10.1016/j.foodchem.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Srichuwong S, Isono N, Jiang H, Mishima T, Hisamatsu M. Freeze-thaw stability of starches from different botanical sources: Correlation with structural features. LWT-Food Sci. Technol. 2012;87:1275–1279. [Google Scholar]
- 18.Nara S, Komiya T. Studies on the relationship between water-saturated state and crystallinity by the diffraction method for moistened potato starch. Starch/Stärke. 1983;35:407–410. doi: 10.1002/star.19830351202. [DOI] [Google Scholar]
- 19.Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fraction. Eur. J. Clin. Nutr. 1992;46:30–50. [PubMed] [Google Scholar]
- 20.Li DX, Zhu F. Physicochemical properties of kiwifruit starch. Food Chem. 2017;220:129–136. doi: 10.1016/j.foodchem.2016.09.192. [DOI] [PubMed] [Google Scholar]
- 21.Goñi I, Garcia-Alonso A, Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- 22.Zheng YF, Wang Q, Li BY, Lin LM, Tundis R, Loizzo MR, Zheng BD, Xiao JB. Characterization and prebiotic effect of the resistant starch from purple sweet potato. Molecules. 2016;2016:932–943. doi: 10.3390/molecules21070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copeland L, Blazek J, Salman H, Tang MC. Form and functionality of starch. Food Hydrocolloid. 2009;23:1527–1534. doi: 10.1016/j.foodhyd.2008.09.016. [DOI] [Google Scholar]
- 24.Nor Nadiha MZ, Fazilah A, Bhat R, Karim AA. Comparative susceptibilities of sago, potato and corn starches to alkali treatment. Food Chem. 2010;121:1053–1059. doi: 10.1016/j.foodchem.2010.01.048. [DOI] [Google Scholar]
- 25.Gerbygiovanna R, Flavio FF. Physical-chemical and functional properties of maca root starch (Lepidium meyenii Walpers) Food Chem. 2009;114:492–498. doi: 10.1016/j.foodchem.2008.09.076. [DOI] [Google Scholar]
- 26.Cheetham NWH, Tao L. Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohyd. Polym. 1998;36:277–284. doi: 10.1016/S0144-8617(98)00007-1. [DOI] [Google Scholar]
- 27.Singh J, Singh N. Studies on the morphological, thermal and rheological properties of starch separated from some Indian potato cultivars. Food Chem. 2001;75:67–77. doi: 10.1016/S0308-8146(01)00189-3. [DOI] [Google Scholar]
- 28.Lan H, Hoover R, Jayakody L, Liu Q, Donner E, Baga M, Asare EK, Hucl P, Chibbar RN. Impact of annealing on the molecular structure and physicochemical properties of normal, waxy and high amylose bread wheat starches. Food Chem. 2008;111:663–675. doi: 10.1016/j.foodchem.2008.04.055. [DOI] [Google Scholar]
- 29.Ashwar BA, Gani A, Wani LA, Shah A, Masoodi FA, Saxena DC. Production of resistant starch from rice by dual autoclaving retrogradation treatment: In vitro digestibility, thermal and structural characterization. Food Hydrocolloid. 2016;56:108–117. doi: 10.1016/j.foodhyd.2015.12.004. [DOI] [Google Scholar]
- 30.Wang SJ, Liu HY, Gao WY, Chen HX, Yu JG, Xiao PG. Characterization of new starches separated from different Chinese yam (Dioscorea opposita Thunb.) cultivars. Food Chem. 2006;99:30–37. doi: 10.1016/j.foodchem.2005.07.008. [DOI] [Google Scholar]
- 31.Zeng F, Chen FQ, Kong FS, Gao QY, Aadil RM, Yu SJ. Structure and digestibility of debranched and repeatedly crystallized waxy rice starch. Food Chem. 2015;187:348–353. doi: 10.1016/j.foodchem.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 32.Zeng F, Zhu SM, Chen FQ, Gao QY, Yu SJ. Effect of different drying methods on the structure and digestibility of short chain amylose crystals. Food Hydrocolloid. 2016;52:721–731. doi: 10.1016/j.foodhyd.2015.08.012. [DOI] [Google Scholar]




