Abstract
This study was carried out to find a method to control tyrosine decarboxylase activity (TDC) of a strain of Enterococcus faecium capable of producing high levels of tyramine. To select a TDC inhibitor, enzyme assay was first performed using purified TDC enzyme and 0.1% of TDC inhibiting chemicals. When 0.23% of nicotinic acid was added, tyramine content (363 ug/mL) was lower than that of the control group (873 ug/mL). At the same time, bacterial growth was decreased 1 log cycle from 8.62 to 7.56 log CFU/mL. TDC expression level in E. faecium was measured by using RT-qPCR. Lower expression level (below 0.7) was observed after the addition of 0.23% nicotinic acid (in vitro). When cheonggukjang was manufactured with addition of nicotinic acid, tyramine contents were decreased from 698.67 to 117.27 mg/kg when the concentration of nicotinic acid added was increased from 0.10 to 0.30%. These results suggest that nicotinic acid could be used as an agent (TDC inhibitor) to reduce tyramine content in cheonggukjang.
Keywords: Tyramine, Biogenic amine, TDC, TDC inhibitor, Choenggukjang
Introduction
Tyramine is a biogenic amine (BA) produced in protein-rich fermented foods such as cheese, salami, dry sausages, and soybean pastes [1–3]. Adverse effects due to large amounts of tyramine ingestion through diet are known as ‘cheese reaction’. They can result in migraine, hypertension, vasoconstriction, increased cardiac output, increased respiration, elevated blood glucose, and release of norepinephrine [4, 5]. Hence, upper limits of tyramine in foods have been suggested to be 100–800 mg/kg [6].
In fact, tyramine is produced in fermented foods through the decarboxylation of tyrosine by microorganisms. Tyrosine decarboxylase (TDC) activity has been produced by a variety of lactic acid bacteria (LAB), including several strains of Enterococci and Lactobacilli actively present during the manufacture of most fermented foods [7, 8]. In most of previous studies, tyramine contents in Korean fermented soybean pastes such as cheonggukjang are up to 2000 mg/kg [3, 9]. And other studies have reported that Enterococcus faecium was detected in cheonggukjang as tyramine high-producer [3, 10]. Also, more than 90% of E. faecium strains isolated from dairy products were identified as tyramine producers. Similar results were reported for E. faecalis and E. durans [11]. However, Enterococci are ubiquitous microorganisms with heat tolerance. They can survive under adverse environmental conditions [12]. Therefore, it is difficult to control Enterococci.
To effectively inhibit tyramine in Enterococci, TDC activity should be selectively controlled. Zhang and Ni [13] have also reported that some mineral ions such as Cu2+, Fe2+, and Al3+ can severely diminish TDC activity in LAB. And this result is similar to Gale [14]. In addition, amino acid decarboxylase inhibition was observed in the culture treated by sugars, acids, and sulphur dioxide (SO2) [15, 16].
However, there is little information on the relationship between inhibition of TDC activity in Enterococci and tyramine reduction. Therefore, the objective of this study was to determine the inhibitor effect of chemicals on TDC enzyme based on our previous study [17]. After selecting TDC inhibitors, the growth of bacterial cell and the TDC activity were observed through in vitro tests. Gene expression levels were determined by realtime quantitative PCR (RT-qPCR). These results might help us manufacture tyramine controlled cheonggukjang.
Materials and methods
Bacterial strains and reagents
Tyrosine decarboxylase (TDC: EC 4.1.1.25), l-tyrosine, tyramine hydrochloride (99%), and dansyl chloride as derivatizing reagent and acetonitrile as mobile phase were purchased from Sigma-Aldrich (St. Louis, MO, USA). TDC gene information of E. faecium was obtained from Genbank (NCBI). Enterococcus faecium KCCM 12118 (ATCC 19434) having TDC gene and Bacillus subtilis KCTC 3014 used for the in situ test were purchased from Korean Culture Center of Microorganisms (KCCM, Seoul, Korea) and Korea Collection for Type Cultures (KCTC, Jeongeup, Korea). These strains were inoculated into tryptic soy broth (TSB, Difco, Detroit, MI, USA) and incubated at 35 ± 2 °C for 24 h. These strains were stored as 60% glycerol stock at −70 °C.
Enzymatic activity assay and HPLC analysis
Enzyme activity assay was used to select inhibition substances that could affect the activity of TDC as described by Zhang and Ni [13]. Enzymatic activity of TDC was determined in a reaction mixture solution (0.2 M sodium acetate buffer, pH 5.4, 2.5 mM l-tyrosine, 0.1% of chemical candidate) in a final volume of 1 mL. The reaction mixture was incubated at 37 °C for 20 min. Then 0.4 M perchloric acid was added to the mixture and incubated for 3 h to inactivate the enzyme and extract tyramine. Derivatization of tyramine was carried out according to procedures developed by Ben-Gigirey et al. [18]. Briefly, 1 mL of extract solution prepared above was mixed with 200 µL of 2 M NaOH and 300 µL of saturated NaHCO3. Two mL of dansyl chloride solution (10 mg/mL) prepared in acetone was added to the mixture followed by incubation at 40 °C for 45 min. Residual dansyl chloride was removed by adding 100 µL of 25% NH4OH. After incubation at room temperature for 30 min, the volume of mixture was adjusted with acetonitrile to 5 mL. Finally, the mixture was centrifuged at 3000×g for 5 min and the supernatant was filtered through 0.2 µm pore size filters (Advantec. Tokyo, Japan). Tyramine analysis was performed using HPLC system (Waters 2996 photodiode array detector, PDA) at 254 nm equipped with a Nova-pak C18 (4 μm, 150 × 3.9 mm column) obtained from Waters (Waters Corp., Milford, MA, USA).
DNA extraction
DNA was extracted from bacterial culture using G-spin™ Genomic DNA kit (Intron, Seongnam, Korea) according to the manufacturer’s protocol. TDC gene identification was performed after PCR amplification. TDC primers and q-tdc primers were used for reverse transcription PCR and realtime-qPCR (Table 1). The amplification program was as follows: 94 °C for 5 min (initial denaturation), 35 cycles of 94 °C for 1 min (denaturation), 50 °C for 2 min (annealing), and 72 °C for 1 min (extension) followed by a final extension step at 72 °C for 7 min.
Table 1.
Primers used for PCR amplification of tyrosine decarboxylase (TDC) gene and reference gene in Enterococcus faecium KCCM12118 (ATCC 19434)
| Gene | Primer | Sequence(5′–3′) | Base pairs | Accession no. | Reference |
|---|---|---|---|---|---|
| tdc a | F | GTGAATCATTGTCGAAAGAT | 1867 | KF195934 | This study |
| R | GCTTCGCTTGCCAATAATTGG | ||||
| q-tdc b | F | AGACCAAGTAATTCCAGTGCC | 116 | KF195934 | This study |
| R | CACCGACTACACCTAAGATTGG | ||||
| q-gap c | F | ATACGACACAACTCAAGGACG | 139 | NC017960 | This study |
| R | GATATCTACGCCTAGTTCGCC |
atdc specific primer used for reverse transcription PCR
btdc specific primer used for real time qPCR
cgap reference primer used for real time qPCR
RT-qPCR analysis
RT-qPCR was performed to examine mRNA expression level of TDC. Enterococcus faecium KCCM 12118 was cultured in modified TSB medium in the presence of the final selected TDC inhibitor. The strain was harvested by centrifugation at 13,000×g for 1 min and washed with 1 × TEA buffer. Total bacterial RNA was isolated using Easy-Red bacterium/yeast/fungus (BYF) total RNA extraction kit (Intron, Seongnam, Korea) according to the manufacturer’s protocols. Afterwards, cDNA synthesis was performed by using Maxime PCR premix kit (Intron, Seongnam, Korea). The resulting cDNA (2 μL) was mixed with 10 μL of SYBR Premix Ex Taq (Takara, Kyoto, Japan) and primer pairs (5 pmol each qPCR, q-tdc for target gene, q-gap for reference gene) in a 20 μL reaction volume followed by amplification for 40 cycles of the following protocol: a denaturation step at 95 °C for 15 s, an annealing step at 60 °C for 15 s, and an extension step at 72 °C for 33 s in an ABI Prism 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Gene expression levels were calculated using 2−ΔΔCt method [19].
In situ application test
White soybeans were soaked in water at 10 °C for 12 h and steamed at 121 °C for 30 min. Steamed soybeans were cooled to 50 °C. Then an inoculum suspension consisting of B. subtilis KTCT 3014, E. faecium KCCM 12118, and TDC inhibitor were added into cooled soybeans. The cheonggukjang was fermented at 45 °C for 48 h into bamboo baskets/trays (25.5 × 15.5 × 4.7 cm) in a fermentation room, under aerobic condition. After the 48 h of fermentation, samples of cheonggukjang were stored at −70 °C for further tests (Table 2).
Table 2.
TDC inhibition by different inhibition chemicals (enzyme assay)
| Chemicals | TDC inhibition ratio (%) |
|---|---|
| Control | 0 |
| Water-soluble vitamins | |
| Thiaminea (B1) | −23.2 |
| Riboflavin (B2) | 7.4 |
| Nicotinic acid (B3) | 6.3 |
| Pantothenic acid (B5) | 8.7 |
| Pyridoxin (B6) | −0.2 |
| Biotin (B7) | −16.6 |
| Ascorbic acid (C) | 4.0 |
| Organic acids | |
| Tartaric acid | 0.8 |
| Acetic acid | −12.8 |
| Lactic acid | −0.6 |
| Succinic acid | −3.7 |
| Oxalic acid | −13.6 |
| Malic acid | −9.5 |
| Citric acid | −7.3 |
| Shortchain fatty acids | |
| Formic acid | −27.0 |
| Propionic acid (C3:0) | 7.4 |
| Butyric acid (C4:0) | −36.7 |
| Valeric acid (C5:0) | 8.7 |
| Hexanoic acid (C6:0) | 10.9 |
| Sugars | |
| Glucose | −12.8 |
| Lactose | −14.9 |
| Sucrose | 8.3 |
| Salts | |
| K2SO4 | −21.6 |
| FeSO4·7H2O | −32.8 |
| MgSO4 | −25.2 |
| MnSO4.(nH2O) | −30.8 |
| ZnSO4 | −39.5 |
| (NH4)2SO4 | −6.0 |
| CuSO4 | 68.0 |
| CuClb2 | 82.2 |
| CuNO3 | 72.4 |
| CuBr2 | 74.4 |
| Na4P2O7 | 0.8 |
| CaCl2 | 0.6 |
| CaCO3 | −6.7 |
| CaSO4 | −0.6 |
| CaNO3 | −30.6 |
| AgNO3 | 98.6 |
| MgCl | 4.6 |
| CaCl | −6.0 |
| MnCl | 2.7 |
| LiCl | −7.4 |
| NaCl | 18.1 |
| Mimic | |
| ρ-cresol | 4.9 |
aInhibition chemical concentration 0.1%
bException chemicals as toxic effects at in vivo
Statistical analysis
Data are presented as mean ± SD (n = 4). All statistical analyses were performed using SPSS statistical software package (IBM SPSS statistics 20, Inc.,Chicago, IL, USA). Differences between means of individual groups were assessed by Tukey’s multiple range test. Statistical significance was considered at p < 0.05.
Results and discussion
Screening of various chemical substances to identify inhibitor of tyrosine decarboxylase (enzyme assay)
A total of 44 types of TDC inhibitory chemical substances were selected based on previous studies [13–17]. When these chemicals were applied at 0.1% to TDC enzyme, a total of 13 types reduced TDC activity by more than 5% compared to the control, including water soluble vitamins (riboflavin, nicotinic acid, and pantothenic acid), short chain fatty acids (propionic acid, valeric acid, and hexanoic acid), sugars (sucrose), and salts (copper salts, silver nitrate, and sodium chloride). Among water-soluble vitamins, pantothenic acid, riboflavin, and nicotinic acid reduced TDC activities by 8.7, 7.4, and 6.3%, respectively. However, pyridoxine, thiamine, and biotin increased tyramine level by 0.2–23.2% compared to the control. Many previous authors have reported the influence of vitamin B on tyramine production and growth in Enterococci [11, 17, 20]. Copper ions (68.0–82.2%) and silver ion (98.6%) also strongly inhibited TDC activities in the present study, similar to results reported previously [13, 14]. However, silver nitrate and copper chloride have in vivo toxicities. Therefore, they were excluded from in vitro experiments in this study. Our results showed that sucrose reduced TDC activity by 8.3%, similar to results reported by Fernández et al. [15].
Tyramine productivity after application of TDC inhibitory chemicals (in vitro)
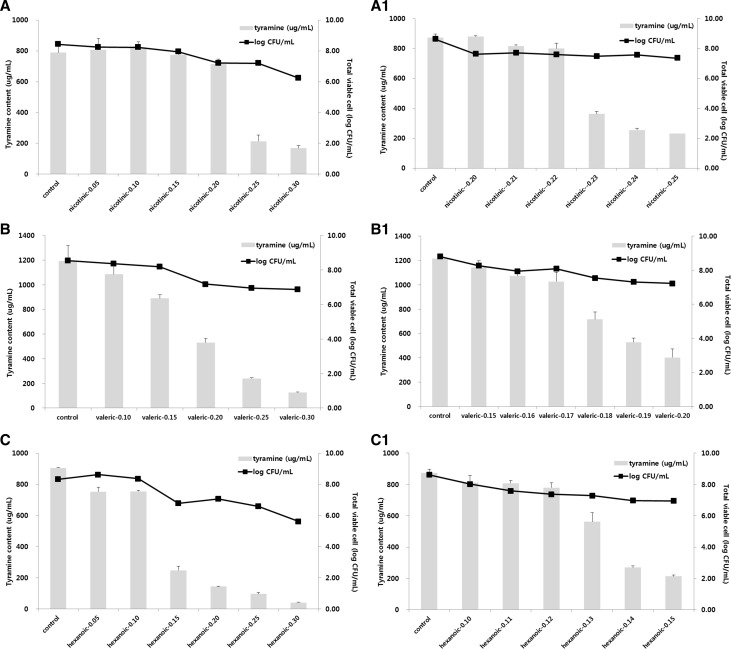
Eleven selected chemical candidates were added into modified TSB medium at different concentrations (0.1, 0.5%) (Table 3). Tyramine high-producing strain (E. faecium KCCM 12118) was then incubated at 35 ± 2 °C for 24 h. When TDC inhibitory chemicals were added at concentration of 0.1%, tyramine production level was in the range of 1037.13–1234.59 µg/mL. Eight chemicals showed similar levels of tyramine production compared to the control (1183.81 µg/mL). However, copper ions inhibited bacterial growth. When the concentration of TDC inhibitory chemicals was increased to 0.5%, tyramine production level was in the range of 12.96–1742.44 µg/mL, which was substantially different from that in the control. Riboflavin (1742.44 µg/mL), pantothenic acid (1292.35 µg/mL), and sucrose (1119.72 µg/mL) increased tyramine production whereas nicotinic acid (211.14 µg/mL), valeric acid (163.97 µg/mL), and hexanoic acid (12.96 µg/mL) decreased tyramine production by more than 50% compared to the control. The optimum concentration of TDC inhibitor was then decided based on the following criteria: when TDC inhibitory chemical was applied, it should affect the growth of bacterial strain at minimum level (<1 log cycle) and its effect of reducing tyramine productivity should be more than 50% compared to that of the control. Results are shown in Fig. 1. When nicotinic acid was added at concentration of 0.23%, tyramine content was at 363.32 µg/mL, which was reduced by 58% compared to that in the control. Cell count was 7.48 log CFU/mL, which was not significantly different from that in the control (8 log CFU/mL). When valeric acid was added at concentration of 0.19%, tyramine content was at 528.25 µg/mL, which was reduced by 56% compared to that in the control. Meanwhile, cell count in this case was 7.31 log CFU/mL, which was less than that when nicotinic acid was applied. When hexanoic acid was added at concentration of 0.14%, tyramine content was 270 µg/mL, which was reduced by 69% compared to that in the control. However, cell count in this case was at 6.97 log CFU/mL. Among these three candidates (nicotinic acid, hexanoic acid, and valeric acid) obtained through secondary selection, nicotinic acid was finally selected due to the fact that hexanoic acid and valeric acid affected bacterial growth more compared to nicotinic acid. In addition, valeric acid induced off flavor.
Table 3.
Tyramine contents and bacterial counts of Enterococcus faecium KCCM12118 grown in modified TSB medium added with 0.1 and 0.5% of inhibition chemicals (in vitro)
| Chemical inhibitors (%) | Tyramine content (ug/mL) | Cell countsa
(log CFU/mL) |
|---|---|---|
| Control | 1183.81 ± 12.15b | 8.30 ± 0.03 |
| Riboflavin-0.1 | 1389.79 ± 18.47 | 8.33 ± 0.01 |
| Riboflavin-0.5 | 1742.44 ± 55.41 | 8.41 ± 0.04 |
| Nicotinic acid-0.1 | 1078.23 ± 23.09 | 8.38 ± 0.10 |
| Nicotinic acid-0.5 | 211.14 ± 27.97 | 6.77 ± 0.11 |
| Pantothenic acid-0.1 | 1141.92 ± 46.50 | 8.05 ± 0.05 |
| Pantothenic aicd-0.5 | 1292.35 ± 64.65 | 8.44 ± 0.01 |
| Propionic acid-0.1 | 1234.59 ± 43.98 | 8.23 ± 0.02 |
| Propionic acid-0.5 | 935.15 ± 40.09 | 6.99 ± 0.05 |
| Valeric acid-0.1 | 1051.32 ± 29.95 | 8.45 ± 0.01 |
| Valeric acid-0.5 | 163.97 ± 10.91 | 6.26 ± 0.01 |
| Hexanoic acid-0.1 | 1158.21 ± 66.57 | 8.03 ± 0.01 |
| Hexanoic acid-0.5 | 12.96 ± 1.29 | 5.41 ± 0.00 |
| Sucrose-0.1 | 1037.13 ± 23.48 | 8.10 ± 0.01 |
| Sucrose-0.5 | 1119.72 ± 0.61 | 8.42 ± 0.02 |
| NaCl-0.1 | 1057.75 ± 39.32 | 8.29 ± 0.02 |
| NaCl-0.5 | 854.98 ± 77.15 | 6.24 ± 0.02 |
| CuSO4-0.1 | NDc | ND |
| CuBr2-0.1 | ND | ND |
| CuNO3-0.1 | ND | ND |
a Enterococcus faecium type strain KCCM 12118 (ATCC 19434)
bMean ± standard deviation
cND not detected (amine level is less than 0.1 ug/mL, cells count is less than 2.0 log CFU/mL)
Fig. 1.
Changes in tyramine contents and bacterial counts of Enterococcus faecium KCCM12118 grown in modified TSB medium with various concentration of TDC inhibition chemicals. (A) Nicotinic acid (0.05–0.30%), (A-1) nicotinic acid (0.20–0.25%), (B) valeric acid (0.10–0.30%), (B-1) valeric acid (0.15–0.20%), (C) hexanoic acid (0.05–0.30%), (C-1) hexanoic acid (0.10–0.15%)
mRNA expression levels of TDC in tyramine-producing strain added with TDC inhibitor based on RT-qPCR
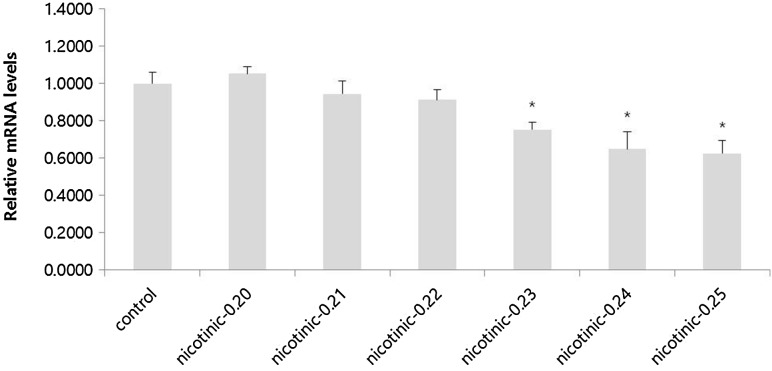
RT-qPCR assay was carried out to monitor the inhibitory effect of nicotinic acid on TDC gene expression in E. faecium. Cycle threshold (Ct) value of target gene was compared to the Ct value of reference gene. Results showed that TDC mRNA expression level in E. faecium grown in modified TSB medium added with nicotinic acid (0.20–0.25%) was generally lower than that in the control without any addition of nicotinic acid. When nicotinic acid was added at more than 0.23%, mRNA expression levels of TDC were gradually and significantly (p < 0.05) decreased compared to that in the control (Fig. 2). Ct values are related to TDC activity. They have been detected in cheeses with higher concentrations of tyramine [21]. According to Zhang and Ni [13], mineral ions could change the relative TDC activity. Landete et al. [16] have also indicated that sugars and organic acids can affect both histidine decarboxylase (HDC) expression and HDC activity. These results suggest that nicotinic acid as TDC inhibitor can affect both TDC expression and tyramine production in E. faecium.
Fig. 2.
Expression level of TDC in Enterococcus faecium KCCM12118 grown in modified TSB medium added with nicotinic acid based on RT-qPCR assay (in vitro). Data are presented as mean ± SD (n = 4). *p < 0.05 compared with control
Using nicotinic acid for tyramine reduction in food system (lab scale)
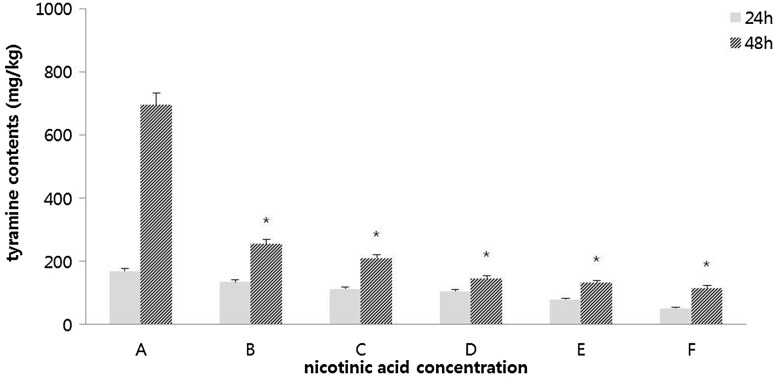
After 24 h of fermentation, both total aerobic cell count and viable cell count of Bacillus spp. showed 9 log CFU/g. These levels lasted until the end of fermentation. However, the number of Enterococcus strains in cheonggukjang with or without addition of nicotinic acid (control) showed different results. When nicotinic acid was added at more than 0.20%, Enterococcus cell was not found after 24 h of fermentation. It was finally found at the end of fermentation. This suggests that the growth of Enterococcus cells might have been initially influenced by nicotinic acid. Such result might be a secondary effect of nicotinic acid. As shown in Table 3, addition of nicotinic acid at 0.5% affected the growth of Enterococcus strains in in vitro test. On the contrary, when nicotinic acid was added at concentration lower than 0.15%, Enterococcus cell count was at 4 log CFU/g after 24 h of fermentation. It was at 8 log CFU/g at the end of fermentation, which was not significantly different from that in the control (data not shown). After 24 h of fermentation, tyramine content was at 113.44 mg/kg in the group with addition of nicotinic acid at 0.15%, which was reduced by more than 30% compared to that in the control (170.06 mg/kg) (Fig. 3). When cheonggukjang was manufactured after addition of nicotinic acid at concentration of 0.15%, tyramine content was further reduced to 212.06 mg/kg, which was reduced by more than 60% compared to that in the control (698.67 mg/kg) at the end of fermentation (48 h). For cheonggukjang added with nicotinic acid at more than 0.20%, tyramine contents ranged from 117.27 to 133.94 mg/kg at the end of fermentation. Tyramine reduction was more influenced by the addition of nicotinic acid at concentration of 0.20% compared to that at concentration of 0.15%. However, 0.15% might be the optimum concentration of nicotinic acid due to its effective reduction of tyramine content without affecting the growth of Enterococcus cells, even when higher numbers of Enterococcus cells are present in the food system.
Fig. 3.
Tyramine contents in cheonggukjang system added with nicotinic acid at several levels (in situ). (A) Bacillus subtilis KCTC3014 (starter strain) + Enterococcus faecium KCCM12118 (tyramine high producing strain), (B) A + nicotinic acid (B3, TDC inhibitor) 0.10%, (C) A + B3 0.15%, (D) A + B3 0.20%, (E) A + B3 0.25%, (F) A + B3 0.30%. Data are presented as mean ± SD (n = 4). *p < 0.05 compared with 48 h of control cheonggukjang
Acknowledgements
This research was supported by the High Value-added Food Technology Development Program (314057-032-SB020) funded by Korea Institute of Planning and Evaluation for Technology in Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Eitenmiller RR, Koehler PE, Reagan JO. Tyramine in fermented sausages: factors affecting formation of tyramine and tyrosine decarboxylase. J. Food Sci. 1978;43:689–693. doi: 10.1111/j.1365-2621.1978.tb02394.x. [DOI] [Google Scholar]
- 2.Joosten HMLJ. The biogenic amine contents of Dutch cheese and their toxicological significance. Neth. Milk Dairy J. 1988;42:25–42. [Google Scholar]
- 3.Oh SJ, Mah JH, Kim JH, Kim YW, Hwang HJ. Reduction of Tyramine by Addition of Schizandra chinensis Baillon in Cheonggukjang. J. Med. Food. 2012;15:1109–1115. doi: 10.1089/jmf.2012.2561. [DOI] [PubMed] [Google Scholar]
- 4.Bodmer S, Imark C, Kneubühl M. Biogenic amines in foods: histamine and food processing. Inflamm. Res. 1999;48:296–300. doi: 10.1007/s000110050463. [DOI] [PubMed] [Google Scholar]
- 5.Shalaby AR. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996;29:675–690. doi: 10.1016/S0963-9969(96)00066-X. [DOI] [Google Scholar]
- 6.Brink B, Damirik C, Joosten HMLJ. Huis in’t Veld JHJ. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990;11:73–84. doi: 10.1016/0168-1605(90)90040-C. [DOI] [PubMed] [Google Scholar]
- 7.Marcobal Á, Martín-Álvarez PJ, Polo MC, Muñoz R, Moreno-Arribas M. Formation of biogenic amines throughout the industrial manufacture of red wine. J. Food Prot. 2006;69:397–404. doi: 10.4315/0362-028X-69.2.397. [DOI] [PubMed] [Google Scholar]
- 8.Coton E, Coton M. Evidence of horizontal transfer as origin of strain to strain variation of the tyramine production trait in Lactobacillus brevis. Food Microbiol. 2009;26:52–57. doi: 10.1016/j.fm.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Mah JH. Evaluation and Reduction of Biogenic Amines in Korean Traditional Fermented Foods. Seoul: Korea University; 2003. [Google Scholar]
- 10.Lee YL. Reduction effect of biogenic amines in Doenjang by combined application of microorganisms and plant extracts. Seoul: Korea University; 2017. [Google Scholar]
- 11.Sarantinopoulos P, Andrighetto C, Georgalaki MD, Rea MC, Lombardi A, Cogan TM, Kalantzopoulos G, Tsakalidou E. Biochemical properties of enterococci relevant to their technological performance. Int. Dairy J. 2001;11:621–647. doi: 10.1016/S0958-6946(01)00087-5. [DOI] [Google Scholar]
- 12.Marcobal Á, de las Rivas B, García-Moruno E, Muñoz R. The tyrosine decarboxylation test does not differentiate Enterococcus faecalis from Enterococcus faecium. System. Appl.Microbiol. 27: 423–426 (2004) [DOI] [PubMed]
- 13.Zhang K, Ni Y. Tyrosine decarboxylase from Lactobacillus brevis: Soluble expression and characterization. Protein Expres. Purify. 2014;94:33–39. doi: 10.1016/j.pep.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Gale E.F. The bacterial amino acid decarboxylases. Adv. Enzymol. Relat. Area Mol. Biol. NY. vol. 6: 1–32 (1946)
- 15.Fernández M, Linares DM, Rodríguez A, Alvarez MA. Factors affecting tyramine production in Enterococcus durans IPLA 655. Appl. Microbiol. Biotech. 2007;73:1400–1406. doi: 10.1007/s00253-006-0596-y. [DOI] [PubMed] [Google Scholar]
- 16.Landete JM, Pardo I, Ferrer S. Regulation of hdc expression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008;105:1544–1551. doi: 10.1111/j.1365-2672.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee BN. Reduction of Biogenic Amines by Application of Degradation Strains and Chemical Compounds to Cheonggukjang. Seoul: Korea University; 2011. [Google Scholar]
- 18.Ben-Giglrey B, Vieites Baptista de Sousa, Juan M, Villa Tomas G. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J. Food Protec. 61: 608–615 (1998) [DOI] [PubMed]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999;53:33–41. doi: 10.1016/S0168-1605(99)00152-X. [DOI] [PubMed] [Google Scholar]
- 21.Ladero V, Martínez N, Martín MC, Fernández M, Alvarez MA. qPCR for quantitative detection of tyramine-producing bacteria in dairy products. Food Res. Int. 2010;43:289–295. doi: 10.1016/j.foodres.2009.10.007. [DOI] [Google Scholar]