Abstract
Persimmon is one of the most common native fruits of South Korea and its leaves are well known to be used in pharmaceuticals, cosmetics as well as beverages. The aim of this study was to compare the antioxidant properties of Korean major persimmon leaves on the basis of drying methods (hot air drying and freeze-drying) and harvesting time. Persimmon leaves from five cultivars (‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Cheongdobansi’, ‘Gabjubaekmok’ and ‘Suhong’) were harvested in late May and late June followed by blanching and drying. Results depicted that, persimmon leaves harvested in late May had the highest amount of antioxidants content compared to the late June. No significant difference was found between HAD and FD treatment with respect to total phenol, total flavonoid, tannin content, ABTS and DPPH radical-scavenging activity. Finally, it can be concluded that ‘Gabjubaekmok’ persimmon leaves collected during late May and dried by hot air are richer in antioxidants.
Keywords: Persimmon leaf, Antioxidant properties, Drying treatment, Harvesting time, Cultivar
Introduction
Persimmon (Diospyros kaki) belongs to the family of Ebenaceae and has been cultivated over hundreds of years around Eastern Asia. The fruit of persimmon is eaten fresh or dry, whereas the leaf is generally used for tea due to their functional properties. In Japan, persimmon leaf is infused with hot water and drunk as a green tea (kakinoha-cha) due to its healing effects in conditions of frostbite, paralysis, burns, as well as in stopping bleeding [1].
Antioxidants are broadly used in the food production and nowadays extra consideration has been paid to common non-poisonous antioxidants with an end goal to shield the human body from free radicals as well as many chronic diseases. Persimmon leaves contain numerous bioactive compounds such as phenols, tannins, flavonoid oligomers, natural acids, ascorbic acid, caffeine and chlorophyll [1, 2]. Particularly, flavonoids in persimmon leaf including kaempferol, quercetin, and catechin demonstrate robust antioxidant activities and are associated with maintaining blood pressure [3]. Moreover, quercetin reveals anti-cancer, anti-allergic and anti-inflammatory activities [4], whereas kaempferol shows the preventive activity against Alzheimer’s disease [5]. In addition, Naoxinqing (NXQ), is a permitted Traditional Chinese Medicine (TCM) prepared from persimmon leaf extract (flavonoid) broadly used in China to cure apoplexy and stroke [6]. Furthermore, the polyphenols in persimmon leaf are broadly analyzed due to their anti-allergic, anti-inflammatory, and antibacterial properties. The major polyphenols in Diospyros kaki leaf are proanthocyanidins, which have vasorelaxant and anti-hypertensive effects [7]. Some in vitro studies have commended the possible valuable effects of polyphenols on diabetes [8, 9]. Beside this, tannin is one of the major components of persimmon leaf, which has various functional properties such as anti-allergic [10], antibacterial [11], reducing blood pressure [12] and scavenging free radicals [13].
Persimmon leaf has become increasingly popular due to its use in the food, pharmaceutical as well as cosmetic industries. People of East Asia continuously use persimmon leaf as a natural food additive due to its functional properties. More notably, the fact that, persimmon leaf contains kaempferol, volatile oil and tannins which play important roles in antimicrobial activity. Persimmon leaf-based ingredients have been incorporated into rice cake [14], cookies [15], athlete’s foot socks and soaps, and also used as a sushi ingredient [16] due to their antimicrobial properties.
Drying techniques are able to change the quantity and quality of antioxidants. The features and quality of persimmon leaf preserved by drying depend to a great extent on their physical, chemical as well as microbiological stability. Moreover, the packaging, transportation as well as storage costs are minimized due to moisture content reduction. However, hot air-drying and freeze-drying are the very common drying methods used in food processing. Freeze-drying is the most superior type of drying in terms of nutritional value preservation as well as color. Therefore, it has been stated that freeze-drying treatment rises the infusion of antioxidants from various goods compared to hot air-drying [17], though it has always been considered as the most expensive method.
To the best of our understanding, limited studies have been reported on the antioxidant properties of persimmon leaves. However, no report has yet been made on the bioactive compounds from Korean major persimmon leaves based on different drying techniques, cultivars as well as harvesting time. This study was thus designed to investigate the antioxidants properties of Korean persimmon leaves on the basis of drying methods (hot air drying at 100°C for 30 min and freeze drying), cultivars (‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Cheongdobansi’, ‘Gabjubaekmok’ and ‘Suhong’) and harvesting time (late May and late June).
Materials and methods
Chemicals and reagents
Folin–Ciocalteu reagent, catechin, and gallic acid were purchased from Sigma Chemicals (St Lous, MO, USA). DPPH and 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) were obtained from TCI Co., Ltd. (Tokyo, Japan). Sodium carbonate, sodium nitrite, and aluminum chloride were obtained from Junsei Chemical Co., Ltd. (Tokyo, Japan). Sodium hydroxide, methanol, and ethanol were bought from Duksan Pure Chemicals Co. Ltd. (Ansan, Korea). Vanillin was obtained from Samchun Chemical Co., Ltd. (Seoul, Korea). All other chemicals were analytical grade.
Samples
Leaves of four cultivars (‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Gabjubaekmok’ and ‘Suhong’) of persimmons (Diospyros kaki) were harvested from persimmon experiment station, Sangju, South Korea on 25th May (flowering stage) and 25th June (fruiting stage) in 2016. At the same time, ‘Cheongdobansi’ persimmon leaves were collected from Cheongdo, South Korea. Prior to sample collection, six rows of trees were selected and six trees within each row labeled for further sample collections.
Drying treatments of raw material
Persimmon leaves were blanched at 100°C for 2 min and then dried using two drying methods. (1) Hot air drying at 100°C for 30 min; (2) freeze-drying for 72 h in a vacuum freeze dryer.
Preparation of aqueous extracts of persimmon leaf
The dried persimmon leaf was ground to a fine powder (particle size ≤ 1 mm) by using a blender (Shinil Industrial SMX-9400MD, South Korea). The extraction was performed with a product to water ratio of 1:10 (w/w) at 90°C for 60 min. The extracts were filtered through Whatman filter paper (4 Whatman™; 150 mm Buckinghamshire, UK). Filtrates were collected and kept at 4°C prior to further investigation.
Analytical determinations
Determination of total phenolic content
The total phenolic content (TPC) was measured by spectrophotometry, according to the method described by Singleton and Rossi [18] with slight modifications. Briefly, 0.2 mL of the sample solution was added to 1 mL of 10% Folin–Ciocalteu’s phenol reagent in the flask. After 10 min, 0.8 mL of 7.5% sodium carbonate (Na2CO3) was equally added into the flask. The flask was then allowed to stand at room temperature for 2 h and the absorbance was measured at 765 nm using a spectrophotometer (Shimadzu Corp., Kyoto, Japan). The standard curve consisted of gallic acid between the concentration arrays of 0–200 ppm for the total phenolic content. Results were expressed as mg gallic acid equivalents per gram of dry matter (mg GAE/g d.m.).
Determination of total flavonoid content
Total flavonoid content was evaluated by using the modified colorimetric technique as stated in Dewanto et al. [19]. Concisely, 0.25 mL of the sample was added to 1 mL of distilled water in a test tube, followed by the mixing of 75 μL of a 5% sodium nitrite (NaNO2) solution. After 6 min, 150 μL of a 10% aluminum chloride (AlCl3) was mixed and the solution was kept for a further 5 min before 0.5 mL of 1 M sodium hydroxide (NaOH) was mixed. Lastly, 2 mL of distilled water was added and the absorbance was measured instantly at 510 nm using a spectrophotometer (Shimadzu Corp., Kyoto, Japan). The results were expressed as mg of (+)-catechin equivalent per gram of dry matter (mg CE/g).
Determination of tannin content
The tannin content was determined following the procedure of Broadhurst and Jones [20] and as described by Xu and Chang [21]. Briefly, 0.5 mL of the extract, 3 mL of a 4% methanol vanillin mixture and 1.5 mL of hydrochloric acid (conc.) were mixed. The solution was kept for 15 min at room temperature. The tannin content was determined spectrophotometrically at 500 nm against methanol as a blank. Results were expressed as mg catechin equivalents per gram of dry matter (mg of CE/g) using the calibration curve of (+)-catechin.
DPPH radical-scavenging activity
DPPH radical-scavenging assay was carried out following the method of Shahidi et al. [22] with some modifications. Briefly, 0.1 mL of the sample diluted in methanol (1 mL extract/5 mL methanol) was mixed with 3.9 mL of a methanolic mixture of DPPH (methanol: water; 80:20) (0.025 mg/mL). The solution was shaken and allowed to stand for 30 min at ambient temperature. Absorbance was measured at 510 nm using the spectrophotometer (Shimadzu Corp., Kyoto, Japan). The antioxidant activity (%) of the samples was calculated according to the following formula:
where Acontrol is the absorbance of DPPH solution without the sample.
ABTS radical-scavenging activity
The ABTS radical-scavenging activity was evaluated using the procedure explained by Thaipong et al. [23] with some modifications. A 7.4 mM ABTS+ (0.203 g/50 mL) solution and 2.6 mM (0.035 g/50 mL) potassium persulfate solution were mixed in equal quantities and allowed to stand in an amber color bottle in the dark for 12 h. Then the ABTS+ stock solution was diluted by adding 1 mL ABTS+ solution with 24 mL ethanol to an absorbance of 1.5 at 734 nm using the spectrophotometer (Shimadzu Corp., Kyoto, Japan). The fresh ABTS solution was set up for each assay. Finally, 0.3 mL sample was added with 2.7 mL of the ABTS solution and the absorbance was measured after 7 min at 734 nm. The scavenging activity was expressed as a percentage (%) according to the following formula:
Statistical analysis
The data were reported as the mean ± standard deviation of triplicate measurements. Analysis of variance (ANOVA) was accomplished to estimate the effect of process variables (harvesting time, drying method, and leaf variety). A significant difference (p < 0.05) within means was analyzed using the Statistical Analysis System (SAS 9.3) program.
Result and discussion
Total phenolic content
The result demonstrated that there were no significant differences between hot air and freeze drying methods with respect to phenolic compounds (Fig. 1). Although, few reports have demonstrated that the freeze-dried sample shows greater antioxidants compared to other drying methods [17, 24]. However, Martínez-Las et al. [25] showed the hot air drying was the optimum method compared to freeze drying regarding phenolic compounds in Spanish persimmon leaves. However, in this present study, similar result was obtained from both methods of drying. Perhaps, during freeze drying enzymatic activity increase upon thawing might have affected degradation of some phenolic compounds [26]. Freeze-drying treatment possibly enriches the browning reaction which is responsible for the oxidation of phenolic compounds by the activity of the enzyme. Also, low temperature during dehydration affected the degradation of phenols for a long time of drying. However, the higher temperature during hot air drying destructs the tissue and allowed more extraction of phenolic compounds. This is because; drying has an ability to break the cellular components of food matrix to release the polyphenol [27].
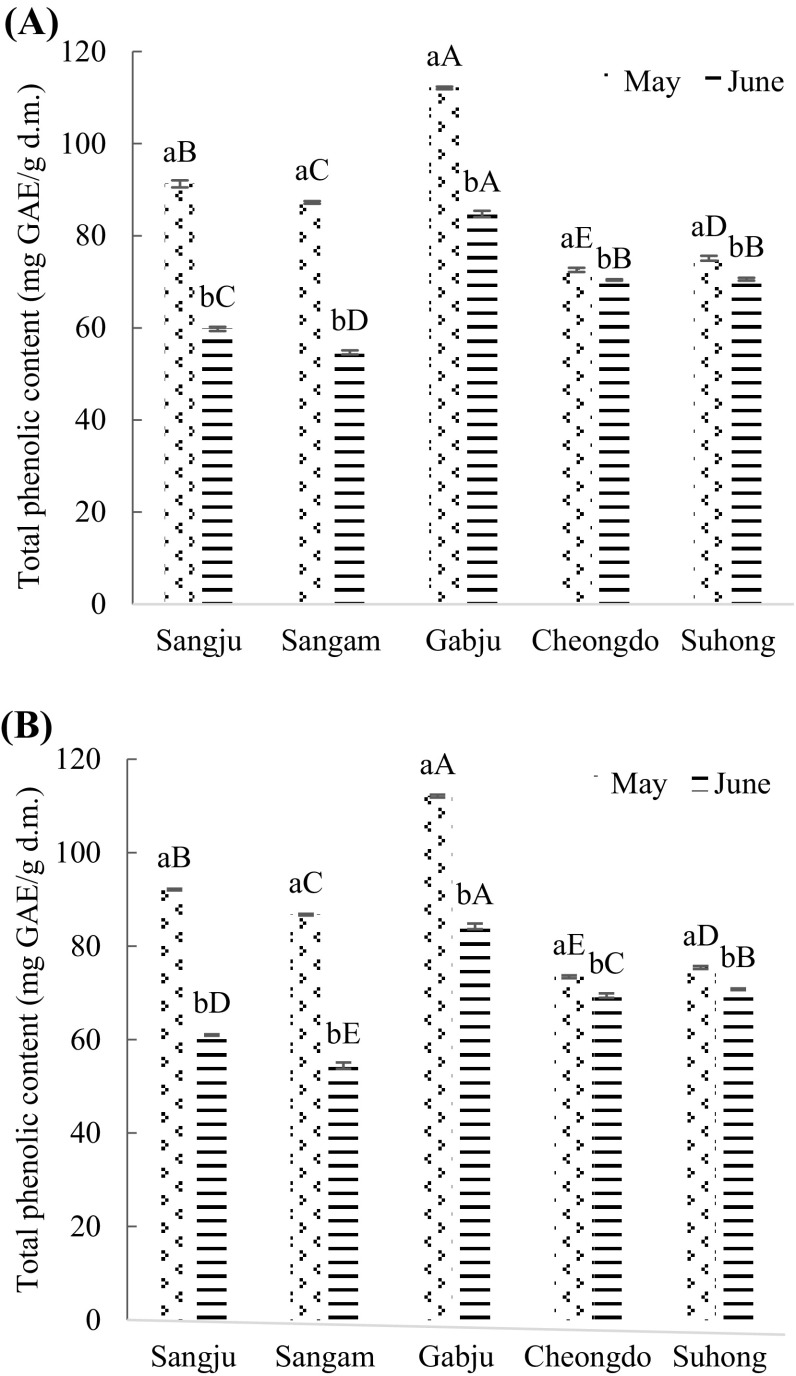
Fig. 1.
Total phenolic content (mg gallic acid/g dry matter) of persimmon leaves extracts. (A) Hot air drying treatment; (B) freeze-drying treatment. Values are mean ± SD of three (n = 3) measurements. Lower case letters (a and b) within bars of the same cultivar with different harvesting times and capital alphabet letters (A–E) within bars of the different cultivars across same harvesting time are significantly different (p < 0.05). Sangju, Sangju-dungsi; Sangam, Sangam-dungsi; Gabju, Gabjubaekmok; Cheongdo, Cheongdobansi
The results showed that the total phenolic content of ‘Gabjubaekmok’ persimmon leaf was the highest (p < 0.05) at both harvesting times (late May and late June) compared to other cultivars by hot air and freeze-drying treatments (Fig. 1). The figures were 112.09 ± 0.3 and 84.72 ± 0.7 (mg GAE/g d.m.) by hot air drying treatment in late May and late June, respectively. Also, 112.09 ± 0.3 and 84.21 ± 0.61 (mg GAE/g d.m.) of phenolic compound were obtained by freeze-drying method in late May and late June, respectively. ‘Cheongdobansi’ and ‘Sangam-dungsi’ had the lowest amount of phenolic compounds during the late May and late June, respectively.
Interestingly, there was a big difference between flowering (late May) and fruiting (late June) stages with respect to phenolic compounds. The highest (p < 0.05) amount of polyphenol was found at the flowering stage compared to the fruiting stage among all cultivars. The results were consistent with the findings of Jung and Jeong [28] and Chung et al. [29]; they found that persimmon leaf harvested in May had the higher amount of phenolic compounds among different harvesting times. Moreover, Zou et al. [30] indicated that mulberry leaves harvested in May had the highest polyphenol contents than April. However, no explanation was provided about that fluctuation of antioxidants. The possible reason for this phenomenon could be the movement of antioxidants from leaves to fruits during fruiting. Another possible reason the differences among harvesting time can be associated to plant physiological characteristics, environmental conditions particularly light intensity, temperature and the various phases of fruit growth and development. It has been stated that the amount of polyphenol may change according to the growth phase, the part of the plant and the environment condition [31].
Total flavonoid content
Similar trends were found in flavonoid content as total phenol, where no significant differences were observed between the both types of drying (Fig. 2). These results are in agreement with the findings reported by Alonzo-macías et al. [32] on dried strawberry, where freeze-dried samples exhibited similar flavonoids as hot air dried samples. Also, Martínez-Las et al. [25] found that the hot air and freeze dried persimmon leaves showed the similar result at 90 °C of extraction with respect to flavonoid content. Generally, freeze-drying considered the optimum method for preserving antioxidant contents, but in this study, it is showing the similar tendency as hot air drying. The loss of flavonoids could be due to the polymerization and oxidation during the freeze-drying process. Horszwald et al. [33] demonstrated that freeze-drying treatment yielded comparatively less flavonoid on aronia powders compared with hot air and oven vacuum drying.
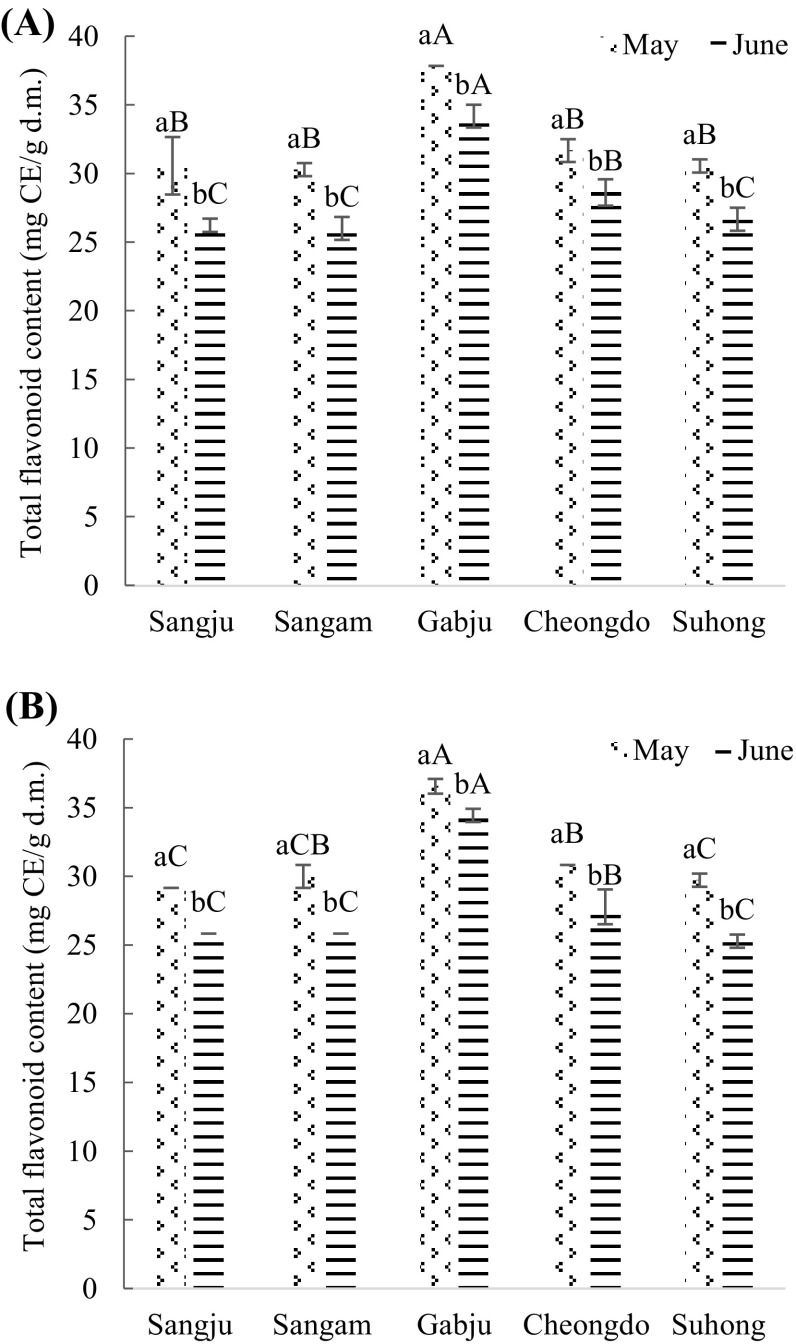
Fig. 2.
Total flavonoid content (mg catechin/g dry matter) of persimmon leaves extracts. (A) Hot air drying treatment; (B) freeze-drying treatment. Values are mean ± SD of three (n = 3) measurements. Lower case letters (a and b) within bars of the same cultivar with different harvesting times and capital alphabet letters (A–C) within bars of the different cultivars across same harvesting time are significantly different (p < 0.05). Sangju, Sangju-dungsi; Sangam, Sangam-dungsi; Gabju, Gabjubaekmok; Cheongdo, Cheongdobansi
‘Gabjubaekmok’ persimmon leaf extracts had the highest (p < 0.05) amount of flavonoid content in late May (flowering) and late June (fruiting) compared to other cultivars when processed by both types of drying method. The figures were 37.83 ± 0 and 34.17 ± 0.83 (mg CE/g of d.m.) by hot air drying method in late May and late June, respectively. Also, 36.55 ± 0.54 and 34.44 ± 0.96 (mg CE/g of d.m.) of flavonoid content were obtained when processed by freeze-drying method in flowering and fruiting stages, respectively. There were no significant differences were observed among ‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Cheongdobansi’, and ‘Suhong’ persimmon leaf extracts during the flowering time when dried by hot air. ‘Sangam-dungsi’ and ‘Sangju-dungsi’ considered the poorer cultivar regarding flavonoid content among all cultivars during flowering and fruiting stages, respectively.
However, the highest (p < 0.05) amount of flavonoid content were obtained in the flowering stage compared to the fruiting stage (Fig. 2). ‘Sangju-dungsi’, ‘Sangam-dungsi’, ‘Gabjubaekmok’, ‘Cheongdobansi’, and ‘Suhong’ leaf extracts were 14.18, 14.13, 9.69, 9.65 and 12.73% respectively richer in flavonoids content in late May compared to late June. It might be the same reason as total phenol, where there is a possible movement of flavonoids from leaves to fruits during fruiting stage. Jung and Jeong [28] and Chung et al. [29] reported that the persimmon leaf contains the highest amount of flavonoid from late May to early June among different harvesting times.
Tannin content
The effect of different drying techniques on tannin content was similar to total phenolic as well as flavonoids. Hot air dried samples yielded the same tannin contents as shown the freeze-dried samples (Fig. 3). Stewart et al. [34] suggested that the freeze-drying should be avoided in tannin research, where air drying was considered as the most desirable drying method. A long time during freeze drying may affect the amount of tannin from persimmon leaves.
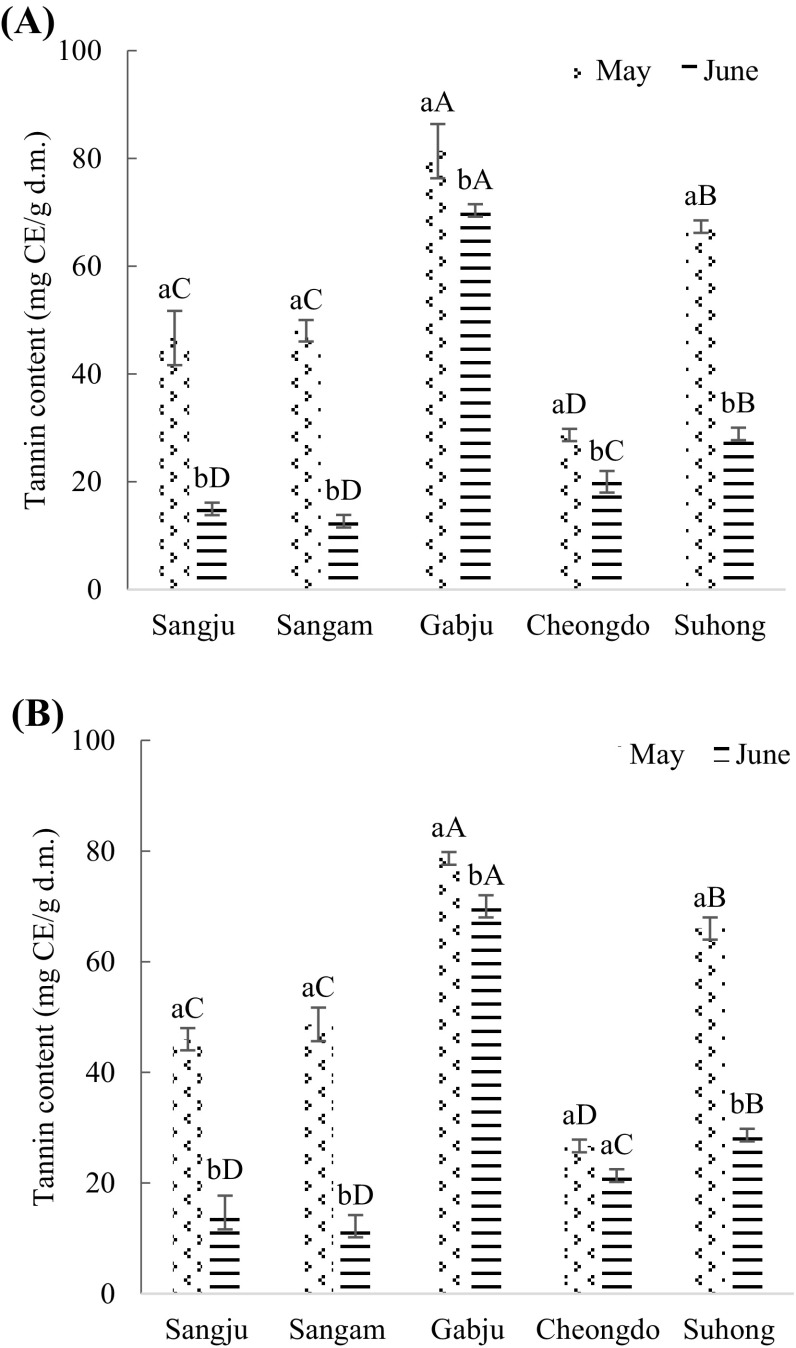
Fig. 3.
Tannin content (mg catechin/g dry matter) of persimmon leaves extracts. (A) Hot air drying treatment; (B) freeze-drying treatment. Values are mean ± SD of three (n = 3) measurements. Lower case letters (a and b) within bars of the same cultivar with different harvesting times and capital alphabet letters (A–D) within bars of the different cultivars across same harvesting time are significantly different (p < 0.05). Sangju, Sangju-dungsi; Sangam, Sangam-dungsi; Gabju, Gabjubaekmok; Cheongdo, Cheongdobansi
Similar characteristics were found in the tannin content, where ‘Gabjubaekmok’ persimmon leaf extracts showed the highest tannin content while the opposite scenario was seen in ‘Cheongdobansi’ leaf extracts during the flowering stage (late May) by both types of drying treatment (p < 0.05). The figures were 81.33 ± 5.03 and 28.67 ± 1.15 (mg CE/g d.m.) by hot air drying and 78.67 ± 1.05 and 26.69 ± 1.15 (mg CE/g d.m.) by freeze-drying treatment, respectively. In comparison, ‘Sangju-dungsi’ and ‘Sangam-dungsi’ were considered the poorer cultivars regarding tannin content in the fruiting stage, while the opposite was true in ‘Gabjubaekmok’ persimmon leaf extracts by both types of drying treatment (p < 0.05).
The tannin content in persimmon leaves reduced dramatically from flowering to fruiting stage among all cultivars (p < 0.05). ‘Sangju-dungsi’ and ‘Sangam-dungsi’ leaf extracts showed almost four times higher tannin content in late May compared to the late June. Chung et al. [29] reported that the persimmon leaves; particularly, ‘Cheongdobansi’ had the highest level of tannin content in early June among different harvesting times. Possibly it could be due to the transformation of tannin content into fruits during fruiting. Scogings et al. [35] stated that the levels of polyphenols and tannins were higher in all species in the early stage.
DPPH radical-scavenging activity
To evaluate the scavenging effects of DPPH in persimmon leaf extracts, percentage (%) of inhibition was investigated and the results are shown in (Fig. 4). DPPH radical-scavenging activity represents the capacity of the antioxidant extract to scavenge free radicals through hydrogen or electron donating mechanisms. The results demonstrate that percentage (%) inhibition of DPPH was similar in hot air and freeze dried samples. The loss of DPPH activity could be due to the enzymatic degradation by the Maillard reaction during freeze-drying. Previous studies have reported that the hot air and freeze dried samples showed similar trends in terms of antioxidants activity from raspberry powders [36] and dried strawberry [32]. However, Martínez-Las et al. [25] reported that the hot air drying was superior compared to freeze drying method with respect to DPPH activity in Spanish persimmon leaves. Probably this might be due to differences in soil types, fertilizers, climate and management and also the duration of freeze drying.
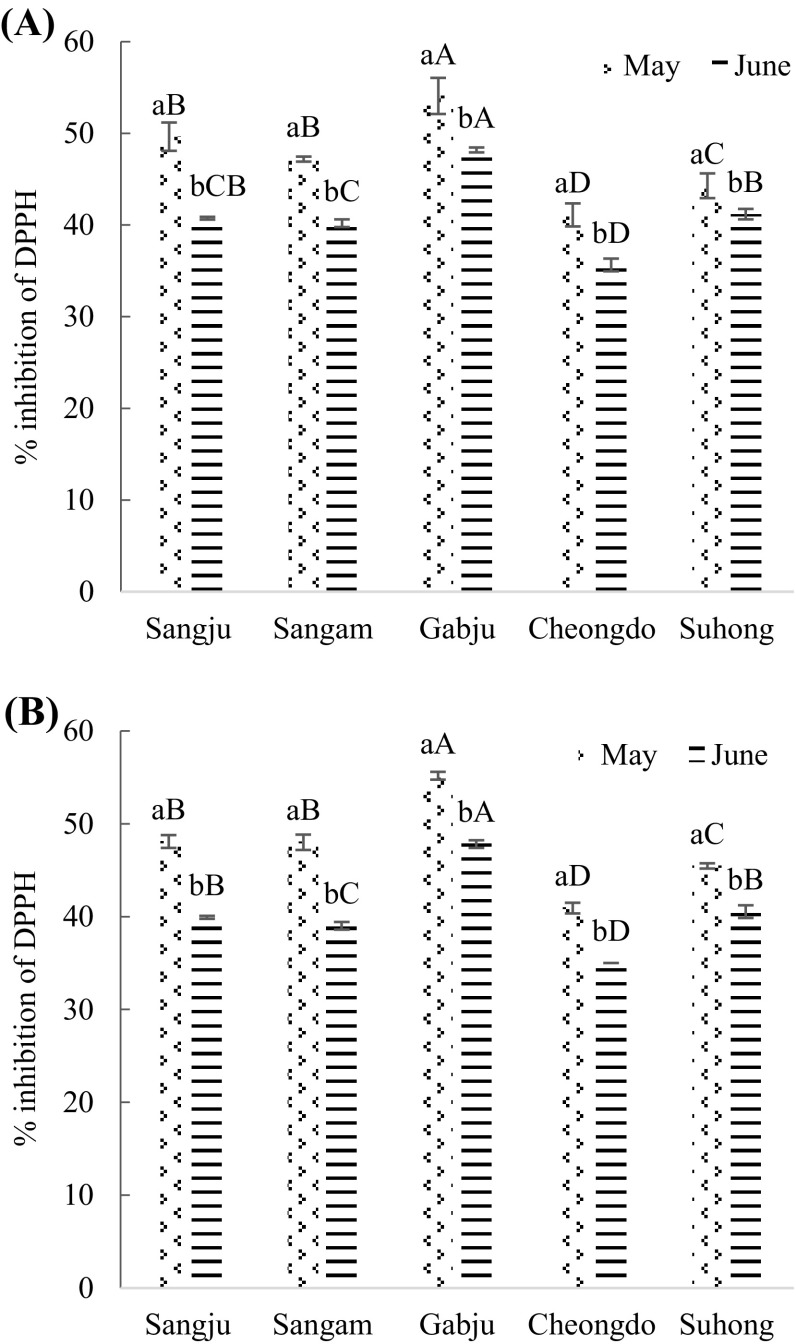
Fig. 4.
DPPH radical- scavenging activity (%) of persimmon leaves extracts. (A) Hot air drying treatment; (B) freeze-drying treatment. Values are mean ± SD of three (n = 3) measurements. Lower case letters (a and b) within bars of the same cultivar with different harvesting times and capital alphabet letters (A–D) within bars of the different cultivars across same harvesting time are significantly different (p < 0.05). Sangju, Sangju-dungsi; Sangam, Sangam-dungsi; Gabju, Gabjubaekmok; Cheongdo, Cheongdobansi
Percentage of DPPH was the highest in ‘Gabjubaekmok’ persimmon leaf extracts while the lowest amount of DPPH was seen in ‘Cheongdobansi’ leaf extracts in both harvesting times by hot air and freeze-drying treatments (p < 0.05). 54.09 ± 1.97 and 48.19 ± 0.27% of DPPH activity were recorded in ‘Gabjubaekmok’ persimmon leaf extracts, while this percentage was 41.11 ± 1.25 and 35.65 ± 0.69 in ‘Cheongdobansi’ leaf extracts by hot air drying treatment in late May and late June, accordingly. ‘Sangju-dungsi’ and ‘Sangam-dungsi’ leaf extracts showed the similar percentages of DPPH activity by both drying methods.
However, during the flowering stage (late May), the highest (p < 0.05) amount of antioxidant activities were observed than the fruiting stage (late June). Though, there were variations in antioxidant activities according to persimmon cultivars. According to the result here, it can be said that flowering time of persimmon leaf was the richest regarding DPPH activity than the fruiting time. A previous report showed that early stage of plants had the highest antioxidant activity compared to the late stage [30]. Also, Wang [37] reported that preharvest condition particularly climate, light intensity, temperature, types of soil, fertilization, compost, the amount of CO2 in the atmosphere, all can affect the antioxidants and antioxidant activity of the harvested fruits. However, the potential reason of these changes of antioxidant activity level in persimmon leaves are still ambiguous. Therefore, it can be said that the probable reason for this transformation could be the movement of antioxidants from leaves to fruits during fruiting.
ABTS radical-scavenging activity
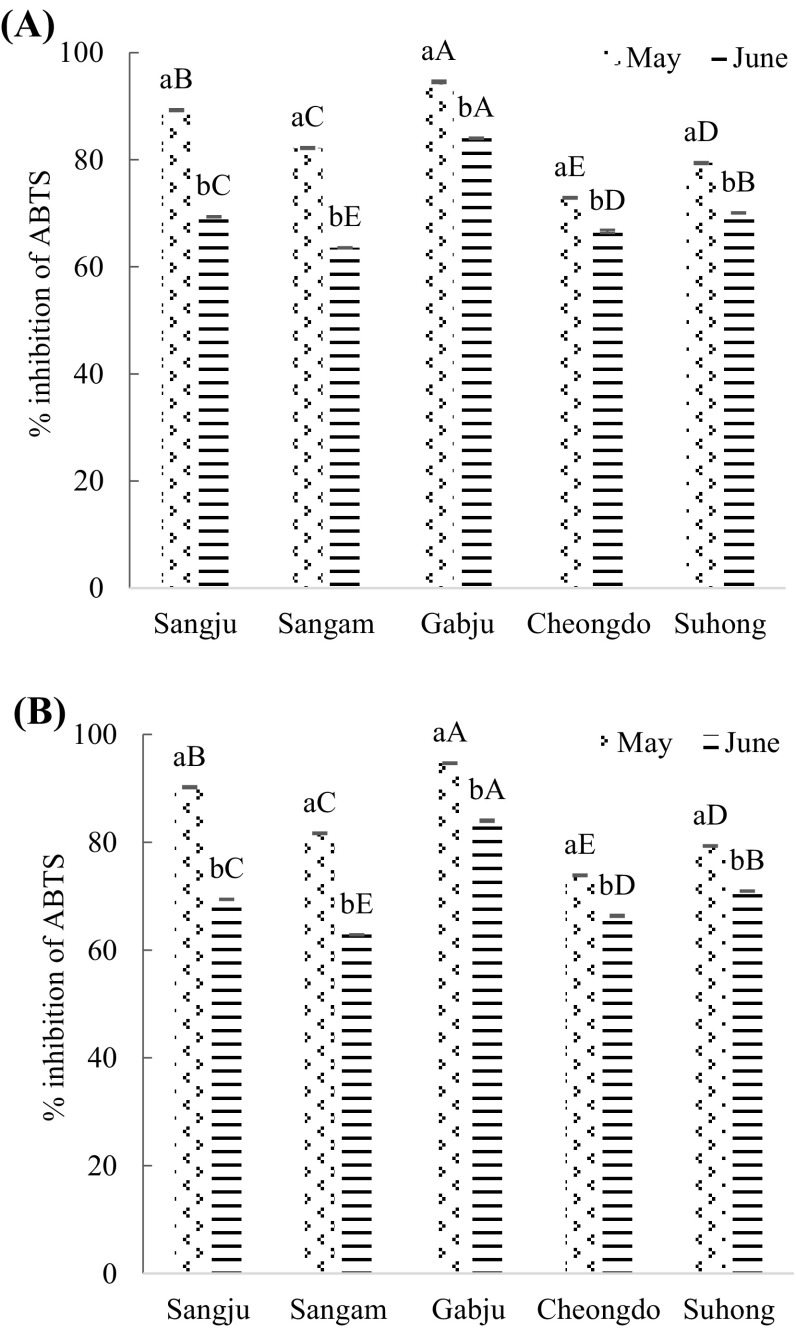
ABTS radical-scavenging activity by hot air and freeze dried samples are illustrated in (Fig. 5). There were no considerable effects detected on the ABTS radical-scavenging activity of freeze dried and hot air dried leaf extracts. Martínez-Las et al. [25] stated that the hot air dried persimmon leaves extracts showed higher antioxidant activities compared to freeze dried extracts. Probably this might be due to the duration of freeze drying. This is because in this present study freeze-drying was done for 72 h instead of 24 h. However, ABTS radical-scavenging activity was higher than those of DPPH radical-scavenging activity at the same condition. Lee et al. [38] reported that ABTS+ have a higher scavenging activity when compared with DPPH radical-scavenging activity from the natural plants extracts.
Fig. 5.
ABTS radical- scavenging activity (%) of persimmon leaves extracts. (A) Hot air drying treatment; (B) freeze-drying treatment. Values are mean ± SD of three (n = 3) measurements. Lower case letters (a and b) within bars of the same cultivar with different harvesting times and capital alphabet letters (A–E) within bars of the different cultivars across same harvesting time are significantly different (p < 0.05). Sangju, Sangju-dungsi; Sangam, Sangam-dungsi; Gabju, Gabjubaekmok; Cheongdo, Cheongdobansi
‘Gabjubaekmok’ persimmon leaves extracts had the highest percentage of ABTS activity in both harvesting times, while the lowest percentage was recorded in ‘Cheongdobansi’ and ‘Sangam-dungsi’ leaf extracts in late May and late June, respectively. Percentage of ABTS ranked in a decreasing order; with the highest percentage being observed in ‘Gabjubaekmok’ followed: ‘Sangju-dungsi’ > ‘Sangam-dungsi’ > ‘Suhong’ > ‘Cheongdobansi’ in late May by both drying methods. In contrast, the order in late June was ‘Gabjubaekmok’ > ‘Suhong’ > ‘Sangju-dungsi’ > ‘Cheongdobansi’ > ‘Sangam-dungsi’ by both drying treatments.
The percentage of ABTS was superior in late May compared to that observed at the late June in all cultivars (p < 0.05), although the variation rate was different according to the cultivars. This fluctuation could be a result of different ecological, morphological, genetic, and environmental factors [39]. However, the actual reasons for these variations during fruiting in persimmon leaves are still vague. This could be attributed to the movement of antioxidant activity from leaves to fruits during fruiting.
Due to antioxidant properties of persimmon leaves, it could be a good way to increase the economic value of this deciduous plant. The application of drying is able to increase the antioxidants content and antioxidant activities of persimmon leaves. The present research introduced the hot air drying as the optimum drying method to evaluate the antioxidant contents from persimmon leaves due to the short time of drying and less energy consumption, while the similar result was obtained from freeze drying method. If the maximum yield can be reached in a shorter time with minimum cost, it will lead to a greater productivity in the industrial point of view. ‘Gabjubaekmok’ persimmon leaf and late May were considered to be a better cultivar and harvest time in the context of antioxidants and antioxidant activity, accordingly. Also, ‘Cheongdobansi’ and ‘Suhong’ persimmon leaves were considered the poorer cultivars with respect to bioactive compounds. These bioactive compounds could have contributed to the functional food as well as cosmetic industries. Further work is required on the identification and quantification of phenolic profiles using HPLC-DAD-MS/MS.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However, the authors are grateful to persimmon experiment station (Sangju) for supplying persimmon leaves during the study.
Contributor Information
Abul Hossain, Email: sakil.hstu@gmail.com.
Hey Kyung Moon, Email: mhk@knu.ac.kr.
Jong-Kuk Kim, Phone: 82-54-530-1305, Email: kjk@knu.ac.kr.
References
- 1.Matsuo T, Ito S. The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki L.) Agric. Biol. Chem. 1978;42:1637–1643. [Google Scholar]
- 2.Jo C, Son JH, Shin MG, Byun MW. Irradiation effects on color and functional properties of persimmon (Diospyros kaki L. folium) leaf extract and licorice (Glycyrrhiza Uralensis Fischer) root extract during storage. Radiat. Phys. Chem. 2002;67:143–148. doi: 10.1016/S0969-806X(02)00443-7. [DOI] [Google Scholar]
- 3.Kameda K, Takaku T, Okuda H, Kimura Y, Okuda T, Hatano T, Agata I, Arichi S. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J. Nat. Prod. 1987;50(4):680–683. doi: 10.1021/np50052a017. [DOI] [PubMed] [Google Scholar]
- 4.Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LFC, Lemos-Senna E, Calixto JB. Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol. Res. 2010;61(4):288–297. doi: 10.1016/j.phrs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Kim JK, Choi SJ, Cho HY, Hwang HJ, Kim YJ, Lim ST, Kim CJ, Kim HK, Peterson S, Shin DH. Protective effects of kaempferol (3,4′,5,7-tetrahydroxyflavone) against amyloid beta peptide (Abeta)-induced neurotoxicity in ICR mice. Biosci. Biotechnol. Biochem. 2010;74(2):397–401. doi: 10.1271/bbb.90585. [DOI] [PubMed] [Google Scholar]
- 6.Xie C, Xie Z, Xu X, Yang D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015;163:229–240. doi: 10.1016/j.jep.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami K, Aketa S, Sakai H, Watanabe Y, Nishida H, Hirayama M. Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011;75(8):1435–1439. doi: 10.1271/bbb.100926. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami K, Aketa S, Nakanami M, Iizuka S, Hirayama M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their α-amylase inhibitory activity. Biosci. Biotechnol. Biochem. 2010;74(7):1380–1385. doi: 10.1271/bbb.100056. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Xu ML, Rasmussen SK, Wang MH. Vomifoliol 9-O-α-arabinofuranosyl (1 → 6)-β-D-glucopyranoside from the leaves of Diospyros Kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011;346(10):1212–1216. doi: 10.1016/j.carres.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Kotani M, Matsumoto M, Fujita A, Higa S, Wang W, Suemura M, Kishimoto T, Tanaka T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/NGa mice. J. Allergy Clin. Immunol. 2000;106(1 I):159–166. doi: 10.1067/mai.2000.107194. [DOI] [PubMed] [Google Scholar]
- 11.Choi C. Studies on investigation into biologically activated substances from Korean persimmon leaves and developing high function beverages. Report of Ministry of Agriculture and Forestry. 149–150 (2000).
- 12.Funayama S, Hikino H. Hypotensive principles of Diospyros kaki leaves. Chem. Pharm. Bull. 1979;27:2865–2867. doi: 10.1248/cpb.27.2865. [DOI] [PubMed] [Google Scholar]
- 13.Uchida S, Edamatsu R, Hiramatsu M, Mori A, Nonaka GY, Nishioka I, Niwa M, Ozaki M. Condensed tannins scavenge active oxygen free radicals. Med. Sci. Res. 1985;15:831–834. [PubMed] [Google Scholar]
- 14.Kim GY, Kim JK, Kang WW, Kim JG, Joo GJ. Shelf-life extension of rice cake by the addition of persimmon leaf tea powder. Food Sci. Biotechnol. 2005;14(2):196–199. [Google Scholar]
- 15.Lim JA, Lee JH. Quality characteristics and antioxidant properties of cookies supplemented with persimmon leaf powder. Korean J. Food Sci. Technol. 2016;48(2):159–164. doi: 10.9721/KJFST.2016.48.2.159. [DOI] [Google Scholar]
- 16.Ladas SD, Kamberoglou D, Karamanolis G, Vlachogiannakos J, Zouboulis-Vafiadis I. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment. Pharmacol. Ther. 2013;37(2):169–173. doi: 10.1111/apt.12141. [DOI] [PubMed] [Google Scholar]
- 17.Pinela J, Barros L, Dueñas M, Carvalho AM, Santos-Buelga C, Ferreira ICFR. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012;135(3):1028–1035. doi: 10.1016/j.foodchem.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 18.Singleton VL, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16(3):144–158. [Google Scholar]
- 19.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 20.Broadhurst RB, Jones WT. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978;29(9):788–794. doi: 10.1002/jsfa.2740290908. [DOI] [Google Scholar]
- 21.Xu BJ, Chang SKC. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007;72(2):159–166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- 22.Shahidi F, Liyana-Pathiran CM, Wall DS. Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem. 2006;99(3):478–483. doi: 10.1016/j.foodchem.2005.08.009. [DOI] [Google Scholar]
- 23.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins-Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19(6–7):669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 24.Effect on antioxidant activity Dorta E, Lobo MG, Gonza´lez M. Using drying treatments to stabilise mango peel and seed. LWT - Food Sci. Technol. 2012;45:261–268. doi: 10.1016/j.lwt.2011.08.016. [DOI] [Google Scholar]
- 25.Martínez-Las HR, Heredia A, Castelló ML, Andrés A. Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves. Food Biosci. 2014;6:1–8. doi: 10.1016/j.fbio.2014.01.002. [DOI] [Google Scholar]
- 26.Ashie INA, Simpson BK. Application of high hydrostatic pressure to control enzyme related fresh seafood texture deterioration. Food Res. Int. 1996;29(5–6):569–575. doi: 10.1016/S0963-9969(96)00012-9. [DOI] [Google Scholar]
- 27.Arslan D, Özcan MM. Study the effect of sun, oven and microwave drying on quality of onion slices. LWT–Food. Sci. Technol. 2010;43(7):1121–1127. [Google Scholar]
- 28.Jung WY, Jeong JM. Change of antioxidative activity at different harvest time and improvement of atopic dermatitis effects for persimmon leaf extract. Korea J. Herbol. 2012;27(1):41–49. doi: 10.6116/kjh.2012.27.1.41. [DOI] [Google Scholar]
- 29.Chung SH, Moon KD, Kim JK, Seong JH, Sohn TH. Changes of chemical components in persimmon leaves during growth for processing persimmon leaves tea. Korean J. Food Sci. Technol. 1994;26(2):141–146. [Google Scholar]
- 30.Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CYO, Sun Y. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in southern China. Int. J. Mol. Sci. 2012;13(12):16544–16553. doi: 10.3390/ijms131216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peixoto STJS, Cardoso KCM, Gomes TLB, Albuquerque UP, Amorim ELC. Validation of spectrophotometric methodology for quantify flavonoid content in Bauhinia cheilantha (Bongard) Steudel. Braz. J. Pharm. Sci. 2008;44(4):683–689. [Google Scholar]
- 32.Alonzo-macías M, Cardador-martínez A, Mounir S, Montejano-Gaitan G, Allaf K. Comparative study of the effects of drying methods on antioxidant activity of dried strawberry (Fragaria Var. Camarosa) J Food Res. 2013;2(2):92–107. doi: 10.5539/jfr.v2n2p92. [DOI] [Google Scholar]
- 33.Horszwald A, Julien H, Andlauer W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013;141(3):2858–2863. doi: 10.1016/j.foodchem.2013.05.103. [DOI] [PubMed] [Google Scholar]
- 34.Stewart JL, Mould F, Mueller-Harvey I. The effect of drying treatment on the fodder quality and tannin content of two provenances of Calliandra calothyrsus Meissner. J. Sci. Food Agric. 2000;80(10):1461–1468. doi: 10.1002/1097-0010(200008)80:10<1461::AID-JSFA672>3.0.CO;2-R. [DOI] [Google Scholar]
- 35.Scogings PF, Dziba LE, Gordon IJ. Leaf chemistry of woody plants in relation to season, canopy retention and goat browsing in a semiarid subtropical savanna. Austral Ecol. 2004;29(3):278–286. doi: 10.1111/j.1442-9993.2004.01347.x. [DOI] [Google Scholar]
- 36.Si X, Chen Q, Bi J, Wu X, Yi J, Zhou L, Li Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2015;96(6):2055–2062. doi: 10.1002/jsfa.7317. [DOI] [PubMed] [Google Scholar]
- 37.Wang SY. Effect of pre-harvest conditions on the antioxidant capacity in fruits. Acta Hortic. 2006;712:299–305. doi: 10.17660/ActaHortic.2006.712.33. [DOI] [Google Scholar]
- 38.Lee BW, Lee JH, Gal SW, Moon YH, Park KH. Selective ABTS radical-scavenging activity of prenylated flavonoids from Cudrania tricuspidata. Biosci. Biotechnol. Biochem. 2006;70(2):427–432. doi: 10.1271/bbb.70.427. [DOI] [PubMed] [Google Scholar]
- 39.Sývacý A, Sökmen M. Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species (Morus alba L. and Morus nigra L.) Plant Growth Regul. 2004;44(3):251–256. doi: 10.1007/s10725-004-4500-4. [DOI] [Google Scholar]