Abstract
The combined mycotoxins zearalenone (ZEA) with ochratoxin A (OTA) or α-zearalenol (α-ZOL) are frequently found together in milk. Toxicological data concerning the combined effects of these mycotoxins are sparse. In present study, individual and combined ZEA, OTA and α-ZOL caused cytotoxicity and oxidative damage, including reductions in intracellular superoxide dismutase and glutathione peroxidase activities and glutathione content, along with increases in malonaldehyde content on human Hep G2 cells after 48 h of exposure. Among individual mycotoxins, OTA had the greatest cytotoxic effect followed by α-ZOL. Compared with individual mycotoxins, combinations produced more serious negative effects, more importantly, ZEA + OTA was antagonistic for these effects, whereas ZEA + α-ZOL was antagonistic at low concentrations, but synergistic at high concentrations of ZEA, which were evaluated by 3 × 3 full factorial analysis and estimated marginal means plots. Our results also demonstrated a significant correlation between cytotoxicity and oxidative damage in response to these combinations.
Keywords: Zearalenone, Ochratoxin A, α-Zearalenol, Combined effect, Full factorial design
Introduction
Mycotoxins are toxic secondary metabolites that are produced by various types of fungi, including those in the Fusarium, Aspergillus, and Penicillium genera [1]. The co-occurrence of different mycotoxins is commonly found in cereal grains. Zearalenone (ZEA) with ochratoxin A (OTA) is one of the most frequently occurring combinations, which is seen in a wide variety of food and feed commodities, sometimes at levels that exceed the European and Chinese regulatory limits [2]. After consumption by livestock, ZEA can be metabolized to α-zearalenol (α-ZOL), which can then co-occur in downstream food products. In the classification of the International Agency for Research on Cancer, OTA is in group 2B as a potential carcinogen [3], because of its mutagenic, teratogenic, neurotoxic, hepatotoxic, and immunotoxic actions [4]. ZEA is known for its strong estrogenic activity, which is caused by competition with 17-β-estradiol for binding to cytosolic estrogen receptors [5], and α-ZOL has an estrogenic activity 3–4 times higher than that of ZEA [6]. Hence, the occurrence of combinations of ZEA with OTA or α-ZOL in food constitutes a potential hazard for human health.
Although epidemiological studies have shown that ZEA and OTA mainly affect the reproductive organs and kidneys, experimental evidence also indicates that they have hepatotoxic and immunotoxic activities [4, 7]. Indeed, the liver could be considered as a target for mycotoxin pathology, because it is the major organ responsible for biotransformation and detoxification of mycotoxins. Previous studies elucidated the hepatotoxic potential of ZEA and OTA (along with other mycotoxins) either separately or in combination, and identified both synergistic and antagonistic interactions [8, 9]. We previously demonstrated an interaction between ZEA, OTA, and α-ZOL that affects cell viability in human hepatoma G2 (Hep G2) cells [10]. However, the mechanism underlying hepatotoxicity caused by combinations of ZEA, OTA, and α-ZOL has not previously been determined.
In the past decade, the importance of oxidative stress in mycotoxin-induced toxicity has been the subject of much research [11, 12]. Li et al. [13] reported that, although the receptors for ZEA and OTA are different, these toxins have some mechanistic overlap, such as in the generation of reactive oxygen species (ROS). Elevation of ROS levels could cause reductions in levels of glutathione (GSH) and activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), leading to the production of malondialdehyde (MDA), the lipid peroxidation product of unsaturated fatty acids [14, 15]. SOD and GSH-Px are the main cellular antioxidant enzymes for the removal of free radicals. To the best of our knowledge, no study has previously determined the interactive effects on cytotoxicity and oxidative damage of the combination of ZEA with OTA or α-ZOL by a method such as full factorial design with estimated marginal means plots, which was applied in assessing the individual and combined developmental toxicity of bisphenol A and genistein [16, 17].
Our hypothesis for this study was that the interactive effects of combinations of ZEA and OTA or α-ZOL on cytotoxicity were consistent with the effects of oxidative damage, and that there is a correlation between cytotoxicity and oxidative damage. Our aim, therefore, was to investigate both cytotoxicity and oxidative damage induced by combinations of mycotoxins. Because both ZEA and OTA are hepatotoxic, the Hep G2 cell line, which is the most commonly used cell system in xenobiotic research, was chosen for testing [18]. Cytotoxicity was determined by cell viability, and oxidative damage was evaluated by the activities of SOD and GSH-Px, and the contents of MDA and GSH. The interactions between viability and oxidative damage were evaluated by 3 × 3 full factorial analysis and plots of estimated marginal means, which indicate mean responses for each factor, adjusted for other variables in the model.
Materials and methods
Chemicals
OTA and ZEA were purchased from Fermentek (Jerusalem, Israel), and α-ZOL was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibiotics, trypsin–EDTA solution, and cell-lysis buffer were purchased from the Beyotime Institute (Shanghai, China). SOD (Cat#A001-3), MDA (Cat#A003-1), GSH-PX (Cat#A005), and GSH (Cat#A006-1) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich.
Cell culture and mycotoxin treatment
The Hep G2 cell line was obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. A 3 × 3 factorial analysis design was applied for combinations of ZEA + OTA and ZEA + α-ZOL, with three different levels of ZEA (0, 30, and 60 μM), OTA (0, 6, and 12 μM), and α-ZOL (0, 5, and 30 μM) (Table 1). These concentrations were selected from the results of previous experiments [10], which showed that the IC50 value of ZEA, OTA and α-ZOL was 39.9, 3.5 and 20.9 μM in Hep G2 cells with 48 h exposure, respectively, to give similar levels of toxicity. Stock solutions (10 mM) of individual mycotoxins were prepared in methanol and stored at −20 °C. They were diluted in DMEM, and the final methanol concentrations in the medium were < 1% (v/v). The control group, with no addition of mycotoxin, was treated with medium containing the same amount of methanol. All treatments were performed in three independent experiments.
Table 1.
3 × 3 two-way ANOVA matrix for evaluating the combined effects of ZEA with OTA or α-ZOL
| Mycotoxin | ZEA (μM) | ||
|---|---|---|---|
| 0 | 30 | 60 | |
| OTA (μM) | |||
| 0 | ZEA 0 + OTA 0 | ZEA 30 + OTA 0 | ZEA 60 + OTA 0 |
| 6 | ZEA 0 + OTA 6 | ZEA 30 + OTA 6 | ZEA 60 + OTA 6 |
| 12 | ZEA 0 + OTA 12 | ZEA 30 + OTA 12 | ZEA 60 + OTA 12 |
| α-ZOL (μM) | |||
| 0 | ZEA 0 + α-ZOL 0 | ZEA 30 + α-ZOL 0 | ZEA 60 + α-ZOL 0 |
| 15 | ZEA 0 + α-ZOL 15 | ZEA 30 + α-ZOL 15 | ZEA 60 + α-ZOL 15 |
| 30 | ZEA 0 + α-ZOL 30 | ZEA 30 + α-ZOL 30 | ZEA 60 + α-ZOL 30 |
ZEA 0, ZEA 30, and ZEA 60 denote the concentrations of ZEA (0, 30, and 60 μM, respectively). OTA 0, OTA 6, and OTA 12 denote the concentrations of OTA (0, 6, and 12 μM, respectively). α-ZOL 0, α-ZOL 15, and α-ZOL 30 denote the concentrations of α-ZOL (0, 15, and 30 μM, respectively)
ZEA zearalenone, OTA ochratoxin A, α-ZOL α-zearalenol
Cytotoxicity assay
Cell proliferation was measured by MTT assays, as previously described [19]. Briefly, cells were seeded in 96-well plates at a concentration of 1 × 105 cells per mL. After 24 h, the medium was discarded and fresh medium containing mycotoxins was added to the wells and incubated for 48 h. The mycotoxin-containing medium was then removed, and 100 μL of 0.5 mg/mL MTT was added and incubated for 4 h. Viable cells convert MTT to formazan, and any formazan crystals that were formed were solubilized by addition of 100 μL of DMSO and gentle agitation for 5 min. Absorbance was measured at 570 nm with a reference wavelength of 630 nm with an automatic ELISA reader (SpectraMax M3, Molecular Devices, Sunnyvale, CA, USA).
Measurement of intracellular SOD, MDA, GSH-Px, and GSH
After treatment with individual or combined mycotoxins for 48 h, cells were harvested by centrifugation (1500 g for 3 min), and then rinsed three times with PBS. Cells were then lysed in PBS containing 20 mM Tris and 1% (v/v) Triton X-100. The contents of SOD, MDA, GSH-PX, and GSH in cell lysates were determined with commercially available kits in accordance with the manufacturers’ instructions.
Evaluation of combined effects
The interactions between mycotoxins were evaluated by 3 × 3 full factorial analysis and estimated marginal means plots. In these plots, the dose–effect curves for increasing doses of ZEA are shown for each dose of OTA or α-ZOL. Parallel lines indicate that the two treatments have additive (no interaction) effects that do not result from interactions between the toxins. Non-parallel lines indicate interaction between the treatments, resulting in synergism or antagonism [20]. In more detail, if the slope of dose–response curve of one chemical does not alter in the presence of other chemicals, we can consider that the combined effect is additivity; reversely, if an interaction exists, the changes of the direction of the slope were used to determine the interaction is synergism or antagonism.
The estimation of marginal means was performed with Excel software (Office 2007, Microsoft, Redmond, WA, USA). Calculation of the slopes of dose–effect curves was carried out with SAS 9.2 statistical software (SAS, Cary, NC, USA).
Statistical analysis
Statistical analysis of the data was carried out with SAS 9.2 statistical software. Data were expressed as the mean ± standard deviation (SD) of three independent experiments. Differences between groups were analyzed with analysis of variance (ANOVA) followed by the Tukey honest significant difference test for multiple comparisons. Interactions between biologically active agents have previously been identified by ANOVA in research studies in toxicology [21, 22]. Our method of analysis enabled us to determine statistically significant interactions between mycotoxins assayed at different concentrations, providing us with F and p values simultaneously. Values of p < 0.05 were considered statistically significant. It would be considered as the interactive effects actually exist if p < 0.05 was shown in the combinations of ZEA + OTA or ZEA + α-ZOL of various endpoints.
Results and discussion
Cytotoxicity and oxidative damage caused by individual and combined mycotoxins
The different levels of cell viability and oxidative damage in Hep G2 cells exposed to individual and combined mycotoxins are shown in Figs. 1 and 2. Exposure to OTA led to significant, dose-dependent reductions in cell viability, MDA content, GSH-Px activity, and GSH content (p < 0.05; Fig. 1A, C, D, and E). Exposure to individual ZEA or α-ZOL led to significant, dose-dependent reductions in cell viability and GSH-Px activity and GSH content (p < 0.05; Fig. 2A, D, and E). Among the individual mycotoxins, OTA had the greatest cytotoxic effect on Hep G2 cells, followed by α-ZOL.
Fig. 1.
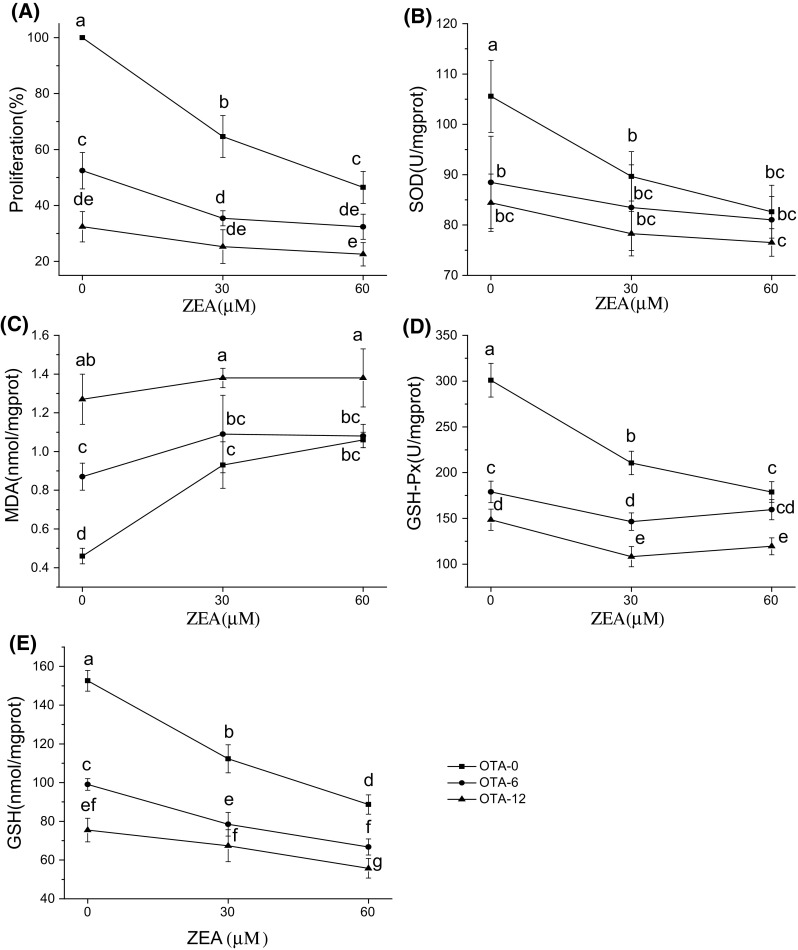
Effects of interactions between ZEA and OTA on Hep G2 cell cytotoxicity (A), SOD (B), MDA (C), GSH-Px (D), and GSH (E) after exposure for 48 h. Profile plots (interaction plots) are shown, with comparative marginal means. Different letters indicate significant differences (p < 0.05) compared with ZEA alone (OTA-0 group). ZEA, zearalenone; OTA, ochratoxin A; SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; GSH, glutathione
Fig. 2.
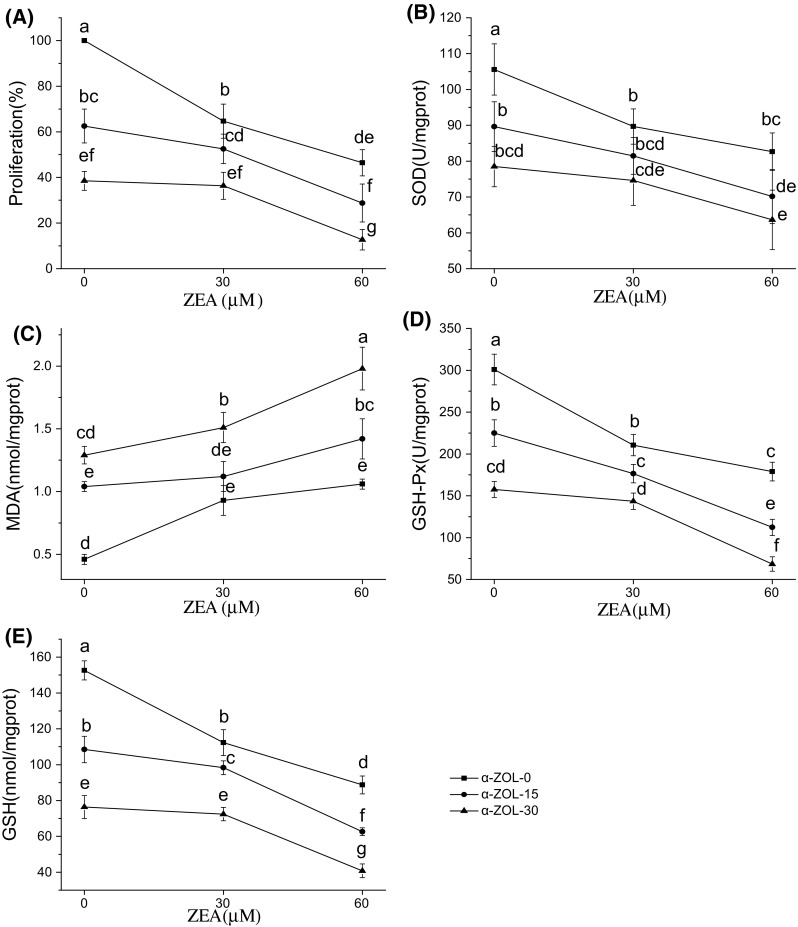
Effects of interactions between ZEA and α-ZOL on Hep G2 cell cytotoxicity (A), SOD (B), MDA (C), GSH-Px (D), and GSH (E) after exposure for 48 h. Profile plots (interaction plots) are shown, with comparative marginal means. Different letters indicate significant differences (p < 0.05) compared with ZEA alone (α-ZOL-0 group). ZEA, zearalenone; α-ZOL, α-zearalenol; SOD, superoxide dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase; GSH, glutathione
From our results, we can conclude that combinations of mycotoxins caused greater cytotoxicity and oxidative damage than individual toxins, indicating that the combined mycotoxins enhanced the toxicology when compared with individual effects. Compared with ZEA individually, treatment with ZEA + OTA overall caused more serious negative effects on Hep G2 cells, more specially, a significant decrease was shown in Hep G2 cell viability and intracellular GSH contents (p < 0.05; Fig. 1A, E). Similarly, the combination of ZEA + α-ZOL significantly reduced cell viability, GSH-Px activity, and GSH content compared with ZEA alone (p < 0.05; Fig. 2A, D, and E).
Prediction and assessment of the combined effects of mycotoxins
Mycotoxins are commonly found in food commodities. Results from some studies show that combinations of mycotoxins cause greater cytotoxicity and oxidative damage than individual mycotoxins [23]. Evaluation of the different types of interaction between mycotoxins is important to understand the mechanisms of cytotoxicity. The present study is the first to have simultaneously determined the effects of combinations of ZEA + OTA and ZEA + α-ZOL on cytotoxicity and oxidative damage in Hep G2 cells, by full factorial analysis with estimated marginal means plots.
For the combinations of ZEA + OTA and ZEA + α-ZOL, results of 3 × 3 factorial ANOVA are presented in Table 2, which reflected whether interactive effects exist in them. And specific interactions between the combined mycotoxins are illustrated by profile plots (interaction plots) with comparative marginal means of cell viability, SOD, MDA, GSH-Px, or GSH (Figs. 1 and 2). The results of further interactive analysis concluded by estimated marginal means plots are presented in Table 3, thereby obtaining the actual interactive effects between ZEA + OTA and ZEA + α-ZOL in various endpoints. For the combination of ZEA + OTA, the overall effect was not equivalent to the sum of the individual effects, as shown by the significant results with ANOVA (p < 0.05, Table 2). More specifically, from Table 2, we can conclude that p < 0.05 was shown in cell proliferation, MDA, GSH-Px, and GSH, indicating that the interaction should be observed in these endpoints, while for the endpoint of SOD, its p value was 0.215, above 0.05, which suggested that there should no interaction between ZEA + OTA in SOD. However, the explicit interactive effects should be combined with 3 × 3 full factorial analysis and estimated marginal means plots, which displayed in Fig. 1 and the detailed results were generated in Table 3. From the non-parallel lines in Fig. 1 in all endpoints, we can get a conclusion that there must be synergism or antagonism for ZEA + OTA in all tested endpoints. Further analysis was applied by the estimated marginal means plot. Take cell viability as an example, the slope of ZEA 30 + OTA 6 or ZEA 30 + OTA 12 was inferior to that of ZEA 30 alone, indicating that the impact of ZEA + OTA was lower than the sum the individual mycotoxins, which represented an antagonistic effect. Similarly, we could analyze other endpoints in different concentrations. Therefore, we can obtain that the interaction between ZEA and OTA was antagonistic in its effect on cell viability, intracellular SOD, and GSH-Px activities, as well as MDA and GSH contents (Table 3). With this antagonism, the effects of the combined ZEA + OTA on these variables were less than the sum of the effects of the individual toxins.
Table 2.
Results of 3 × 3 factorial ANOVA to determine significance of effects of ZEA, OTA, and α-ZOL, alone and their interaction
| Cell viability | SOD (U/mg.prot) | MDA (nmol/mg.prot) | GSH-Px (U/mg.prot) | GSH (nmol/mg.prot) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| ZEA | 68.92 | <0.001 | 10.25 | 0.001 | 20.36 | <0.001 | 63.48 | <0.001 | 102.24 | <0.001 |
| OTA | 167.74 | <0.001 | 10.17 | 0.001 | 52.16 | <0.001 | 173.61 | <0.001 | 190.69 | <0.001 |
| ZEA + OTA | 15.04 | <0.001 | 1.61 | 0.215 | 4.35 | 0.012 | 16.52 | <0.001 | 12.45 | <0.001 |
| ZEA | 88.65 | <0.001 | 19.26 | <0.001 | 57.84 | <0.001 | 174.11 | <0.001 | 194.33 | <0.001 |
| α-ZOL | 105.05 | <0.001 | 22.22 | <0.001 | 112.41 | <0.001 | 171.64 | <0.001 | 242.64 | <0.001 |
| ZEA + α-ZOL | 7.08 | 0.001 | 0.69 | 0.606 | 3.87 | 0.019 | 7.49 | 0.001 | 11.19 | <0.001 |
ZEA + OTA represents the combination of ZEA and OTA. ZEA + α-ZOL represents the combination of ZEA and α-ZOL
ZEA zearalenone, OTA ochratoxin A, α-ZOL α-zearalenol, SOD superoxide dismutase, MDA malondialdehyde, GSH-Px glutathione peroxidase, GSH glutathione
Table 3.
Antagonism and synergism in the combined effects of ZEA + OTA and ZEA + α-ZOL, compared with individual toxins
| Binary mixture (μM) | Cell viability (%) | SOD (U/mg.prot) | MDA (nmol/mg.prot) | GSH-Px (U/mg.prot) | GSH (nmol/mg.prot) |
|---|---|---|---|---|---|
| ZEA 30 + OTA 6 | Antagonism | Antagonism | Antagonism | Antagonism | Antagonism |
| ZEA 30 + OTA 12 | Antagonism | Antagonism | Antagonism | Antagonism | Antagonism |
| ZEA 60 + OTA 6 | Antagonism | Antagonisnm | Antagonism | Antagonism | Antagonism |
| ZEA 60 + OTA 12 | Antagonism | Antagonism | Antagonism | Antagonism | Antagonism |
| ZEA 30 + α-ZOL 15 | Antagonism | Antagonism | Antagonism | Antagonism | Antagonism |
| ZEA 30 + α-ZOL 30 | Antagonism | Antagonism | Antagonism | Antagonism | Antagonism |
| ZEA 60 + α-ZOL 15 | Synergism | Synergism | Synergism | Synergism | Synergism |
| ZEA 60 + α-ZOL 30 | Synergism | Synergism | Synergism | Synergism | Synergism |
ZEA zearalenone, OTA ochratoxin A, α-ZOL α-zearalenol, SOD superoxide dismutase, MDA malondialdehyde, GSH-Px glutathione peroxidase, GSH glutathione
Applying the similar analysis as ZEA + OTA, we can get the combined effects of ZEA + α-ZOL in different endpoints and concentrations. First of all, from the significant results with ANOVA in all tested endpoints except SOD, suggesting that the interaction was existed in the combination (Table 2). Subsequently, the presence of non-parallel lines in Fig. 2 indicated that interaction of ZEA + α-ZOL was not equivalent to the sum of the individual effects. And the particular interaction effects were analyzed by the direction of the slope as ZEA + OTA. The specific combined effects are shown in Table 3. At the lower concentration of ZEA, the combination of ZEA + α-ZOL displayed antagonism with regard to the effect on cell viability, SOD, and GSH-Px activities, as well as MDA and GSH contents. However, at the higher concentration of ZEA, the combination of ZEA + α-ZOL showed synergism, with greater effects than the sum of the effects with individual toxins.
The antagonistic effect of ZEA + OTA could be explained by ROS induction, leading to the p53-dependent apoptotic pathway [24]. At levels of equipotency where antagonism was observed, ZEA and OTA may compete for the same target or receptor site in the induction of apoptosis [25]. As a metabolite of ZEA, α-ZOL has a similar structure and mechanism of toxicity [26], and might also compete for the same receptors as ZEA in the apoptotic pathway, leading to antagonism. Doi and Uetsuka [27] suggested that incorporation of mycotoxins into membrane structures causes various detrimental changes, resulting in alterations in second-messenger systems through damage to membrane receptors. When the concentration of ZEA was increased to 60 μM, we could speculate that, its affinity to cell-membrane transporters was altered, contributing to a higher accumulation of α-ZOL with the higher cytotoxicity depicted by a potentiation of toxicity for the mixture, which lead to a synergistic action [28, 29]. Hence, there is a possibility that the concentration-dependent variation of the interactive effects were associated with the graded levels of saturation of cell receptors from low to high mycotoxin concentrations. Moreover, the biological differences in intrinsic toxicity of mycotoxins could be proposed to explain the various pattern of toxicological interactions with ZEA, OTA and α-ZOL reported in this study.
Correlation coefficients of cytotoxicity and oxidative damage
The interactions between the toxins produced the same effects (antagonism or synergism) on cell viability and the different measures of oxidative damage (Table 3), indicating the presence of a relationship between these effects. There was a significant correlation between cytotoxicity and oxidative damage responses to both ZEA + OTA and ZEA + α-ZOL (Table 4), indicating that oxidative damage has an important role in cytotoxicity. Negative correlation was found between proliferation and MDA content, whereas SOD and GSH-Px activities and GSH content were all positively correlated with proliferation.
Table 4.
Coefficients of the correlations between cytotoxicity and the oxidative measures SOD, MDA, GSH-Px, and GSH
| Mixtures | Proliferation | SOD | MDA | GSH-Px | GSH |
|---|---|---|---|---|---|
| ZEA + OTA | 0.888 (p < 0.05) | −0.846 (p < 0.05) | 0.979 (p < 0.05) | 0.990 (p < 0.05) | |
| ZEA + α-ZOL | 0.958 (p < 0.05) | −0.878 (p < 0.05) | 0.982 (p < 0.05) | 0.990 (p < 0.05) |
ZEA zearalenone, OTA ochratoxin A, α-ZOL α-zearalenol, SOD superoxide dismutase, MDA malondialdehyde, GSH-Px glutathione peroxidase, GSH glutathione
In the present study, compared with individual toxins, the combination of ZEA + OTA displayed antagonism in the induction of oxidative damage, whereas ZEA + α-ZOL showed antagonism at the lower level of ZEA and synergism at the higher ZEA concentration. Previous results show that ROS generation by ZEA + OTA in Hep G2 shifts from an additive interaction to antagonism at high concentrations of toxins [13]. The difference between this result and our observations might be the result of the different concentrations of the toxins we used, in the previous study, the concentrations of OTA (6.61, 16.30, 24.66, and 37.40 μM) and ZEA (1.12, 7.36, 17.44, and 41.28 μM) were applied. In the development of mycotoxin-induced cytotoxicity, overproduction of ROS and disruption of antioxidant defenses have been well documented [30]. Our results showed that ZEA + OTA and ZEA + α-ZOL induce toxicity through depletion of GSH, an increase of MDA content, and reduction of the activities of antioxidant enzymes, which is similar to the mechanisms reported elsewhere [15, 31]. In addition to these effects, oxidative stress could lead to cell death via intrinsic apoptotic pathways [32], such as the MAPK–JNK–c-jun pathway, which is involved in toxin-induced apoptosis [27]. The behavior of combined mycotoxins is complicated, involving interactions with cell receptors and intracellular enzymes [13], and further study is required to fully characterize the mechanisms that are involved.
In studies involving cells from other organs, similar interactions were found to those with liver cells, and oxidative stress was also important. ZEA and α-ZOL injure Chinese Hamster Ovary cells via oxidative stress, leading to the elevation of MDA products [26]. Moreover, in experiments with a central composite design involving porcine kidney cells, a synergistic effect on cell viability and ROS levels was observed after co-exposure with aflatoxin-B1 and ZEA [33]. The consistency of previous results with our observations suggests that induction of oxidative stress by mycotoxins might be the common pathway leading to hepatotoxicity and damage to other organs, including the kidneys and reproductive organs. Furthermore, Gayathri et al. [18] reported that toxins can affect targets that initiate more than one cellular response, thereby affecting homeostasis. As the major organ of biotransformation and detoxification, if the structural integrity of the liver is damaged by mycotoxins, the homeostatic balance in the body will be disrupted, which may result in injury to other organs including the kidneys and reproductive organs.
In conclusion, we found that Hep G2 cells were more sensitive to OTA than α-ZOL, and α-ZOL was more cytotoxic than ZEA. Antagonism was shown in the effects of the combination of ZEA + OTA on cytotoxicity and oxidative damage, whereas the combination of ZEA + α-ZOL showed antagonism at a low level of ZEA and synergism at a high concentration of ZEA. The level of cytotoxicity that was observed with ZEA alone was significantly increased by the addition of OTA or α-ZOL. This increased toxicity could represent a threat to health, as it is common to find co-occurrence of the three mycotoxins in food. Hence, efforts should be made to fully assess the potential safety risks of different combinations of mycotoxins, and to determine the molecular mechanisms underlying these toxicological interactions. Our results showed that there is a significant correlation between cytotoxicity and oxidative damage in response to the combinations of ZEA + OTA and ZEA + α-ZOL, indicating that oxidative damage has an important role in the induction of cytotoxicity.
Acknowledgements
This study was supported by special fund for agro-scientific research in the public interest (201403071), introduce international advanced agriculture science and technology plan (2014-Z3) and National Natural Science Foundation of China (31501399).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Jestoi M. Emerging fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin: a review. Crit. Rev. Food Sci. 2008;48:21–49. doi: 10.1080/10408390601062021. [DOI] [PubMed] [Google Scholar]
- 2.Soleimany F, Jinap S, Faridah A, Khatib A. A UPLCeMS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control. 2012;25:647–653. doi: 10.1016/j.foodcont.2011.11.012. [DOI] [Google Scholar]
- 3.International Agency for Research on Cancer (IARC). Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Aflatoxins. WHO. IARC Monogr. Eval. Carcinog. Risks Humans. 1993;56:245–395. [Google Scholar]
- 4.Pfohl-Leszkowicz A, Manderville RA. Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper-Goodman T, Scott PM, Watanabe H. Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 1987;7:253–306. doi: 10.1016/0273-2300(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 6.Fink-Gremmels J, Malekinejad H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Tech. 2007;137:326–341. doi: 10.1016/j.anifeedsci.2007.06.008. [DOI] [Google Scholar]
- 7.Abbès S, Ouanes Z, Ben Salah-Abbès J, Houas Z, Oueslati R, Bacha H, Othman O. The protective effect of hydrated sodium calcium aluminosilicate against haematological, biochemical and pathological changes induced by zeralenone in mice. Toxicon. 2006;5:567–574. doi: 10.1016/j.toxicon.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Qi XZ, Yang X, Chen SY, He XY, Dweep H, Guo MZ, Cheng WH, Xu WT, Luo YB, Gretz N, Dai Q, Huang KL. Ochratoxin A induced early hepatotoxicity: new mechanistic insights from microRNA, mRNA and proteomic profiling studies. Sci. Rep.-UK 4:5613 (2014).
- 9.Sun LH, Lei MY, Zhang NY, Zhao L, Krumm CS, Qi DS. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014;74:289–293. doi: 10.1016/j.fct.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 10.effects of individual and combined treatment Wang HW, Wang JQ, Zheng BQ, Li SL, ZhangYD, Li FD, Zheng N. Cytotoxicity induced by ochratoxin A, zearalenone, and alpha-zearalenol. Food Chem. Toxicol. 2014;71:217–224. doi: 10.1016/j.fct.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Abid-Essefi S, Zaied C, Bouaziz C, Salem IB, Kaderi R, Bacha H. Protective effect of aqueous extract of Allium sativum against zearalenone toxicity mediated by oxidative stress. Exp. Toxicol. Pathol. 2012;64:689–695. doi: 10.1016/j.etp.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Halbin KJ. Low level of Ochratoxin A enhances Aflatoxin B1 Induced Cytotoxicity and Lipid Peroxydation in both human intestinal (Caco-2) and Hepatoma (HepG2) Cells Lines. Int. J. Food Sci. Nutr. 2013;2:294–300. doi: 10.11648/j.ijnfs.20130206.15. [DOI] [Google Scholar]
- 13.Li Y, Zhang B, He X, Cheng WH, Xu W, Luo Y, Liang R, Luo H, Huang K. Analysis of individual and combined effects of ochratoxin A and zearalenone on HepG2 and KK-1 cells with mathematical models. Toxins. 2014;6:1177–1192. doi: 10.3390/toxins6041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusi E, Rebucci R, Pecorini C, Campagnoli A, Pinotti L, Saccone F, Cheli F, Purup S, Sejrsen K, Baldi A. Alpha-tocopherol counteracts the cytotoxicity induced by ochratoxin a in primary porcine fibroblasts. Toxins. 2010;2:1265–1278. doi: 10.3390/toxins2061265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim Y, Choi Y, Ham H, Jeong HS, Lee J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013;137:136–141. doi: 10.1016/j.foodchem.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kong D, Xing L, Liu R, Jiang JJ, Wang WY, Shang LQ, Wei XT, Hao WD. Individual and combined developmental toxicity assessment of bisphenol A and genistein using the embryonic stem cell test in vitro. Food Chem. Toxicol. 2013;60:497–505. doi: 10.1016/j.fct.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y, Liu R, Xing L, Xu YJ, Shang LQ, Hao WD. Combined developmental toxicity of bisphenol A and genistein in micromass cultures of rat embryonic limb bud and midbrain cells. Tocivol. In Vitro. 2011;25:153–159. doi: 10.1016/j.tiv.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Gayathri L, Dhivya R, Dhanasekaran D, Periasamy VS, Alshatwi AA, Akbarsha MA. Hepatotoxic effect of ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015;83:151–163. doi: 10.1016/j.fct.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival. Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Gennings C, Carter WH, Jr, Carchman RA, Teuschler LK, Simmons JE, Carney EW. A unifying concept for assessing toxicological interactions: changes in slope. Toxicol. Sci. 2005;88:287–297. doi: 10.1093/toxsci/kfi275. [DOI] [PubMed] [Google Scholar]
- 21.Harvey MJ, Klaassen CD. Interaction of metals and carbon tetrachloride on lipid peroxidation and hepatotoxicity. Toxicol. Appl. Pharmacol. 1983;71:316–322. doi: 10.1016/0041-008X(83)90018-2. [DOI] [PubMed] [Google Scholar]
- 22.Brennan JF, Jastreboff PJ. Interaction of salicylate and noise results in mortality of rats. Experientia. 1989;45:731–734. doi: 10.1007/BF01974571. [DOI] [PubMed] [Google Scholar]
- 23.Wan LY, Turner PC, El-Nezami H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013;57:276–283. doi: 10.1016/j.fct.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 24.Bouaziz C, Sharaf El Dein O, El Golli E, Abid-Essefi S, Brenner C, Lemaire C, Bacha H. Different apoptotic pathways induced by zearalenone, T-2 toxin and ochratoxin A in human hepatoma cells. Toxicology. 2008;254:19–28. doi: 10.1016/j.tox.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Fernandez-Franzon M, Font G, Ruiz MJ. Toxicity evaluation of individual and mixed enniatins using an in vitro method with CHO-K1 cells. Toxicol. In Vitro. 2013;27:672–680. doi: 10.1016/j.tiv.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Tatay E, Meca G, Font G, Ruiz MJ. Interactive effects of zearalenone and its metabolites on cytotoxicity and metabolization in ovarian CHO-K1 cells. Toxicol. In Vitro. 2014;28:95–103. doi: 10.1016/j.tiv.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Doi K, Uetsuka K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 12: 5213–5237 (2011). [DOI] [PMC free article] [PubMed]
- 28.Alassane-Kpembi I, Puel O, Oswald IP. Toxicological interactions between the mycotoxins deoxynivalenol, nivalenol and their acetylated derivatives in intestinal epithelial cells. Arch. Toxicol. 2015;89:1337–1346. doi: 10.1007/s00204-014-1309-4. [DOI] [PubMed] [Google Scholar]
- 29.Prosperini A, Font G, Ruiz MJ. Interaction effects of Fusarium enniatins (A, A1, B and B1) combinations on in vitro cytotoxicity of Caco-2 cells. Toxicol. In Vitro. 2014;28:88–94. doi: 10.1016/j.tiv.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Hou YJ, Zhao YY, Xiong B, Cui XS, Kim NH, Xu YX, Sun SC. Mycotoxin-Containing Diet Causes Oxidative Stress in the Mouse. PLoS One. 2012;8:e60374. doi: 10.1371/journal.pone.0060374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abid-Essefi S, Bouaziz C, Golli-Bennour EEl, Ouanes Z, Bacha H. Comparative study of toxic effects of zearalenone and its two major metabolites α-Zearalenol and β-Zearalenol on cultured human Caco-2 Cells. J. Biochem. Mol. Toxic. 23: 233–243 (2009). [DOI] [PubMed]
- 32.Dinu D, Bodea GO, Ceapa CD, Munteanu MC, Roming FI, Serban AI, Hermenean A, Costache M, Zarnescu O, Dinischiotu A. Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon. 2011;57:1023–1032. doi: 10.1016/j.toxicon.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Lei M, Zhang N, Qi D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013;65:1149–1157. doi: 10.1016/j.etp.2013.05.007. [DOI] [PubMed] [Google Scholar]


