Abstract
This study aimed to determine the effects of Metschnikowia pulcherrima yeast on storage quality of ‘Tainong’ mango, and elucidate it’s possible anti–disease mechanism. The results showed that M. pulcherrima could inhibit the changes in peel colour, fruit firmness, the contents of total soluble solids, total acid and vitamin C, and maintain the storage quality of mango fruits. An investigation of the mechanism showed that M. pulcherrima competed not only for the primary carbon source, but also for living space with Colletotrichum gloeosporioides. In addition, M. pulcherrima promoted the activities of defence-related enzymes, including ß-1,3-glucanase(GLU) and chitinase (CHT), and secreted a small amount of antimicrobial substances composed of volatile and nonvolatile anti-fungal compounds. The results strongly demonstrated that antagonistic yeast M. pulcherrima could be applied as a biocontrol agent for deducing the spoilage and decay of mango fruit.
Keywords: Mango fruit, Metschnikowia pulcherrima, Storage quality, Anti-disease mechanism, Enzyme activity
Introduction
Postharvest diseases cause immeasurable losses to fruits and vegetables during harvest, transportation and marketing, severely reduce shelf life and economic benefits [1]. Rhizopus stolonifer, Penicillium expansum, Botrytis cinerea, Penicillium digitatum and other species cause a variety of fruit diseases [2, 3]. Anthracnose is one of the most important postharvest diseases and causes decay in many fruits, including mangoes, avocadoes, apples, bananas, and others [4–6]. Therefore, research on postharvest anthracnoses in fruits and vegetables has received considerable attention worldwide.
Anthracnose caused by Colletotrichum gloeosporioides is a major disease of mango after harvest, and the loss of mangoes to decay can reach 20–30%. Several types of diseases have brought huge losses to the mango industry. The traditional method for controlling postharvest anthracnose in mangoes is to use chemical fungicides. Although their sterilization effect is strong, chemical fungicides have many disadvantages [7, 8]. For instance, the frequent use of chemical fungicides promote resistance among pathogenic microorganisms, the residual fungicide on the surface of fruits and vegetables threatens human health, and fungicides can cause serious and irreversible environmental pollution [9, 10]. Therefore, preventing and controlling the disease through biological approaches have received increasing attention because such methods are potentially safer than using gungicides for human health and the environment. In a variety of biological control studies, research on antagonistic yeast has received increasing attention, and the positive effects of many antagonistic yeasts on not only controlling disease but also maintaining fruit quality have been reported [11–13].
In recent years, application of antagonistic yeast has been studied by many researchers, and some antagonistic yeasts with obvious antagonistic effects on the main diseases of mango fruits have been isolated [14, 15]. Many studies have confirmed that there is a dramatic difference in the effectiveness of biological control versus use of chemical fungicides. However, the use of antagonistic yeasts in mango fruits is still in laboratory stages [16, 17]. Metschnikowia pulcherrima was one of the most resistant to diphenylamine (DPA). BIOLOG test separated the strains into one major cluster containing four strains and four scattered clusters. Among different antagonistic yeasts, M. pulcherrima is an important yeast species which has been successfully applied to control for a number of pathogens on fruits and vegetables. The main reason for this on-going research is that the inhibition mechanism of antagonistic yeast against postharvest pathogens is unclear, the interactions between antagonistic yeast and the pathogens have yet to be elucidated, and it is very difficult to get antagonistic yeast to exert high antibacterial effects [18, 19].
The antagonistic mechanism is the theoretical basis for using antagonistic microorganisms to control various pathogens. Parafati et al. [20] have confirmed multiple mechanisms of Wickerhamomyces anomalus against Botrytis cinerea, including forming colonizing wounds and a biological protective layer and producing hydrolytic enzymes and volatile organic compounds. In addition, the attachment efficacy of yeasts depends on the secretion abilities of lytic enzymes, and this adhesion might be involved in the biocontrol efficacy of antagonistic yeasts [21, 22]. Our previous studies demonstrated that a strain of yeast, Debaryomyces nepalensis had significant inhibitory effects on anthracnose occurrence in postharvest mango fruits [23]. Therefore, the objective of this study was to evaluate the effects of another antagonistic yeast—M. pulcherrima on the storage quality of ‘Tainong’ mango, and elucidate the antimicrobial mechanisms of M. pulcherrima against anthracnose caused by C. gloeosporioides.
Materials and methods
Materials preparation
C. gloeosporioides Penz. was isolated in our laboratory by Luo et al. [23]. C. gloeosporioides Penz. was activated in potato dextrose agar (PDA), incubated at 28 °C for 7 days and subsequently maintained at 4 °C for storage. For the bioassays, a suspension of fungus spores was obtained by picking up mycelium portions with a loop and placing them in 10 mL of sterile distilled water, which was subsequently filtered with gauze and adjusted to a concentration of 1 × 105 spores/mL using a haemocytometer.
M. pulcherrima was isolated from the soil of a mango orchard in our laboratory in 2015. M. pulcherrima was grown in potato dextrose broth (PDB) on a rotary shaker at 28 °C and 110 rpm for 72 h. The culture was sterilized at 121 °C for 20 min to achieve yeast sterilization. The culture was centrifuged at 12,000×g for 20 min, and the yeasts were resuspended in distilled water to achieve yeast suspension. The resulting supernatant was filtered through a 0.22 µm membrane to obtain yeast filtrate. The concentration was adjusted to 1 × 108 cells/mL using a haemocytometer.
Mangoe fruits (Cv. ‘Tainong’) were harvested at physiological maturity and with no apparent mechanical injury, pests or diseases. Fruits were superficially disinfected with 0.5% sodium hypochlorite for two min, washed with tap water, allowed to dry at room temperature (28 °C) and prepared for use.
Effects of M. pulcherrima on storage quality of mango fruit
In primary experiment, we compared the preservation effects of four different concentrations (106, 107, 108 and 109 cells/mL), the results showed that 108 cells/mL was most suitable (data not shown). So, suspension of M. pulcherrima with 108 cells/mL was used in the study.
Selected mango fruits (the variety of Tainong) were randomly divided into 2 groups of 150 fruits each for the following treatments. The first group was immersed in suspension of M. pulcherrima with 108 cells/mL, the second group was the control, immersed in sterile distilled water, all treated fruits were air-dried at the room temperature (approximately 25 °C). Then every group was stored at 15 °C and 90% relative humidity. In order to evaluate the effect of M. pulcherrima on fruit quality of mango stored at 15 °C, quality parameters were measured. Samples were picked randomLy, to be analyzed at 5-day intervals. All assays were performed at ambient temperature (approximately 20 °C). This experiment was done 3 times, each with 3 replicates (3 fruits for 1 replicate). The testing methods used are described below.
Nine individual fruits for 1 treatment were selected for the determination of peel color, which was measured at 4 points around the equatorial zone of the fruit by a colorimeter (Konica Minolta, CM-700d, Osaka, Japan), using b* values to represent the change from green to yellow. The average of 4 readings was taken as measure of b* value for this fruit. Flesh firmness was measured using a hand-held penetrometer (FMH-1, Takemura Motor Manufacturing, Matsumoto, Japan) equipped with a conical probe (12 mm in diameter) and recorded as N (Newtons). Measurements were taken at the peeled equatorial surface on 2 sides of the fruit. The average of 2 readings was taken as a measure of firmness for this fruit. The TSS (%) in fruit flesh were determined using a temperature-compensated digital refractometer (ATAGO, Japan). The titratable acidity content, or total acid (TA) was measured by acid–base titration, and the content of vitamin C (VC) was determined by 2,6-dichloroindophenol according to Shao et al. [24].
The mechanism of M. pulcherrima against C. gloeosporioides
Assessment of the in vitro antagonism effect
The plates were flooded with 20 μL of a conidial suspension (1 × 105 spores/mL) of C. gloeosporioides followed by M. pulcherrima (1 × 108 cells/mL) streaked onto the centre of each plate. The widths of the inhibition zones were measured after 4 days of incubation at 25 °C. The experiments were repeated three times.
Assessment of competition for nutrients
Forty healthy mango fruits were washed, disinfected and drilled (8 mm in diameter and 1 cm deep) to evaluate each of the following substrates, 2% of sucrose (S), glucose (G) or fructose (F) as carbon sources and 0.3% nitrate potassium (K) as the nitrogen source. For evaluation, the wounds were inoculated with 25 μL of the yeast suspension containing 1 × 108 cells/mL (A), 25 μL of a pathogen spore suspension containing 1 × 105 spores/mL (P) and 50 μL of the nutrients. For the control, the nutrients were replaced with 50 mM phosphate solution (pH 6.7) to compare the diameter of the lesion caused by the disease against the treatments with added sugars or potassium nitrate. The fruits were stored at 25 and 15 °C with high relative humidity (above 90%) for 6 days. The diameters of the lesions produced by the fungus in each fruit were observed and measured according to Bautista-Rosales’s method [4]. An increase in the development of the disease was calculated with the following equation,
LDR diameters of the lesions (treatment group), LDC diameters of the lesions (A + P).
Assessment of competition for space
Thirty healthy mangoes as one treatment were washed, disinfected and drilled (8 mm in diameter and 1 mm deep). There were 6 treatments, (1) Control fruit, 25 μL sterile distilled water and 25 μL of C. gloeosporioides at the same time; (2) the yeast was inoculated 12 h earlier than C. gloeosporioides; (3) the yeast was inoculated 24 h earlier than C. gloeosporioides; (4) C. gloeosporioides was inoculated 12 h earlier than the yeast; (5) C. gloeosporioides was inoculated 24 h earlier than the yeast; (6) both the yeast and C. gloeosporioides were inoculated at the same time. All treatments included two groups, one was stored at 15 °C with high relative humidity (above 90%) for 6 days; the other was stored at 25 °C with high relative humidity (above 90%) for 6 days. The diameters of the lesions produced by the fungus in each fruit were observed and measured. The inhibitory rate (IR) of antagonistic yeast on the injury was calculated with the following equation:
LDC diameters of the lesions (control group), LDR diameters of the lesions (treatment group).
Determination of enzyme activities
All mangoes were washed, disinfected and drilled (8 mm in diameter and 1 mm deep). There were four treatments, i.e. CK, inoculated with sterile distilled water; A, inoculated with M. pulcherrima and C. gloeosporioides; B, inoculated with M. pulcherrima; C, inoculated with C. gloeosporioides.
The fruit pulp and mesocarp tissue at 3–10 mm below the skin and 5–15 mm from the edge of the inoculated lesion were removed with a stainless steel knife and cut into small pieces every 2 days during storage. Each treatment replicate at each sampling time contained 3 fruits, with 3 replicates for each treatment. The tissue samples were stored in a −80 °C ultra-low temperature freezer.
Two-gram samples of frozen mango tissue taken from three fruits, with 3 replicates for each treatment, were ground with a mortar and pestle on ice and homogenized with various pre-cooled extracting buffers as follows, 5 mL of 100 mM sodium acetate buffer (pH 5.5) containing 4% (w/v) polyvinylpyrrolidone; 5 mL of 100 mM boric acid buffer (pH 8.8) containing 4% (w/v) polyvinylpyrrolidone; and 5 mL of 50 mM sodium acetate buffer (pH 5.2) containing 1 mM EDTA, 5 mM ß-mercaptoethanol and 5 mM ascorbic acid. The extracts were then centrifuged at 12,000×g for 20 min at 4 °C. The supernatants were used for the enzyme assays.
ß-1,3-glucanase (GLU) activity assays were performed by measuring the amount of reducing sugars released from laminarin according to the method of Ippolito et al. [25] with some modifications. Enzyme extract was dialyzed for 12 h at 4 °C and then centrifuged at 12,000×g for 20 min at 4 °C. The supernatant (1 mL) was incubated with 0.1 mL of laminarin (0.5%, w/v) for 40 min at 37 °C. Afterward, 1 mL of the mixture was diluted at a ratio of 1,1 with distilled water. The reaction was stopped by the addition of 3,5-dinitrosalicylate (1.5 mL) and boiled for 5 min in a water bath. The solution was diluted to 25 mL with distilled water, and the amount of reducing sugars was measured spectrophotometrically at 540 nm.
Chitinase (CHT) activity was measured following the method of Wirth and Wolf [26] with some modifications. One millilitre of enzyme extract was mixed with 2 mL of 1% carboxymethylchitin in 50 mM sodium acetate buffer (pH 5.0). After incubation at 37 °C for 1 h, the reaction was terminated by adding 0.1 mL of 1.0 M HCl. The mixture was cooled on ice and centrifuged at 12,000×g for 10 min. The absorbance of the supernatant was recorded at 550 nm.
Detection of volatile anti-fungal compounds
Volatile anti-fungal compounds were assayed using the method of Mackie and Wheatley [27]. When the pathogen hypha extended to the plate edge, the diameter of the pathogen hypha that inoculated the biocontrol yeast was observed and measured. The inhibitory rate (bacteriostasis rate) of antagonistic yeast on pathogen growth was calculated with the following equation,
CDC diameter of the colony (control group), CDR diameter of the colony (treatment group).
Detection of nonvolatile anti-fungal compounds
Five groups of healthy mangoes (20 mangoes for one group) were wounded with a puncher (8 mm) to a 1 mm depth with two wounds per mango. Four different yeast treatment fluids were used. Within the wounds, 25 μL of different treatment fluids was inoculated and allowed to incubate for 1 h. After incubation, 25 μL aliquots of a C. gloeosporioides fungus spore suspension (1 × 105 spores/mL) were added to the wound. Additionally, 25 μL of sterile distilled water and 25 μL C. gloeosporioides fungus spore suspension (1 × 105 spores/mL) were added to the wounds as negative controls. There were five treatments, (1) fruits treated with the yeast culture to a concentration of 1 × 108 cells/mL and C. gloeosporioides to a concentration of 1 × 105 spores/mL, (2) fruits treated with the yeast suspension to a concentration of 1 × 108 cells/mL and C. gloeosporioides to a concentration of 1 × 105 spores/mL, (3) fruits treated with the yeast sterilization and C. gloeosporioides to a concentration of 1 × 105 spores/mL, (4) fruits treated with the yeast filtrate and C. gloeosporioides to a concentration of 1 × 105 spores/mL, and (5) fruits treated with sterile distilled water and C. gloeosporioides to a concentration of 1 × 105 spores/mL. The subsequent steps were the same as described in section of ‘Determination of the effect of yeast on the severity of anthracnose’. The IR of antagonistic yeast on the injury was calculated as above formula.
Statistical analysis
Statistical analysis was performed using the Origin statistical software package (OriginVersion 8.5, Hampton, Massachusetts, USA). All experiments were run in triplicate, and all the results were expressed as mean ± standard deviation (SD) of three independent experiments. The experimental data were assessed by one-way analysis of variance (ANOVA), and significance of differences among treatment was obtained by the least significant difference (LSD). A p value < 0.05 was considered statistically significant.
Results and discussion
Effects of M. pulcherrima on storage quality of mango fruit
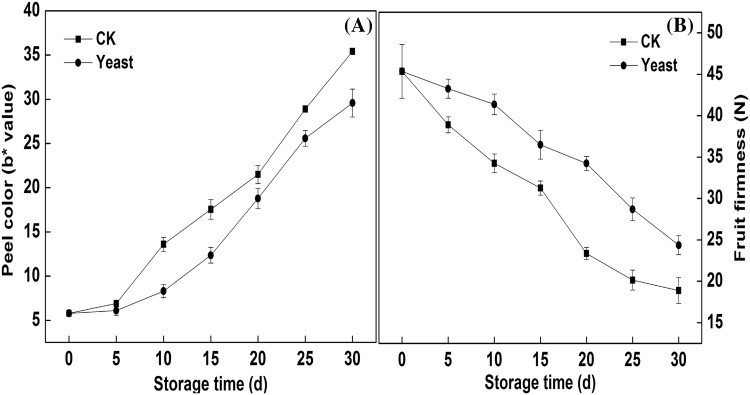
Color and pulp firmness are very important quality factors for stored fruits. Figure 1 showed the changes in b* value and fruit firmness of the mango fruits stored at 15 °C. The changes in b* value and fruit firmness of CK fruits were more slowly than those of antagonist yeast treated fruits during the whole storage time. When stored for 15 days, the b* value of CK was 41% higher than that of treatment fruits, and the treated fruits were by almost 5.231 N firmer than the untreated fruits, the significant difference between CK and the treatment groups was observed (p < 0.05). It indicated that the antagonist yeast (M. pulcherrima) had the significant effects on both delaying peel color change and inhibiting the fruit softening of mango fruits.
Fig. 1.
The effects of antagonist yeast treatment on the b* value (A), fruit firmness (B) of mango fruits stored at 15 °C. Each data point represents a mean ± standard error (n = 3). Vertical bars on symbols represent ± SE of the mean
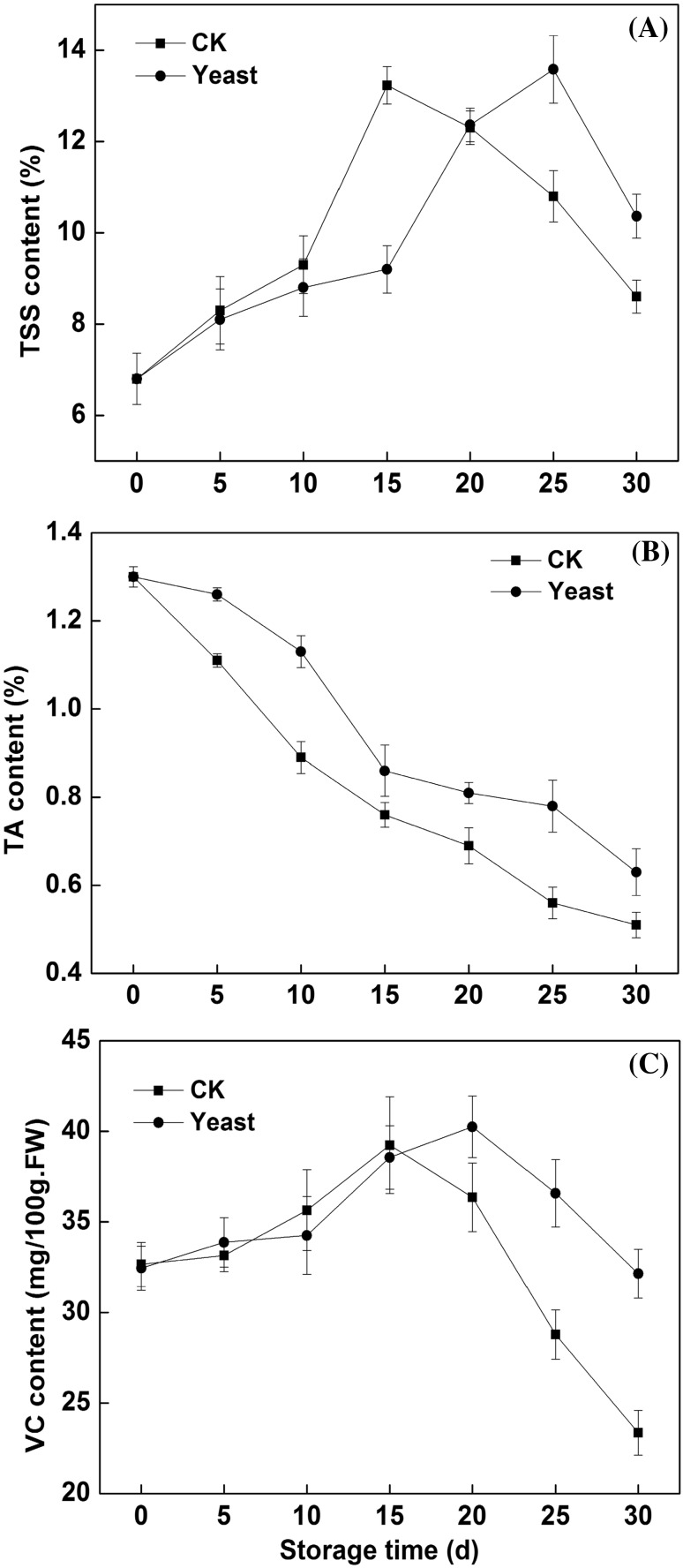
The changes in TSS, TA and VC of mango fruits were shown in Fig. 2. During the storage of mango fruits, TSS and VC exhibited the tendency of increasing then decreasing. The peak time of TSS and VC of yeast treated fruits were delayed 10 days (Fig. 2A) and 5 days (Fig. 2C), respectively, and the peak values of TSS and VC of treated fruit were 0.31% and 1.01 mg/100 g FW higher, respectively, as compared with untreated fruits. There were significant differences in TSS and VC between treated and untreated fruits (p < 0.05).
Fig. 2.
The effects of antagonist yeast treatment on TSS content (A), TA content (B) and VC content (C) of mango fruits stored at 15 °C. Each data point represents a mean ± standard error (n = 3). Vertical bars on symbols represent ± SE of the mean
The change in TA content was suppressed greatly by yeast treatment, as compared with untreated fruits (Fig. 2B). After 5 and 25 days of storage, TA content of antagonist yeast-treated fruits was 0.15 and 0.22% higher, respectively, as compared with untreated fruits and the significant differences in TA content between treated and untreated fruits were observed (p < 0.05). This data suggested M. pulcherrima yeast treatment could inhibit the changes in TSS, TA and VC, and significantly maintains the storage quality of mango fruits.
The mechanism of M. pulcherrima against C. gloeosporioides of mango fruit
The in vitro antagonism effect
M. pulcherrima has obvious antagonism effect on C. gloeosporioides in vitro. The conidial of C. gloeosporioides was not germinated. The strain of M. pulcherrima showed an inhibition zone of 8.5 mm when cultured with C. gloeosporioides in PDA (data not shown). The mycelium of C. gloeosporioides near the yeast side growed slowly and sparsely.
The competition for nutrients
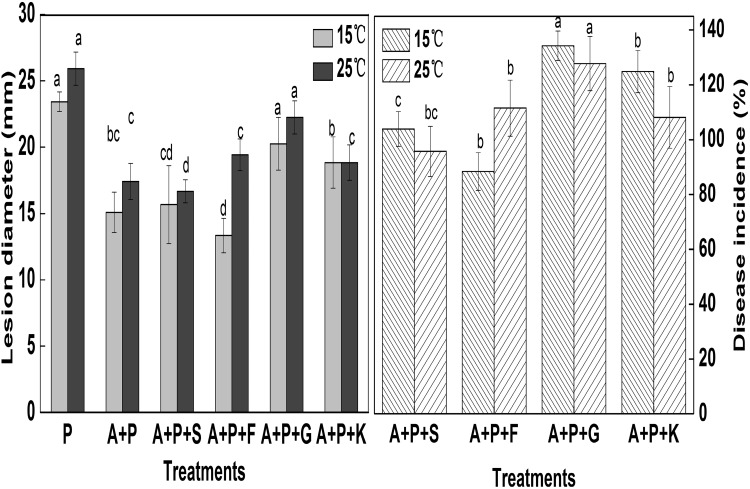
After 10 days of incubation of treated fruits, the diameters of the disease lesions caused by the fungus at 15 and 25 °C were measured (Fig. 3, left). M. pulcherrima showed different levels of competition for nutrients under in vivo conditions. When glucose was added to wounds as an exogenous nutrient (A + P+G), the diameters of the disease lesions were 5.0 and 6.0 mm higher than those of only A + P treated fruits at 15 and 25 °C, respectively. In addition, the development of the disease in A + P + G treated group was increased by 134.28 and 127.73% at 15 and 25 °C, respectively, relative to A + P treatment (p < 0.05) (Fig. 3, right panel). When fructose was added to wounds as an exogenous nutrient (A + P + F) at 25 °C, the diameter of the disease lesions was 6.5 mm higher than that of only A + P treatment. As shown in Fig. 4, when sucrose and potassium nutrients were added, there was no significant difference in the lesion diameters between A + P + S, A + P + R and A + P treatments (p > 0.05). This finding indicated that M. pulcherrima could compete for nutrients, especially glucose, and rapidly assimilate and absorb the basic carbohydrate. This pattern is similar to the conclusions of Sharma et al. [28] and Spadaro et al. [29] that the yeast can use a variety of monosaccharides and disaccharides.
Fig. 3.
The diameters of the lesions (left) and the disease incidence (right) of all treatments. P pathogen, A antagonist, S sucrose, F fructose, G glucose, K potassium nitrate. Each value is the mean of 3 experiments. Bars on the top of columns represent the standard error of the mean. Data in columns with the different letters are significantly different according to Duncan’s multiple range test at p = 0.05
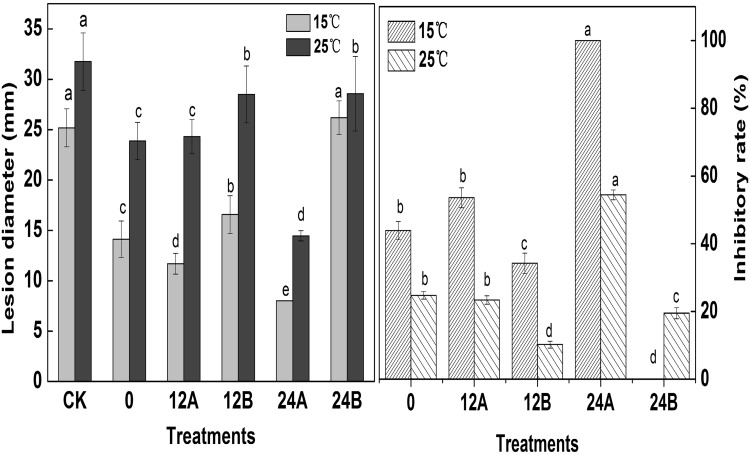
Fig. 4.
The diameters of the lesions (left) and the inhibitory rate (right) of all treatments. (0) The pathogen and M. pulcherrimaat the same time. (12A) M. pulcherrimainoculated 12 h earlier than the pathogen. (12B) The pathogen inoculated 12 h earlier than M. pulcherrima. (24A) M. pulcherrimainoculated 24 h earlier than the pathogen. (24B) The pathogen inoculated 24 h earlier than M. pulcherrima. Each value is the mean of 3 experiments. Bars on the top of columns represent the standard error of the mean. Data in columns with the different letters are significantly different according to Duncan’s multiple range test at p ≤ 0.05
The competition for space
Competition for space was detected based on the inoculation of pathogens and yeast in chronological order and length [30]. The lesion diameters associated with different inoculation times of yeast cells and the pathogen are shown in Fig. 4, left panel. For all treatments, the lesion diameters when storage at 25 °C were larger than those observed at 15 °C. For treatment 12B (pathogen inoculated 12 h earlier than M. pulcherrima), the lesion diameters were 5.5 and 4.5 mm larger than those of 12A (M. pulcherrima inoculated 12 h earlier than the pathogen) stored at 15 and 25 °C, respectively. The IRs (inhibitory rate) were 33.21 and 15.89% for 12B stored at 15 and 25 °C, respectively (Fig. 4, right panel). The earlier time for the yeast inoculation, the shorter diameters for the lesion, suggests that the inhibition of M. pulcherrima against the pathogen increased with incubation time without pathogens. This observation is the same as results of Ferraz et al. [31]. When M. pulcherrima was inoculated 24 h after the inoculation with the pathogen (24B), the IR reached 100 and 55% at 15 and 25 °C, respectively (p < 0.05) (Fig. 4, right panel). A reasonable explanation for this behaviour was that when M. pulcherrima was inoculated prior to the wounding of the mango, it could grow and develop rapidly, the yeast became the dominant microorganism at the wound and adapted to the environment of the wound, and the pathogens lost the ability to seize space, the anthracnose pathogens gradually died because there was no growth space [32]. Therefore, competition for nutrition and space became one of the effective ways for the antagonistic yeast M. pulcherrimato inhibiting C. gloeosporioides.
The changes in activities of defence-related enzymes
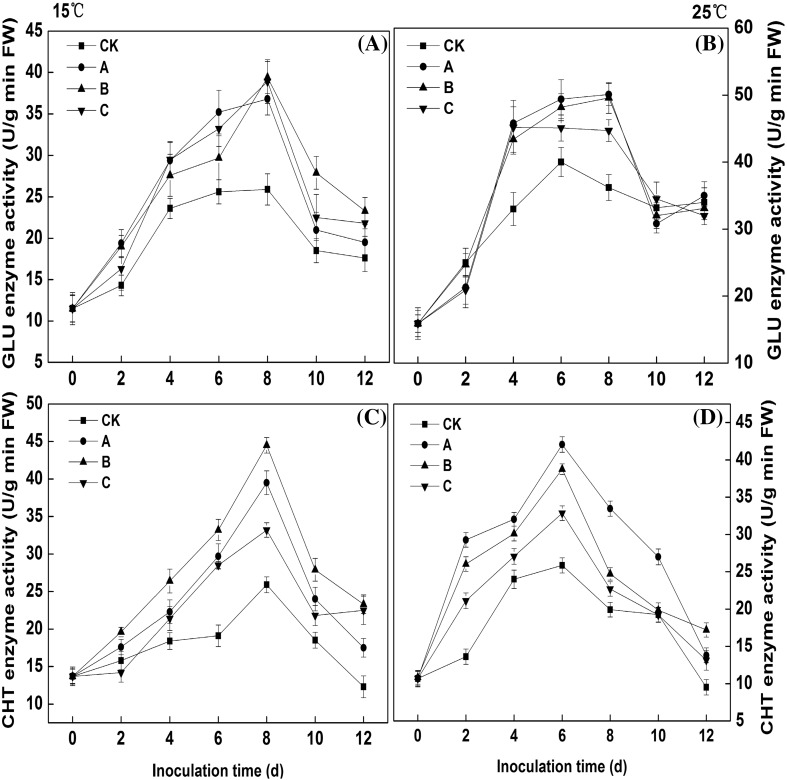
GLU and CHT are two typical disease-process proteins which play important role in the interaction between the host and pathogen [33]. GLU activity in wounded mango fruits showed an increase and then a decreasing tendency at 15 and 25 °C (Fig. 5A, B). At 15 °C, the GLU activity peaks of all treatments appeared on day 8, but the peak values were dramatically different between treated and control groups. The peaks of the treated groups were 36.8, 39.4, and 38.91 U/g/min FW, and the peak of the control group was 25.9 U/g/min FW. The activities of A (inoculated with M. pulcherrima and C. gloeosporioides), B (inoculated with M. pulcherrima) and C (inoculated with C. gloeosporioides) treatment groups were much higher than that of the control (p < 0.05). When stored for 8 days at 25 °C, the GLU activities in the control, A, B, and C treatment groups were 36.2, 50.1, 49.6 and 44.7 U/g/min FW, respectively. This finding showed that the GLU activities of the treated groups were higher than those of the control group both at 15 and 25 °C.
Fig. 5.
Changes in GLU (A and B) and CHT (C and D) activities in wounded mango fruits inoculated by different treatments. (CK) inoculated with sterile distilled water. (A) inoculated with M. pulcherrima and C. gloeosporioides. (B) inoculated with M. pulcherrima. (C) inoculated with C. gloeosporioides. Each data point represents a mean ± standard error (n = 3)
CHT activity showed a trend towards an increase and then a decrease at 15 and 25 °C (Fig. 5C, D). CHT activity increased to a maximum at 6 days at 25 °C but increased to a maximum at 8 days at 15 °C. When the treated mango fruits were stored at 15 °C, the peak values of the CHT activities of A, B and C groups were 39.5, 44.5 and 33.2 U/g/min FW, respectively, but the peak value of the control group was 25.9 U/g/min FW; a significant difference between treatment and control groups was observed (p < 0.05). When the treated mango fruits were stored at 25 °C, the peak values of the CHT activities in A treatment group were the highest, followed by B treatment group. The peaks of CHT activity in the A, B and C treatment groups were 16.2, 13.0 and 7.0 U/g/min FW higher than that of the control, respectively. This finding suggested that all treatments could increase the CHT activity of wounded mango fruits.
The data of the current study suggested that the activities of defence enzymes (GLU, and CHT) in mango fruits could be induced by yeasts when only inoculated with the yeast, and anthracnose could induce the increase of defensive enzymes in mango fruit when only inoculated with the anthracnose pathogen. However, when the mango fruit was inoculated with yeasts and anthracnose pathogens at the same time, the defence-related enzyme activities were not superimposed. GLU can directly hydrolyse the cell wall of the fungus and may also indirectly assist in enhancing resistance by releasing oligosaccharides in the plant [34]. CHT plays a crucial role in degrading chitin [35]. So, the increased activities of GLU and CHT by M. pulcherrima is one important reason for inhibiting anthracnose caused by C. gloeosporioides in mango fruits.
The changes in volatile anti-fungal compounds and nonvolatile anti-fungal compounds
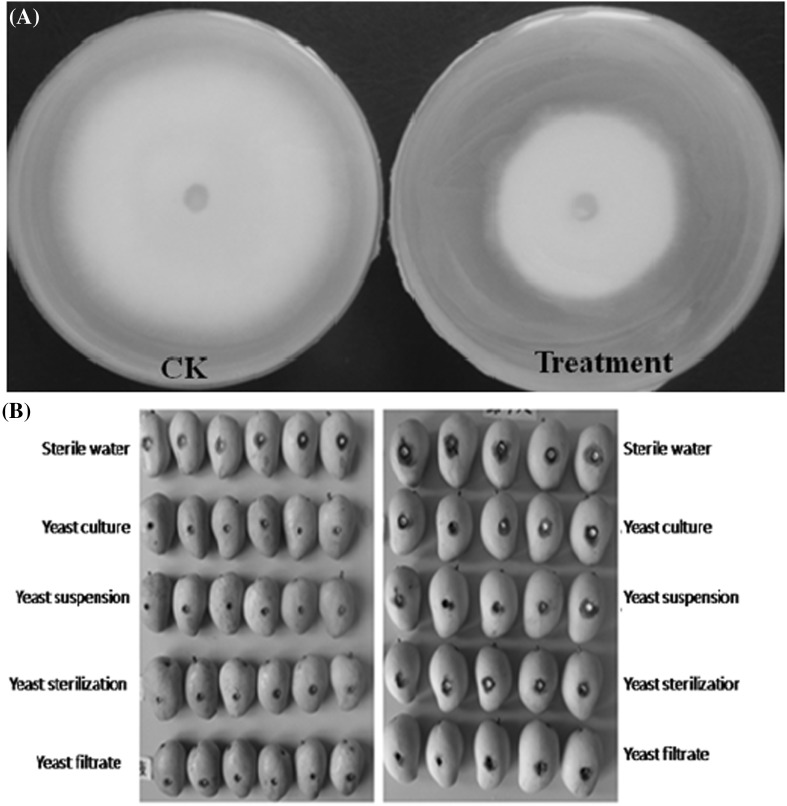
As shown in Fig. 6(A), after 4 days of incubation at 28 °C, the colony diameter of the control group (left panel) was 70 mm, whereas the colony diameter of the treatment group (right panel) was 48.5 mm (p < 0.05). The bacteriostasis rate (BR) reached 30.71%. This finding suggested that M. pulcherrima produced certain volatile substances that could suppress the growth of C. gloeosporioides.
Fig. 6.
The production of volatile antifungal compounds was achieved via incubation at 28 °C (A). CK, Standoff plate coated sterile water; Treatment, Standoff plate coated with M .pulcherrima. Production of nonvolatile antifungal compounds (B). Left panel stored at 15 °C; right panel stored at 25 °C
The effects of different yeast treatments on mango anthracnose are shown in Fig. 6(B). Four types of treatments had different antibiotic effects on anthracnose, but no significant differences between treatment groups were found (p ≥ 0.05). However, the antibiotic effect of four treatments were observed compared to the control group (p ≤ 0.05). The inhibition effects of yeast culture and the filtrate were better than the other treatments. The inhibition rates of the yeast suspension and the filtrate were 24 and 23%, respectively, when stored at 15 °C. In addition, the inhibition rates of the yeast suspension and the filtrate were 31 and 39%, respectively, when stored at 25 °C. These data revealed that M. pulcherrima produced certain nonvolatile substances that could influence and suppress the growth of C. gloeosporioides.
Da Silva Felix et al. [36] reported that antagonistic microorganisms play a role in controlling disease by competing for space and nutrition, producing antimicrobial substances and inducing resistance in the host. Kumar et al. [37] found that 2,4-Di-tert-butylphenol produced by Streptomyces had antibacterial activity. Kai et al. [38] reported that small organic volatile compounds (VOCs) emitted from bacterial antagonists negatively influence the mycelial growth, such as Bacillus subtilis, Serratia odorifera. Here, we did find M. pulcherrima could produce a variety of antimicrobial substances, but the precise substances and the content of each component remain unknown. This information needs to be investigated in further studies. Nevertheless, we can conclude for the first time that the antimicrobial substance contains certain non-protein substances and a certain volatile gas. We did find that the antimicrobial substances produced by antagonistic yeast were very weak, and the possible reasons for this may be as follows: first, the growth of mycelium was fast and the secretion of antibacterial substances was not as fast as that of its growth; second, the concentration of antimicrobial substances secreted by the antagonistic yeast was too low. Therefore, finding a medium to promote the secretion of yeast substances, optimizing extraction and purifying antibacterial substances will be the important work in the future.
Theis and Stahl [39] mentioned that the interaction between yeast-pathogens and plant-pathogens depends on the signal recognition system between yeast cells, fungal hyphae and fruit cells. Castoria et al. [40] aLso showed that antagonistic yeast may cause direct and severe damage to Botrytis cinerea hyphae. However, we found that M. pulcherrima did not attach to C. gloeosporioides and did not cause any damage to the spores and hyphae. This finding indicates the possibility of the practical application of M. pulcherrima yeast.
In a word, this work confirmed that M. pulcherrima could inhibit the changes of quality parameters and maintain the storage quality of‘Tainong’ mango. M. pulcherrima could control anthracnose caused by C. gloeosporioides by competing for space and nutrition, producing antimicrobial substances and inducing resistance in mango fruits. M. pulcherrima proved to be an effective antagonistic yeast and could be applied as a potential biocontrol agent for deducing the spoilage and improving postharvest quality of mango fruits.
Acknowledgements
This work was funded by the Project of the National Natural Science Foundation of China (No. 31660587), and it represents part of work for the project of the Key Research and Development of Science and Technology Department in Hainan Province (ZDYF2016043).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Wen Li, Email: Liwen9-210@163.com.
Yuan-zhi Shao, Email: s.yz123789@163.com.
References
- 1.Mahunu M, Zhang HY, Yang QY, Zhang XY, Li DD, Zhou YX. Improving the biocontrol efficacy of Pichia caribbica with phytic acid against postharvest blue mold and natural decay in apples. Biol. Control. 2016;92:172–180. doi: 10.1016/j.biocontrol.2015.10.012. [DOI] [Google Scholar]
- 2.Zhang HY, Ma LC, Jiang S. SaLicylic acid enhances biocontrol efficacy of Rhodotorula glutinis against postharvest Rhizopus rot of strawberries and the possible mechanisms involved. Int. J. Food Microbiol. 2010;141:122–125. doi: 10.1016/j.ijfoodmicro.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 3.Zhu RY, Lu LF, Lu HP. Postharvest control of green moLd decay of citrus fruit using combined treatment with sodium bicarbonate and Rhodosporidium paludigenum. Food Biopro. Technol. 2013;6:2925–2930. doi: 10.1007/s11947-012-0863-0. [DOI] [Google Scholar]
- 4.Bautista-Rosales PU, Calderon-Santoyo M, Servín-Villegas R, Ochoa-Álvarez NA, Ragazzo-Sánchez JA. Action mechanisms of the yeast Meyerozyma caribbica for the control of the phytopathogen Colletotrichum gloeosporioides in mangoes. Biol. Control. 2013;65:293–301. doi: 10.1016/j.biocontrol.2013.03.010. [DOI] [Google Scholar]
- 5.Jat BL, Sharma P, Gour HN. Production of Enzymes and Culture Filtrates by Colletotrichum gloeosporioides Penz. Causing Banana Fruit Rot. Production of Enzymes and Culture Filtrates 83:177–180 (2013).
- 6.Campos-Martínez A, Velazquez-del Valle MG, Flores-Moctezuma HE, Suarez-Rodríguez R, Ramírez-TrujiLLo JA, Hernandez-Lauzardo AN. Antagonistic yeasts with potential to control Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Colletotrichum acutatum J.H. Simmonds on avocado fruits. Crop Prot. 2016;89:101–104. doi: 10.1016/j.cropro.2016.07.001. [DOI] [Google Scholar]
- 7.Guo J, Fang WW, Lu HP, Zhu RY, Lu LF, Zheng XD, Yu T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus Laurentii. Postha. Biol. Technol. 2014;88:72–78. doi: 10.1016/j.postharvbio.2013.09.008. [DOI] [Google Scholar]
- 8.Grzegorczyk M, Żarowska B, Restuccia C, Cirvilleri G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017;61:93–101. doi: 10.1016/j.fm.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Tang JM, Liu YQ, Li HH, Wang LM, Huang K, Chen ZX. Combining an antagonistic yeast with harpin treatment to control postharvest decay of kiwifruit. Biol. Control. 2015;89:61–67. doi: 10.1016/j.biocontrol.2015.04.025. [DOI] [Google Scholar]
- 10.Ponsone ML, Nally MC, Chiotta ML, Combina M, KöhL J, Chulze SN. Evaluation of the effectiveness of potential biocontrol yeasts against black sur rot and ochratoxin A occurring under greenhouse and field grape production conditions. Biol. Control. 2016;103:78–85. doi: 10.1016/j.biocontrol.2016.07.012. [DOI] [Google Scholar]
- 11.Graeme M, Walke R, Anne H, Mcleod Valerie J, Hodgson K. Interactions between killer yeasts and pathogenic fungi. FEMS Micro. Lett. 1995;127:213–222. doi: 10.1111/j.1574-6968.1995.tb07476.x. [DOI] [PubMed] [Google Scholar]
- 12.Tian SP, Fan Q, Xu Y, Jiang AL. Effects of caLcium on biocontrol activity of yeast antagonists against the postharvest fungal pathogen Rhizopus stolonifer. Plant Pathol. 2002;51:352–358. doi: 10.1046/j.1365-3059.2002.00711.x. [DOI] [Google Scholar]
- 13.Usall J, Torres R, Teixido N. Biological control of postharvest diseases on fruit: a suitable alternative. Curr. Opin Food Sci. 2016;11:51–55. doi: 10.1016/j.cofs.2016.09.002. [DOI] [Google Scholar]
- 14.Jamalizadeh M, Etebarian HR, Aminian H, Alizadeh A. A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. EPPO Bull. 2011;41:65–71. doi: 10.1111/j.1365-2338.2011.02438.x. [DOI] [Google Scholar]
- 15.Bautista-Rosales PU, CaLderon-Santoyo M, Servín-Villegas R, Ochoa-Álvarez NA, Ragazzo-Sánchez JA. Biocontrol action mechanisms of Cryptococcus Laurentii on Colletotrichum gloeosporioides of mango. Crop Prot. 2014;65:194–201. doi: 10.1016/j.cropro.2014.07.019. [DOI] [Google Scholar]
- 16.Chi MS, Li GK, Liu YS, Liu GQ, Li M, Zhang XJ, Sun ZQ, Sui Y, Liu J. Increase in antioxidant enzyme activity, stress tolerance and biocontrol efficacy of Pichia kudriavzevii with the transition from a yeast-like to biofilm morphology. Biol. Control. 2015;90:113–119. doi: 10.1016/j.biocontrol.2015.06.006. [DOI] [Google Scholar]
- 17.Jin P, Zheng C, Huang YP, Wang XL, Luo ZS, Zheng YH. Hot air treatment activates defense responses and induces resistance against Botrytis cinerea in strawberry fruit. J. Integ. Agric. 2016;15:2658–2665. doi: 10.1016/S2095-3119(16)61387-4. [DOI] [Google Scholar]
- 18.Kwon MJ, Wei N, Millerick K, Popovic J, Finneran K. Clostridium geopurificans strain MJ1 sp. nov., a strictly anaerobic bacterium that grows via fermentation and reduces the cyclic nitramine explosive hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) Curr. Microbiol. 2014;68:743–750. doi: 10.1007/s00284-014-0531-x. [DOI] [PubMed] [Google Scholar]
- 19.Hartman GL, Pawlowski ML, Chang HX, Hill CB. Successful Technologies and Approaches Used to Develop and Manage resistance against Crop disease and Pests. Emerging Technologies for Promoting Food Security. 2016;8:43–66. doi: 10.1016/B978-1-78242-335-5.00003-2. [DOI] [Google Scholar]
- 20.Parafati L, Vitale A, Restuccia C, Cirvilleri G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing postharvest bunch rot of table grape. Food Microbiol. 2015;47:85–92. doi: 10.1016/j.fm.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Chan ZL, Tian SP. Interaction of antagonistic yeast against postharvest pathogens of apple fruit and possible mode of action. Postha. Biol. Technol. 2005;36:215–223. doi: 10.1016/j.postharvbio.2005.01.001. [DOI] [Google Scholar]
- 22.Spadaro D, Droby S. Development of biocontrol products for postharvest diseases of fruit, The importance of elucidating the mechanisms of action of yeast Antagonists. Tre. Food Sci. Technol. 2016;47:39–49. doi: 10.1016/j.tifs.2015.11.003. [DOI] [Google Scholar]
- 23.Luo SS, Wan B, Feng SH. ShaoYZ. BiocontroL of Postharvest Anthracnose of Mango Fruit with Debaryomyces Nepalensis and Effects on Storage Quality and Postharvest physiology. J. Food Sci. 2015;80:2555–2563. doi: 10.1111/1750-3841.13087. [DOI] [PubMed] [Google Scholar]
- 24.Shao YZ, Xie JH, Chen P, Li W. Changes in some chemical components and in the physiology of rambutan fruit (Nephelium lappaceum L.) as affected by storage temperature and packing material. Fruits. 2013;68(1):15–24. doi: 10.1051/fruits/2012045. [DOI] [Google Scholar]
- 25.Ippolito A, Ghaouth AE, Wilson CL, Wisniewski M. Control of postharvest decay of apple fruit with Candida saitoana and induction of defense responses. Phytopathol. 2003;93:344–348. doi: 10.1094/PHYTO.2003.93.3.344. [DOI] [PubMed] [Google Scholar]
- 26.Wirth SJ, Wolf GA. Dye-Labelled substrates for the assay and detection of chitinase and lysozyme activity. J. Micro. Meth. 1990;11:197–205. doi: 10.1016/0167-7012(90)90031-Z. [DOI] [Google Scholar]
- 27.Mackie AE, Wheatley RE. Effects and incidence of volatile organic compound interactions between soil bacterial and fungal isoLates. Soil Biol. Biochem. 1999;31(3):375–385. doi: 10.1016/S0038-0717(98)00140-0. [DOI] [Google Scholar]
- 28.Sharma RR, Singh D, Singh R. Biological control of postharvest diseases offruits and vegetables by microbial antagonists: a review. Biol. Control. 2009;50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 29.Spadaro D, Ciavorella A, Zhang D, Garibaldi A, Gullino ML. Effect of culture media and pH on the biomass production and biocontrol efficacy of a M. pulcherrimastrain to be used a fungicide for postharvest disease control. Can. J. Microbiol. 2010;56:128–137. doi: 10.1139/W09-117. [DOI] [PubMed] [Google Scholar]
- 30.Keshavarz-Tohid V, Taheri P, Taghavi SM, Tarighi S. The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. J. Plant Physiol. 2016;199:29–38. doi: 10.1016/j.jplph.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Ferraz LP, da Cunha T, da Silva AC, Kupper KC. Biocontrol ability and putative mode of action of yeasts against Geotrichum citri-aurantii in citrus fruit. Microbiol. Res. 2016;188:72–79. doi: 10.1016/j.micres.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Esposito-Polesi NP, de Abreu-Tarazi MF, de ALmeida CV, de Almeida ML. Investigation of Endophytic Bacterial Community in Supposedly Axenic Cultures of Pineapple and Orchids with Evidence on Abundant Intracellular Bacteria. Curr. Microbiol. 2017;74:103–113. doi: 10.1007/s00284-016-1163-0. [DOI] [PubMed] [Google Scholar]
- 33.Yang JL, Sun C, Zhang YY, Fu D, Zheng KD, Yu T. Induced resistance in tomato fruit by c-aminobutyric acid for the control of aLternaria rot caused by Alternaria alternate. Food Chem. 2017;221:1014–1020. doi: 10.1016/j.foodchem.2016.11.061. [DOI] [PubMed] [Google Scholar]
- 34.Zhang ZK, Yang DQ, Yang B, Gao ZY, Li M, Jiang YM, Hu MJ. ß-Aminobutyric acid induces resistance of mango fruit to postharvest anthracnose caused by Colletotrichum gloeosporioides and enhances activity of fruit defense mechanisms. Sci. Horti. 2013;160:78–84. doi: 10.1016/j.scienta.2013.05.023. [DOI] [Google Scholar]
- 35.Roberti R, Veronesi A, Cesari A, Cascone A, Di Berardino I, Bertini L, Caruso C. Induction of PR proteins and resistance by the biocontrol agent Clonostachys rosea in wheat plants infected with Fusarium culmorum. Plant Sci. 2008;175:339–347. doi: 10.1016/j.plantsci.2008.05.003. [DOI] [Google Scholar]
- 36.Da Silva Felix K, da Silva CL, de Oliveira WJ, de Lima Ramos Mariano R, de Souza EB. Calcium-mediated reduction of soft rot disease in Chinese cabbage. Europ. J. Plant Pathol. 147:73–84 (2017) DOI: 10.1007/s10658-016-0980-0.
- 37.Kumar PS,Duraipandiyan V,Ignacimuthu S.Isolation,screening and partial purification of antimicrobial antibiotics from soil Streptomyces sp. SCA 7.Kaohsiungd MedSci. 30: 435–446 (2014). [DOI] [PMC free article] [PubMed]
- 38.Kai M, Effmert U, Berg G, Piechulla B. Volatiles ofbacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol. 2007;187:351–360. doi: 10.1007/s00203-006-0199-0. [DOI] [PubMed] [Google Scholar]
- 39.Theis T, Stahl U. Antifungal proteins, targets, mechanisms and prospective applications. Cellu Mole. Life Sci. Cmls. 2004;61:437–455. doi: 10.1007/s00018-003-3231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castoria R, Curtis FD, Lima G, Cicco VD. ß -1,3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases. Postha. Biol. Techno. 1997;12:293–300. doi: 10.1016/S0925-5214(97)00061-6. [DOI] [Google Scholar]