Abstract
The antioxidant and anti-adipogenic activities of the water extract (WE) and methanol extract (ME) of the shell and kernel of Castanopsis cuspidata var. thunbergii (CCT) nuts were evaluated. The shell extracts showed higher DPPH and ABTS radical scavenging activities (RSAs) than did the kernel extracts. Furthermore, the RSA of the ME was higher than that of the WE, regardless of the part. The total phenolic contents (TPCs) of the ME of the shell and kernel were 71.38 and 10.56 mg gallic acid equivalent (GAE)/100 mg extract, respectively. The TPCs of the WE of the shell and kernel were 17.44 and 9.27 mg GAE/100 mg extract, respectively. The WE inhibited 3T3-L1 adipogenesis more effectively than did the ME, and the shell extracts suppressed 3T3-L1 adipogenesis more effectively than did the kernel extracts. These results suggest that CCT nut kernels (ME) and shells (WE) may be strategically used to enhance antioxidant or and anti-obesity materials.
Keywords: Castanopsis cuspidata var. thunbergii, Nut, Antioxidant, Anti-adipiogenic
Introduction
Castanopsis cuspidata, which is native in southern Korea and southern Japan, belongs to the Fagaceae family and is a medium-sized evergreen tree related to beech and oak that grows in woods and ravines, especially near the sea. Its leaves are long elliptical, and the cotyledons of the nut are eaten boiled or roasted. Several mushroom species including shiitake (Castanopsis mushroom) use the dead wood of C. cuspidata for growth. C. cuspidata var. sieboldii (CCS) is known to exhibit antioxidant, antimicrobial, and anti-inflammatory activities [1–4]. The ethylacetate fraction of the ethanolic extract of CCS leaves was found to exhibit significant antioxidant and antimicrobial activities [3], while the n‑hexane, dichloromethane, and ethylacetate fractions inhibited the pro-inflammatory cytokine mRNA expression [4]. Moreover, the kernel of CCS nuts has been reported to contain 54 mg% of monosaccharide and several types of organic acids including oxalic, formic, malic, and citric acids [2]. However, little information is available on the physiological activities of C. cuspidata var. thunbergii (CCT), despite its widespread usage in Korea. There are CCT colonies at Yokji Island, which has been designated as the 343rd natural monument in Korea. The CCT nut is referred to as pine nut-chestnut in Korea, as it resembles a pine nut. The CCT nut kernel is consumed by itself or as an additive to other foods such as rice cake by the residents of Yokji Island.
Meanwhile, reactive oxygen species (ROS) are formed in the human body as natural byproducts of oxidative metabolism. They can adversely affect several easily oxidizable cellular components, which may subsequently lead to diverse diseases such as cancer, Alzheimer’s disease, atherosclerosis, stroke, and diabetes [5]. Apart from the self-protection mechanisms employed by cells against oxidative damage, several antioxidants have been investigated for their protective role against ROS-mediated oxidative damage. Although artificial antioxidants have been widely used because of low cost and convenience, their toxicity and carcinogenicity are of concern [6]. There is therefore a need for safer alternatives in the form of natural antioxidants. Among natural antioxidants, phenolic compounds are particularly interesting owing to their wide distribution in the plant kingdom and in agro-industrial by-products [7, 8]. Polyphenolic compounds are distributed in higher amounts in the outer layers than in the inner parts of plants, to possibly protect the important inner parts, such as the seed or embryo [9].
As the CCT nuts contain valuable nutritional compounds, in this study, we evaluated their potential as a source of high-value antioxidant and antiobesity compounds. We determined the total phenol content (TPC) of the water extract (WE) and methanol extract (ME) of the CCT nut kernels and shells. We also investigated the ability of the extracts to scavenge DPPH and ABTS and their anti-adipogenic activity in 3T3-L1 cells.
Materials and methods
Reagents
Dulbecco’s Modified Eagle’s Medium (DMEM), bovine calf serum (BCS), and penicillin–streptomycin were purchased from Welgene Inc. (Daegu, Korea). Fetal bovine serum (FBS) was obtained from GE Healthcare Bio-Sciences Co. (Piscataway, NJ, USA). Folin–Ciocalteu reagent was supplied from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Preparation of extracts
CCT nuts were collected at colony in Yokji Island (Gyeongnam, Republic of Korea) (34°6′34″N, 128°2′69″E) in September 2015. The nuts were dried in a dryer (G-DF2, HY Industry Co., Incheon, Korea) at 50 °C for 12 h, and their shells and kernels were divided and collected. The shells were ground using a blender (Samyang Co., Gimpo, Korea). The resulting powder, with a particle diameter smaller than 710 μm (25 mesh), was used in the following extraction preparation. The kernel powder was obtained in same way. The shell and kernel powders were extracted by water or methanol, respectively. Water extract (WE) was prepared with deionized water (1 g/100 mL) at 100 °C for 1 h, and methanol extract (ME) was made with methanol (1 g/100 mL) at 80 °C for 12 h according to the reflux extraction method described by Custódio et al. [10]. The extracts were centrifuged at 2260×g for 10 min, and supernatants were filtered through Whatman No. 3 paper (Toyo Roshi Kaisha Ltd., Tokyo, Japan) under reduced pressure. The filtrates were dried by evaporation in vacuo, then the dried extracts were dissolved in dimethyl sulfoxide (DMSO) and stored in a deep freezer (Operon Co., Seoul, Korea) at −70 °C for further experiments.
Total phenolic content (TPC)
TPC in the extract was determined according to the method of Gutfinger [11]. Each extract (1.0 mL) was mixed with 1.0 mL of 2% Na2CO3, and then the mixture was allowed to stand at room temperature for 3 min. After the addition of 0.2 mL of 50% Folin–Ciocalteu reagent, the reaction was kept for 30 min in the dark room, followed by centrifugation at 13,400×g for 10 min. The absorbance of supernatant was measured at 750 nm by using a spectrophotometer (UV-1601, Shimadzu Co., Kyoto, Japan), and TPC were expressed as mg gallic acid equivalents (GAE) per 100 mg extracts.
DPPH radical scavenging activity (RSA)
To determine the DPPH RSA of the extracts, the extract (0.1 mL) was mixed with 0.9 mL of 0.041 mM DPPH dissolved in ethanol for 30 min, and then its absorbance was measured at 517 nm using a spectrophotometer (UV-1601, Shimadzu) [12]. Ascorbic acid was used as a positive control and all tests were carried out in triplicate. RSA was expressed as percentage inhibition and was calculated by the following formula:
where Acontrol is the absorbance of the control reaction (the mixture of 0.1 mL of DMSO and a solution of 0.9 mL of DPPH was used as the control), and Asample is the absorbance of the reaction under the presence of extracts.
ABTS RSA
The ABTS RSA was modified as described by Re et al. [13]. Each extract (0.1 mL) was mixed with sodium phosphate buffer (125 μL, 0.1 M, pH 5.0) and hydrogen peroxide (10 μL, 10 mM), and then pre-incubated at 37 °C for 5 min. After pre-incubation, ABTS (15 μL, 1.25 mM, in 50 mM phosphate–citrate buffer, pH 5.0) and peroxidase (30 μL, 10 unit/mL) were added to the mixture and then it was incubated at 37 °C for 10 min. The absorbance was measured at 405 nm using a multiplate reader (Sunrise RC/TS/TS Color-TC/TW/BC/6Filter; Tecan Austria GmbH, Grdig, Austria). Ascorbic acid was used as a positive control and all tests were carried out in triplicate. The ABTS RSA was calculated by the following formula:
where Asample is the absorbance in the presence of extracts and Acontrol is the absorbance of the control reaction (DMSO was used instead of sample).
Cell viability
The effects of CCT extracts treatment on cell viability were evaluated using 3T3-L1 preadipocytes with previously reported MTT assay [14] with slight modification. After reaching confluency in 100 mm culture dish, 3T3-L1 preadipocytes were treated with 10, 50, 100, and 200 μg/mL CCT extracts for 24 h and the DMEM medium containing 10%(v/v) BCS and 100 unit/mL penicillin–streptomycin cocktail was removed. Then, 1 mL of MTT-medium (DMEM including 0.2 mg/mL MTT) was added to 3T3-L1 preadipocytes in 12-well culture plate, for an additional 1 h in CO2 incubator (Thermo Forma Steri Cycle 370; Thermo Scientific, Fremont, USA). After 1 h of incubation, absorbance values were measured at a wavelength of 570 nm using a microplate reader (VersaMax; Molecular Devices, Sunnyvale, CA, USA). Cell viability was calculated as a percentage of viability versus untreated cells.
3T3-L1 cell culture and adipocytes differentiation
3T3-L1 cells were purchased from the Korean Cell Line Bank (KCLB, Seoul, Korea). The 3T3-L1 cells were cultured in DMEM supplemented with 10%(v/v) BCS and 100 unit/mL penicillin–streptomycin cocktail at 37 °C in a 5% CO2 incubator. The DMEM medium was changed every 2 days. The 3T3-L1 preadipocytes were treated with each concentration of CCT extracts during day −2 to day 6. Two days after reaching confluence in 12-well culture plate, designated as day 0, the 3T3-L1 cells were cultured with differentiation medium (DM) supplemented with hormonal cocktail of 500 μM 3-isobutyl-1-methylxanthine (IBMX), 5.2 μM dexamethasone (DEX), and 167 nM insulin. After differentiation, the DM medium was replaced with post-differentiation medium (Post-DM) containing 167 nM insulin for another 2 days. Thereafter, the cells were cultured in normal DMEM and the medium was changed every 2 days.
Oil red O (ORO) staining
Differentiated 3T3-L1 adipocytes in 12-well culture plate were washed using PBS (phosphate buffered saline, pH 7.2) and the cells were fixed with 3.7%(v/v) formaldehyde for 30 min at 25 °C. Formaldehyde fixed-3T3-L1 adipocytes were washed 3 times with tap water and the mature 3T3-L1 adipocytes were then stained with 3 mg/mL ORO solution dissolved in isopropanol for 15 min at 25 °C. After 15 min, the plates were washed three times with tap water, cells were photomicrographed. The ORO stained lipid droplets were dissolved in 300 μL DMSO and transferred to a 96-well clear plate. The absorbance of each well was quantified using a microplate reader at 510 nm.
Statistical analysis
All data are presented as means ± SDs. Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA). The significant differences between groups was assessed with one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test or Student’s t-test. A p value < 0.05 was considered to indicate statistical significance.
Results and discussion
Antioxidant activity of the extracts
The TPC of the CCT nutshell extract was relatively higher than that of the kernel extract (Table 1). The TPC of the ME of CCT nutshells was 71.38 mg gallic acid equivalent (GAE)/100 mg extract, while that of the CCT nut kernels was 10.56 mg GAE/100 mg extract, implying that phenolic compounds constituted approximately 71.38 and 10.56% (w/w), respectively, of the extracts. The TPC of the WE of CCT nutshells and kernels was 17.44 mg GAE/100 mg extract and 9.27 mg GAE/100 mg extract, respectively. The phenolic compounds in the shells of CCT nuts might play a role in protecting the kernels (embryos) against environmental changes. Additionally, the TPC of the ME was higher than that of the WE, indicating that methanol-soluble phenolics are more prevalent than water-soluble ones in CCT nuts. While there is a dearth of information on the phytochemical profile of CCT, we found that the WE and ME of the kernels of CCS, a plant that is taxonomically very similar to CCT, contained 27.69 and 10.30 mg % phenolic compounds, respectively [2]. Similarly, chestnut shells have been reported to contain high amounts of phenolics, with the TPC of the WE and ME being 55.8 and 32.5 g GAE/100 g extract, respectively [15]. Acorn shells have also been reported to contain high amounts of phenolics, with the TPC of the WE and ME being 375.96 and 288.01 mg GAE/g extract, respectively [16].
Table 1.
Total phenolic contents of extracts from nuts of Castanopsis cuspidate var. thunbergii (unit: mg GAE/100 mg extract)
| Sample | Kernel | Shell |
|---|---|---|
| WE | 9.27 ± 0.66b | 17.44 ± 1.36a, y |
| ME | 10.56 ± 0.82b | 71.38 ± 2.57a, z |
Data represents the mean ± SD values (n = 3) obtained from three individual experiments
a,bDifferent letters within a row indicate significant differences at p < 0.05 by Duncan’s multiple range test
y,zDifferent letters within a column indicate significant differences at p < 0.05 by Duncan’s multiple range test
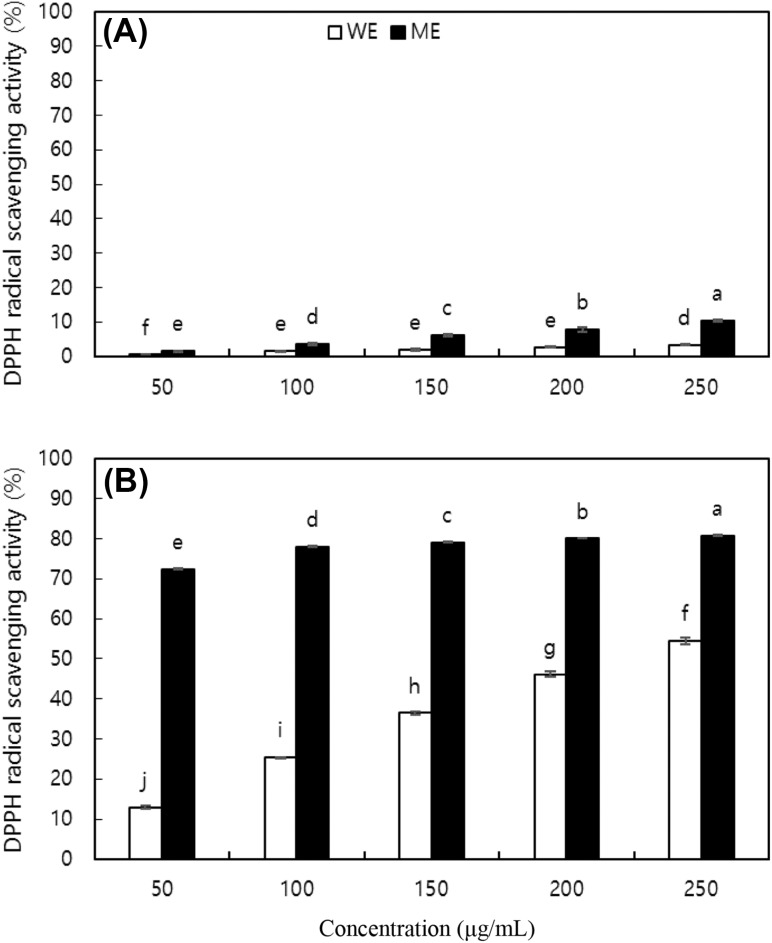
The antioxidant activity of CCT extracts was evaluated by estimating their free radical scavenging activity (RSA). The WE and ME of CCT nut kernels and shells (50–250 μg/mL) scavenged the DPPH radicals in a concentration-dependent manner (Fig. 1). The DPPH RSA of shell extracts was higher than that of kernel extracts. Similarly, the DPPH RSA of the ME was higher than that of the WE, regardless of the part. At 100 μg/mL, the WE and ME of the CCT kernels scavenged 1.60 and 3.66% DPPH, respectively, while those of the shell scavenged 25.37 and 78.03% DPPH, respectively. In comparison, the DPPH RSA of ascorbic acid, a positive control, was 56.71% at 40 μg/mL. Further, the DPPH RSA of the WE of the outer shell of Portuguese chestnuts was reported to be 93.8% at 100 μg/mL [17]. Thus, CCT nuts and their shells in particular showed strong antioxidant activity, considering that the extracts used in this study were a crude mixture of a variety of compounds. The IC50 values of the WE and ME of CCS nut kernels were 869.67 and 155.00 μg/mL, respectively [2].
Fig. 1.
DPPH radical scavenging activities (RSA) of water extract (WE) and methanol extract (ME) from (A) kernel and (B) shell of nuts of Castanopsis cuspidate var. thunbergii. Each value is mean ± SD. Values with different letters on the bars in each figure are significantly different by Duncan’s multiple range test at p < 0.05
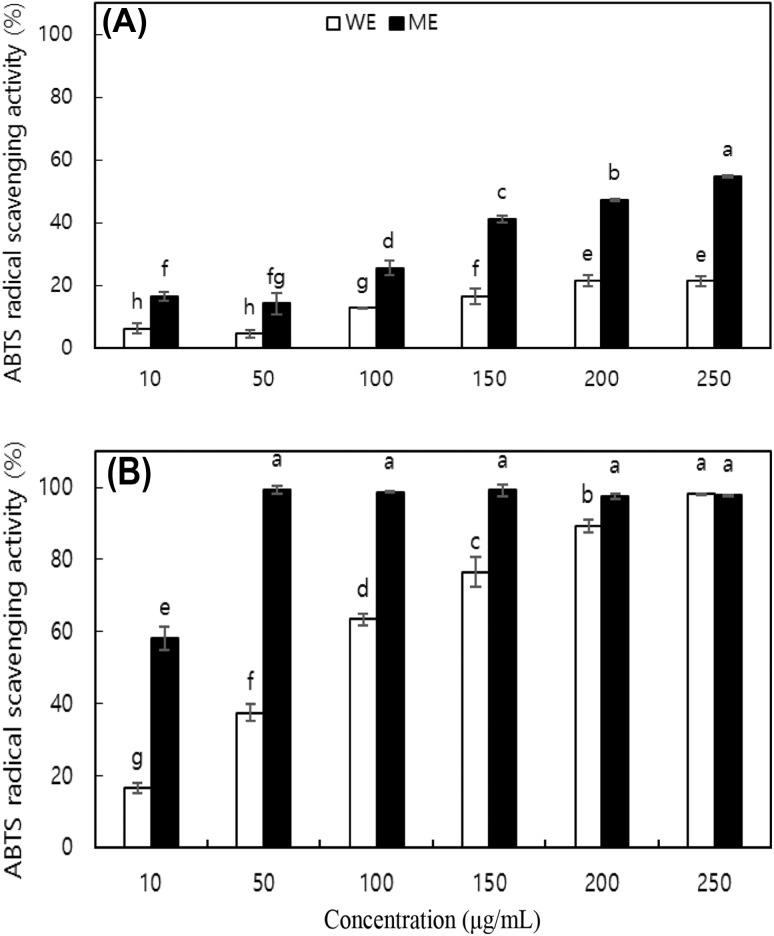
The ABTS RSA of the extracts was also determined. All extracts except the ME of the shells of CCT nuts scavenged ABTS in a dose-dependent manner (Fig. 2). The ME of shells exhibited saturation kinetics with respect to the ABTS RSA. While the DPPH and ABTS RSAs of the extracts followed the same trend, the scavenging activity against ABTS was stronger than that against DPPH. Ascorbic acid, the positive control, scavenged 70.94% of the ABTS radicals at 20 μg/mL. The high purity of ascorbic acid may have contributed to the significantly stronger activity. The difference in the scavenging activity of the extracts against DPPH and ABTS may be due to differences in the nature of the free radicals, as DPPH is uncharged and stable and ABTS is cationic. Alternatively, the difference may be due to differences in experimental selectivity, as ABTS assays measure the activity of both hydrophilic and hydrophobic antioxidants, while DPPH assays measure only the hydrophobic antioxidant activity [18]. Therefore, some phenolic compounds that show strong ABTS RSA are undetectable in a DPPH assay [19].
Fig. 2.
ABTS radical scavenging activities (RSA) of water extract (WE) and methanol extract (ME) from (A) kernel and (B) shell of nuts of Castanopsis cuspidate var. thunbergii. Each value is mean ± SD. Values with different letters on the bars in each figure are significantly different by Duncan’s multiple range test at p < 0.05
Anti-adipogenic activity
The effect of CCT nut kernel and shell extracts on the viability of 3T3-L1 preadipocytes was examined by the MTT assay. The cells were treated with 10, 50, 100, and 200 μg/mL of the extracts for 24 h. There was no change in the viability of the 3T3-L1 preadipocytes that were treated with up to 200 μg/mL of the ME of CCT nut kernels and shells for 24 h (Table 2). However, the viability was significantly decreased (p < 0.05) upon exposure to 200 μg/mL of the WE of CCT nut kernels and shells and 10 μg/mL of the WE of CCT shells alone, compared with the control (untreated cells) viability. The cytotoxicity observed upon treatment with 10 μg/mL of the WE of CCT nut shells needs to be investigated further. Interestingly, we observed that the 50 μg/mL of the WE (CCT nut kernels) and 200 μg/mL of the ME (CCT nut shells) significantly increased the 3T3-L1 viabilities when compared with the control cells. The previous study showed the selenite possessed cytotoxic ambiguity depending on its concentration range [20] and another research suggest that increased cell proliferation is closely associated with Rex1 expression [21]. Further study would be focused that the elucidating molecular mechanisms of enhanced 3T3-L1 viabilities by CCT extract treatments. In this study, the WE and ME prepared from the kernels and shells of CCT nuts were chosen for further experiments at concentrations up to 100 μg/mL, as this was observed to be a relatively safe concentration range for 3T3-L1 preadipocytes.
Table 2.
Effect of CCT extracts treatment on the 3T3-L1 viability (Unit: % of control)
| Extract | Concentration (ug/mL) | Kernel | Shell |
|---|---|---|---|
| WE | 10 | 95.91 ± 7.10 | 78.08 ± 4.52* |
| 50 | 119.26 ± 10.60* | 86.67 ± 6.21 | |
| 100 | 95.42 ± 6.27 | 90.43 ± 5.47 | |
| 200 | 82.32 ± 6.23* | 79.12 ± 4.68* | |
| ME | 10 | 84.48 ± 5.60 | 102.92 ± 7.20 |
| 50 | 85.38 ± 4.26 | 96.23 ± 6.45 | |
| 100 | 99.35 ± 4.33 | 106.12 ± 6.80 | |
| 200 | 89.81 ± 5.64 | 136.16 ± 5.62* |
Corresponding letters indicate significant differences based on Student’s t-test (* p < 0.05)
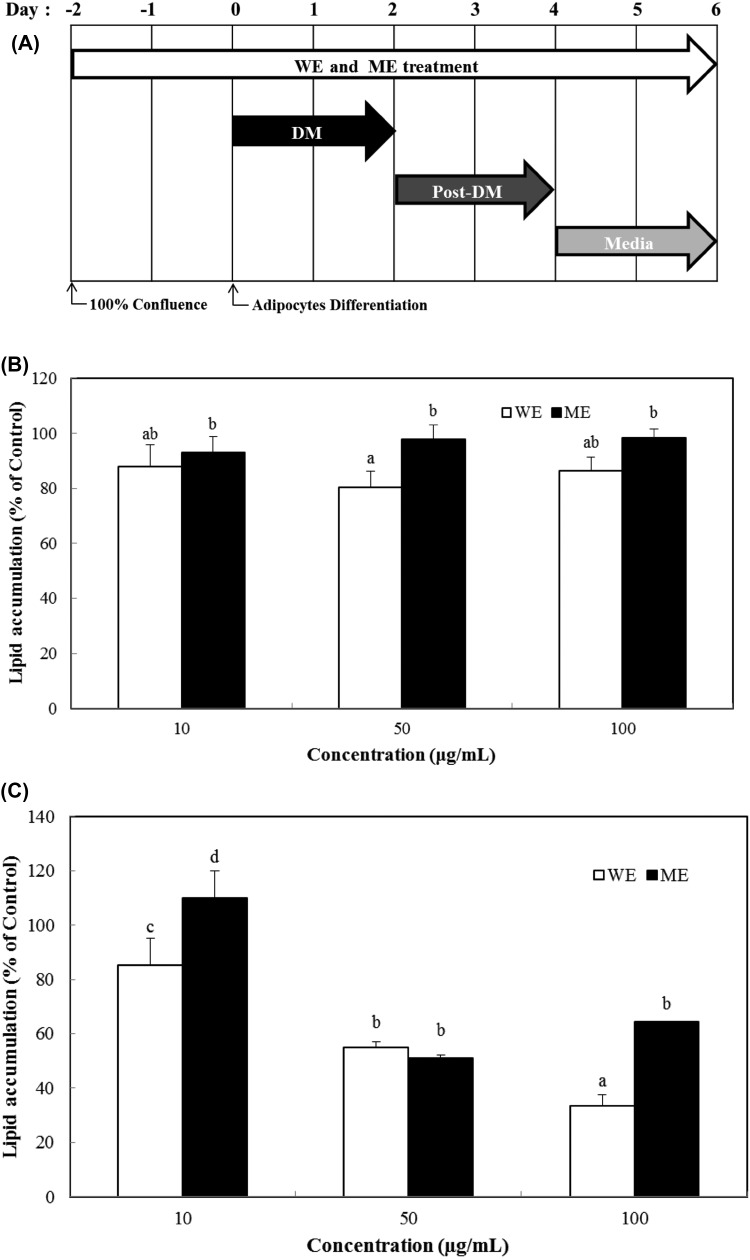
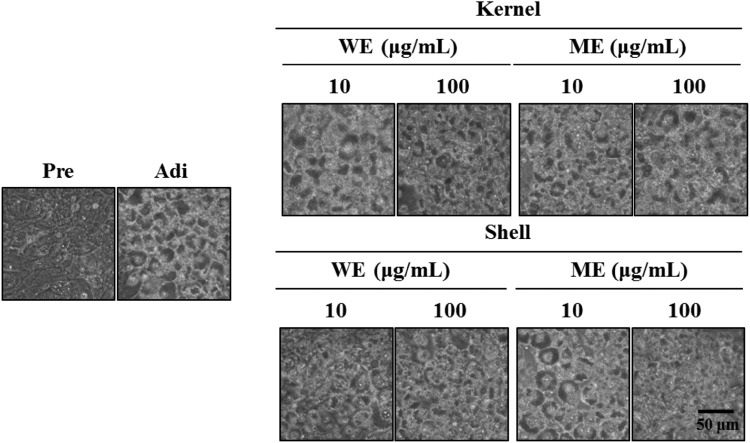
The 3T3-L1 preadipocytes were exposed to differentiation medium to induce adipogenesis in the presence or absence of 10, 50, and 100 μg/mL of the WE and ME of CCT nut kernels and shells, in order to determine whether the extracts could modulate adipogenesis. The effect of the extracts on adipogenesis was investigated 6 days after adipocyte differentiation by oil red O (ORO) staining (Fig. 3A). The inhibition of 3T3-L1 adipogenesis by the WE was stronger than that by the ME (Fig. 3B, C). The treatment with 10, 50, and 100 μg/mL of the WE of CCT nutshells inhibited 3T3-L1 adipogenesis by 85.15, 54.87, and 33.37%, respectively. Similarly, treatment with 10, 50, and 100 μg/mL of the ME of CCT nutshells suppressed the adipogenesis by 109.92, 51.15, and 64.30%, respectively. The CCT nutshell extracts suppressed 3T3-L1 adipogenesis more effectively than the kernel extracts (Fig. 3B, C). These results were correlated with decreased lipid droplet formation (Fig. 4), as microscopic observation revealed that the intracellular lipid droplet formation was dramatically inhibited by the WE and ME of the CCT nut kernels and shells. Collectively, these results demonstrate a CCT nut extract-mediated inhibition of 3T3-L1 adipogenesis with no cytotoxic effects.
Fig. 3.
Effects of CCT extracts treatment on the adipogenesis in 3T3-L1 cells. Scheme of CCT (WE and ME) treatment and 3T3-L1 adipocytes differentiation (A). Lipid accumulation levels of 3T3-L1 adipocytes treated with WE and ME prepared from kernel (B) and shell (C) by ORO staining at day 6. Each value was expressed by mean ± SD. Values with different letters on the bars in each figure indicate significant differences by Duncan’s multiple range test at p < 0.05
Fig. 4.
Inhibitory effects of CCT extracts treatment on the number and size of lipid droplets in ORO-stained 3T3-L1 cells at day 6. Pre, preadipocytes; Adi, mature 3T3-L1 adipocytes (Day 6)
Obesity, which is an abnormal accumulation of body fat caused by an increase in the size and number of adipocytes, has become a serious public health problem globally [22]. Several natural plant extracts have been reported to have anti-adipogenic activities with their bioactive components playing important roles in the inhibition of adipogenesis [23]. Recent studies have reported the anti-adipogenic activities of plant-based materials such as amur grape roots (Vitis amurensis Ruprecht), chocolate vine leaves (Akebia quinata D.), and black ginseng [24, 25], which indicate the immense potential of plants as a source of antiobesity compounds. The increased lipid accumulation in 3T3-L1 adipocytes has been observed to be correlated with elevated ROS levels which indicate that the ROS production is closely linked with lipid accumulation in adipocytes [26]. Moreover, fucoxanthin and methanolic extracts of certain wild Korean herbs have been reported to effectively inhibit both lipid accumulation and elevation in ROS levels [27, 28]. Additionally, the increase in ROS production during adipocyte differentiation has been suggested to occur owing to the activation of peroxisome proliferator-activated receptor gamma (PPARγ) [29]. These studies indicate that enriching the diet with antioxidants and anti-adipogenic compounds is an effective dietary strategy for maintaining human health and preventing obesity. Our study showed that the CCT nut kernels and shells extracts exhibited strong antioxidant and anti-adipogenic activity, however, they showed different functional patterns in antioxidant activity using DPPH assays and anti-adipogenic activities depending on their extraction solvent and part of CCT nut. Further study would be conducted in the elucidating phytochemical profile of CCT kernels and shells extracts by different extraction solvent and major bioactive components in contribution of antioxidant and anti-adipogenic activities respectively.
Adipogenic hormones such as insulin, drugs such as 3-isobutyl-1-methylxanthine (IBMX) and dexamethasone (DEX), and several growth factors affect the induction of transcription factor expression in 3T3-L1 adipocytes. The differentiation of preadipocytes into adipocytes is controlled by the adipogenic transcriptional cascade comprised of CCAAT/enhancer-binding protein (C/EBP), PPARγ, and adipocyte lipid-binding protein (aP2), which promote the expression of adipose-specific genes, such as lipoprotein lipase (LPL) and fatty acid synthase (FAS), to trigger the synthesis of fatty acids and triglycerides [30, 31]. The WE of the inner shells of chestnuts has been reported to demonstrate anti-adipogenic activity at concentrations below 100 μg/mL by suppressing the triglyceride content in 3T3-L1 adipocytes [32]. Similarly, blueberry peel extracts have been observed to exhibit DPPH RSA and suppress the expression of aP2 and FAS by dose-dependently suppressing C/EBPα/β and PPARγ expression in 3T3-L1 adipocytes [33]. Thus, our results and previous studies on the anti-adipogenic potential of chestnut [8] and blueberry peel extracts [34] indicate a positive correlation between the antioxidant and anti-adipogenic activities of the CCT nut extracts [8, 34].
Although the specific bioactive constituents of the CCT nut extracts were not revealed in this study, our results strongly suggest that the extracts contain several phenolic compounds such as gallic acid, castalagin, and acutissimin B based on chestnut phenolic components [35]. Our results also indicate that the CCT nutshell extracts possess potent antioxidant and anti-adipogenic activities compared to the kernel extracts. Collectively, our results demonstrate that extracts prepared from the shells of CCT nuts have immense potential as an ingredient for the development of functional foods for obesity prevention. Future studies should therefore focus on determining the in vivo anti-adipogenic effects of the CCT nutshell extracts in animals and molecular mechanisms underlying the observed effects.
Acknowledgements
This work was supported by Kyungnam University Foundation Grant, 2017.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kang KA, Lee KH, Zhang R, Piao MJ, Kang MJ, Kwak YS, Yoo BS, You HJ, Hyun JW. Protective effects of Castanopsis cuspidate through activation of ERK and NF-kappaB on oxidative cell death induced by hydrogen peroxide. J. Toxicol. Environ. Health A. 2007;70:1319–1328. doi: 10.1080/15287390701429315. [DOI] [PubMed] [Google Scholar]
- 2.Lee SJ, An KW, Choi TS, Jung HS, Moon JH, Park KH. Component analysis and antioxidative activity of Castanopsis cuspidata var. sieboldii nut. Korean J. Food Preserv. 17: 139–144 (2010)
- 3.Kim JY, Yoon WJ, Yim EY, Park SY, Kim YJ, Song G. Antioxidative and antimicrobial activities of Castanopsis cuspidata var. sieboldii extracts. Korean J. Plant Res. 24: 200–207 (2011)
- 4.Ko YJ, Song SM, Hyun WC, Yang SK, Song CK, Lee DS, Yoon WJ. Anti-inflammatory effect of Castanopsis cuspidata extracts in murine macrophage RAW 264.7 cells. Korean J. Plant Res. 27: 439–446 (2014)
- 5.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 6.Ito N, Hirose M, Fukushima G, Tauda H, Shira T, Tatematsu M. Studies on antioxidant. Their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem. Toxicol. 1986;24:1071–1081. doi: 10.1016/0278-6915(86)90291-7. [DOI] [PubMed] [Google Scholar]
- 7.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 8.Youn UY, Shon MS, Kim GN, Katagiri R, Harata K, Ishida Y, Lee SC. Antioxidant and anti-adipogenic activities of chestnut (Castanea crenata) byproducts. Food Sci. Biotechnol. 2016;25:1169–1174. doi: 10.1007/s10068-016-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon BF, Perez-Ilzarbe J, Hernandez T, Gomez-Cordoves C, Estrella I. Importance of phenolic compounds for the characterization of fruit juices. J. Agr. Food Chem. 1992;40:1531–1535. doi: 10.1021/jf00021a012. [DOI] [Google Scholar]
- 10.Custódio L, Patarra J, Alberício F, Neng NR, Nogueira JMF, Romano A. Extracts from Quercus sp. acorns exhibit in vitro neuroprotective features through inhibition of cholinesterase and protection of the human dopaminergic cell line SH-SY5Y from hydrogen peroxide-induced cytotoxicity. Ind. Crop. Prod. 2013;45:114–120. [Google Scholar]
- 11.Gutfinger T. Polyphenols in olive oil. J. Am. Oil. Chem. Soc. 1981;58:966–968. doi: 10.1007/BF02659771. [DOI] [Google Scholar]
- 12.Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam KC, Ahn DU. Effect of far-infrared radiation on the antioxidant activity of rice hulls. J. Agr. Food Chem. 2003;51:4400–4403. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- 13.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evan C. Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Vázquez G, Fontenla E, Santos J, Freire MS, González-Álvarez J, Antorrena G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crop Prod. 2008;28:279–285. doi: 10.1016/j.indcrop.2008.03.003. [DOI] [Google Scholar]
- 16.Youn UY, Shon MS, Kim GN, Katagiri R, Harata K, Kamegai M, Ishida Y, Lee SC. Antioxidant and anti-adipogenic activities of acorn shells. Food Sci. Biotechnol. 2016;25:1183–1187. doi: 10.1007/s10068-016-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barreira JCM, Ferreira ICFR, Oliveira MBPP, Pereira JA. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008;107:1106–1113. doi: 10.1016/j.foodchem.2007.09.030. [DOI] [Google Scholar]
- 18.Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011;24:1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 19.Wang M, Li J, Rangarajan M, Shao Y, LaVoie EJ, Huang TC, Ho CT. Antioxidative phenolic compounds from sage (Salvia officinalis) J. Agr. Food Chem. 1998;46:4869–4873. doi: 10.1021/jf980614b. [DOI] [Google Scholar]
- 20.Kim CY, Kim GN, Wiacek JL, Chen CY, Kim KH. Selenate inhibits adipogenesis through induction of transforming growth factor-β1 (TGF- β1) signaling. Biochem. Bioph. Res. Co. 2012;426:551–557. doi: 10.1016/j.bbrc.2012.08.125. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Lee MR, Jee MK, Kang SK. IFATS collection: selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells. 2008;26:2724–2734. doi: 10.1634/stemcells.2008-0184. [DOI] [PubMed] [Google Scholar]
- 22.Barness LA, Opitz JM, Gilbert-Barness E. Obesity: genetic, molecular, and environmental aspects. Am. J. Med. Genet. 2007;143A:3016–3034. doi: 10.1002/ajmg.a.32035. [DOI] [PubMed] [Google Scholar]
- 23.Jeon YS, You YH, Jun WJ. Anti-obesity effects of extracts from young Akebia quinata D. leaves. J. Korean Soc. Food Sci. Nutr. 43: 200–206 (2014)
- 24.Park HS, Kim GH. Inhibitory effects of Sasa borealis on mechanisms of adipogenesis. J. Korean Soc. Food Sci. Nutr. 2013;42:837–843. doi: 10.3746/jkfn.2013.42.6.837. [DOI] [Google Scholar]
- 25.Park HJ, Kim AJ, Cheon YP, Lee MS. Anti-obesity effects of water and ethanol extracts of black ginseng. J. Korea Soc. Food Sci. Nutr. 2015;44:314–323. doi: 10.3746/jkfn.2015.44.3.314. [DOI] [Google Scholar]
- 26.Liu GS, Chan EC, Higuchi M, Dusting GJ, Jiang F. Redox mechanisms in regulation of adipocyte differentiation: Beyond a general stress response. Cells. 2012;1:976–993. doi: 10.3390/cells1040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YJ, Kim DB, Lee JS, Cho JH, Kim BK, Choi HS, Lee BY, Lee OH. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules. 2013;18:12937–12950. doi: 10.3390/molecules181012937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo MJ, Seo YJ, Pan CH, Lee OH, Kim KJ, Lee BY. Fucoxantin suppresses lipid accumulation and ROS production during differentiation in 3T3-L1 cells. Phytother. Res. 2016;30:1802–1808. doi: 10.1002/ptr.5683. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Zhang Y, Lu W, Liu K. Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PloS One. 2015;10:e0120629. doi: 10.1371/journal.pone.0120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JW, Kim SS. Ginsenside Rc promotes anti-adipogenic activity on 3T3-L1 aidpocytes by down-regulating C/EBPα and PPARγ. Molecules. 2015;20:1293–1303. doi: 10.3390/molecules20011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim GS, Park HJ, Woo JH, Koh PO, Min W, Ko YG, Kim CH, Won CK, Cho JH. Citrus aurantium flavonoids inhibit adipogenesis through the Akt signaling pathway in 3T3-L1 cells. BMC Complemnt. Altern. Med. 2012;12:31–40. doi: 10.1186/1472-6882-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SG. Effects of chestnut inner shell extract on 3T3-L1 preadipocyte differentiation. Korean J. Orient. Physiol. Pathol. 2010;24:266–271. [Google Scholar]
- 33.Li C, Zhou L. Inhibitory effect 6-gingerol on adipogenesis through of the Wnt/β-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol. In Vitro. 2015;30:394–401. doi: 10.1016/j.tiv.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Song Y, Park HJ. Kang SN, Jang SH, Lee SJ, Ko YG, Kim GS, Cho JH. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PloS One. 2013;8:e69925. doi: 10.1371/journal.pone.0069925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasconcelos MCBM, Bennett RN, Quideau S, Jacquet R, Rosa EAS, Ferreira-Cardoso JV. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crop. Prod. 2010;31:301–311. [Google Scholar]