Abstract
The ability of lactic acid bacterial starter cultures to produce gamma-aminobutyric acid (GABA) during sausage fermentation was studied. Among 305 strains of lactic acid bacteria isolated from kimchi samples, 11 strains were selected as starter candidates based on the following criteria: growth speed, pH lowering ability, and biogenic amine productivity including GABA-producing activity. During in vitro tests, the Y8 (Lactobacillus brevis), O52, and KA20 strains produced 39.00 ± 1.36, 49.73 ± 3.80, and 64.59 ± 0.61 mg/kg of GABA, respectively. Interestingly, although isolate Y8 showed low productivity in vitro, the GABA content it produced during in situ tests (61.30 ± 2.61 mg/kg) was similar to that produced by isolate PM3 (L. brevis) used as positive control (69.64 ± 2.20 mg/kg). Therefore, isolate Y8 was selected as the best functional starter culture for the production of fermented sausage because it exhibited rapid growth, safety, and abundant GABA productivity.
Keywords: Gamma-aminobutyric acid (GABA), Fermented sausage, Functional starter culture, Lactic acid bacteria (LAB)
Introduction
Fermented sausage is defined as a mixture of comminuted fat and lean meat, salt, nitrate and/or nitrite, sugar, and spices (mostly oregano and black pepper), which are stuffed into casings, subjected to lactic acid fermentation, and then allowed to dry [1]. As lactic acid bacteria (LAB) play an important role in fermenting sausages, various LAB species have been identified as regular catalysts in the process. The history of starters begins with the selection of yeasts for the brewing of beer, following the introduction of LAB for manufacturing dairy products. For producing fermented sausages, various starter cultures such as genus Lactobacillus (L. plantarum, L. pentosus, and L. curvatus) and Pediococcus (P. pentosaceus and P. acidilactici) can be used with the starter cultures available in the market [1, 2]. Most studies that have examined starter cultures for the production of fermented sausage have focused on the microbiological, physiochemical, and technological aspects of fermentation, with only a few of them focusing on starter cultures with gamma-aminobutyric acid (GABA) activity.
GABA is a nonprotein amino acid that is widely distributed among microorganisms, plants, and animals [3]. Since it has the potential to be used as a bioactive ingredient in pharmaceuticals and functional foods, the development of functional foods containing GABA has attracted significant interest [4]. Various foods and plants have been reported to contain GABA-producing LAB in large amounts, such as L. paracasei and L. plantarum from Italian cheeses [5], L. brevis from alcohol distillery lees [6], L. senmaizukei from Japanese pickle [7], and Lactococcus lactis from cheese starters [8]. In addition, several GABA-producing LAB, including L. buchneri and L. sakei, have been isolated from kimchi, a traditional Korean fermented food [9, 10].
Kimchi is a functional food that contains various ingredients and fermentation products such as dietary fiber, vitamins, ginger, garlic, salted fish, lactic acid, and LAB [11]. In addition, bioactive materials such as various organic acids, GABA, and ornithine are formed in kimchi as a result of the active growth of LAB. Some strains of LAB catalyze the decarboxylation of glutamate, resulting in the release of GABA and CO2 as the end products [12]. Thus, kimchi is expected to contain a large amount of GABA owing to the abundance of LAB in it. Functional starter cultures offer an additional functionality(lower cholesterol, bacteriocins, GABA, conjugated linoleic acids, decarboxylases and oligosaccharides) compared with classical starter cultures and provide a way of improving and optimizing the food fermentation process and achieving tastier, safer, and healthier food products [2, 13]. Selecting strains with interesting properties for use as a new type of functional starter culture may lead to improvements in the fermentation process and enhance the quality of the end product.
In this study, we established a method of selecting starter cultures with a functional property (GABA) through in vitro and in situ tests for using them in the production of fermented sausages. We selected isolate Y8 as the starter culture since it exhibited rapid growth, safety, and abundant GABA productivity it is the most suitable starter candidate.
Materials and methods
Samples and bacterial isolation
A total of six kimchi samples (three types: Korean Cabbage Kimchi, Nabak Kimchi, and Dongchimi) were purchased from retail outlets in Sejong City, Korea. The samples were serially diluted in a sterile saline solution; plated onto de Man, Rogosa and Sharpe (MRS) agar (Difco, Le Pont de Claix, France); supplemented with 0.0006% bromocresol purple (BCP, Sigma, USA); and incubated for 48 h. Then, the serially diluted sterile saline solution was spread on plate count agar (PCA) at 37 °C for 48 h. The number of microorganisms was expressed as the log10 of colony forming units (CFU) per g. In the first experiment, the detection of GABA in the kimchi samples was preliminarily performed, and kimchi samples that had a high GABA content were intensely isolated from the other samples; 305 strains of LAB were randomly picked from the isolated primary colonies of each sample and maintained on MRS agar slant for further identification. The stock culture of each isolate was maintained at −70 °C in MRS broth with 60% glycerol (Merck, Darmstadt, Germany).
Screening of LAB producing gamma-aminobutyric acid (GABA)
The LAB in the 305 strains were screened for the functionality of GABA as secondary selection. The high-GABA-producing L. brevis PM3 used in previous studies [19] was used as the positive control. Thin-layer chromatography (TLC) was performed at 37 °C for 24 h using a silica gel plate (Kieselgel 60, F254, 0.2 mm, Merck, Germany) to confirm GABA production in MRS broth containing 1% mono sodium glutamate (MSG) and 1% meat extract. The solvent system used n-butanol:acetic acid:water (3:2:1, v/v). The plate was spotted with 2 μl of culture supernatant, and the spotted plate was developed in the solvent. The plate was removed from the development chamber and dried. Amino-acid-containing compounds were visualized via spraying using ninhydrin, and the Rf values were measured.
Growth speed and acid production capacity
Based on the results, the selection criteria were established using a growth speed of more than 107 cells/mL in modified MRS broth with 1% meat extract and pH lowering activity to provide less than pH 5.0 at 37 °C for 24 h. In a previous study [19], L. brevis YG331 was used as the positive control owing to its high growth speed and ability to produce large amounts of acid.
16S rRNA gene sequence analysis
Genomic DNA was extracted from the bacterial cultures using the genomic DNA extraction kit (Intron Biotecnology Inc., Sungnam, Korea) according to the manufacturers’ instructions. Purified genomic DNA was used as the template DNA for polymerase chain reaction (PCR) amplification of the 16S rRNA gene with two universal primers (27F, 5′-AGAGTTTGATCCTGGCTCAG-3′; 1492R, 5′-GGTTACCTTGTTACGACTT-3′). The PCR condition was as follows: an initial denaturation at 95 °C for 15 min followed by 30 cycles of denaturation at 95 °C for 20 s, annealing at 50 °C for 40 s, extending at 72 °C for 1.5 min, and the final extension at 72 °C for 5 min. The identities of the sequences were determined using the BLAST of the National Center for Biotechnology Information (NCBI).
Analysis for the identification of the glutamate decarboxylase (GAD) gene
To identify strains that possess the GAD gene, genomic DNA was extracted from the bacterial cultures using the genomic DNA extraction kit (Intron Biotechnology Inc., Sungnam, Korea) according to the manufacturer’s instructions. Purified genomic DNA was used as the template DNA for the PCR amplification of the GAD gene with two specific primers (gad F, 5′-AGT CGC GTT GGT TGC ACT TAC GGT ATC CTA-3′; gad R, 5′- GTA ATG TAA CGG TGA GGT CTT GCC GTT CTG-3′). The PCR condition was as follows: an initial denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 2 min, extension at 72 °C for 1 min, and the final extension at 72 °C for 7 min.
Analysis of GABA
GABA contents were evaluated according to the AccQ • Tag method for amino acid analysis using a high-performance liquid chromatography (HPLC) system (Waters 515 series; Waters, Milford, MA, USA) equipped with a scanning Photodiode Array Detector (PDA) detector (Waters 2996, Waters). Samples were derived using amino quioly-N-hydroxysuccinimidylcarbonate (AQC, Waters). The derivatives were then separated using an AccQ • Tag™ column (3.9 × 150 mm, 4-µm particle size, Nova-Pak C18; Waters) at 35 °C. The mobile phase was composed of AccQ • Tag eluent A (Waters) and 60% aqueous acetonitrile (ACN, JTBaker, Holland). The elution flow rate was 1.0 mL/min. GABA contents were calculated using a commercial GABA standard based on a standard curve.
Detection of biogenic amine (BA) contents
BA contents in the bacterial cultures and fermented sausage were evaluated using the procedure developed by Bover-Cid and Ben-Gigirey [14, 15].
Manufacturing of fermented sausage
Fermented sausage was manufactured according to the following formulation: 200 g/kg lean beef, 600 g/kg lean pork, 200 g/kg pork back fat, 30 g/kg salt, 5 g/kg glucose, 0.07 g/kg NaNO2, bacterial cultures, and spices. The selected strains were incubated in MRS broth for 24 h at 30 C, subsequently, the resulting 7 log CFU/g of culture was added to sausages for fermentation. In addition, commercial starter cultures Staphylococcus carnosus and L. pentosus (Bacterferm™ C-P-77S, Chr. Hansen, Denmark) were added to raw sausage material to ensure color formation. Raw sausage material was stuffed into 40-mm-diameter fibrous casings and fermented for 1 day at 23 °C and at a relative humidity (RH) of 95%. This was followed by a gradual reduction in temperature (from 22 to 19 °C) and RH (from RH 94 to 89%) during the next seven days. After seven days of fermentation, the sausages were ripened for 35 days at 18 °C (RH 75%). Four different experimental series were prepared with each experimental strain.
Physicochemical measurements
Several properties of the fermented sausage samples were measured. Ten grams of the samples were homogenized in a stomacher with 90 mL of distilled water, and the pH of the homogenate was measured using a pH meter (Orion 3 star pH Benchtop; Thermo Scientific, Waltham, MA, USA). Water activity was measured using an electric hygrometer (Thermoconstanter, Novasina, Talstrasse, Switzerland).
Sensory evaluation
Sensory evaluation for the fermented sausage samples was performed by 10 panelists using a 7-level scoring method based on the organoleptic properties of olfaction, gustation, texture, juiciness, and overall evaluation.
Statistical analysis
All values of the experimental data were obtained in triplicate and analyzed using the Statistical Analysis System (SAS Institute Inc., Cary, NC). Multiple comparisons were made for all data using Duncan’s multiple-range tests at p < 0.05.
Results and discussion
Isolation and selection of starter candidates
Three hundred and five strains of LAB were primarily isolated from the kimchi samples. Starter candidates were screened through secondary selection based on growth speed, pH lowering ability, and safety (BA) in vitro. The candidate strains should be able to grow in sausage mix; thus, the isolates were incubated in the modified MRS broth with meat extract. The growth rate and pH lowering activity of all the starter candidates were similar to those of the positive control strain (L. brevis YG331) used herein. In the first experiment for primary selection, the GABA content in the kimchi samples was evaluated; the results are shown in Table 1. The GABA content in kimchi samples ranged from not detected (ND) to 52.77 mg/kg. A total of 305 strains were obtained as the first isolates from sample C (Korean Cabbage Kimchi) and sample F (Korean Cabbage Kimchi), which had the highest GABA content among the six samples. Out of the 305 LAB strains, the starter candidates of 11 strains were obtained using TLC. The Rf value of GABA was 0.41 on the TLC plate, while the Rf for glutamic acid was 0.39. The liquid culture of these strains was expressed as the same value. Subsequently, 11 LAB strains were selected in vitro owing to their fastest growth properties, lowest pH, highest safety, and the presence of functional properties for GABA production. The 11 selected strains showed a range of pH 4.92–5.13 after 12 h of incubation. The LAB count was 4.27–4.69 log CFU/mL. All strains showed a pH range of 4.30–4.43 after 24 h of incubation, and the LAB count was 7.01–7.16 log CFU/mL. The pH of isolate YG331 decreased to 4.40 after 12 h of incubation, and the viable cell count of LAB was 8.34 log CFU/mL. In addition, the value of pH decreased further to 4.00 after 24 h of incubation, and the LAB count was 8.40 log CFU/mL. Compared with the positive control strain (L. brevis YG331), the 11 isolates showed a rather low growth speed and pH lowering ability after 12 h of incubation in the modified MRS broth; moreover, the viable cell count (growth speed) of LAB was considerably low (Table 2).
Table 1.
GABA contents in commercial kimchi products
| Kimchi samples | GABA content (mg/kg) |
|---|---|
| A | 9.26 ± 7.23a |
| B | 17.6 ± 6.03 |
| C | 52.77 ± 0.52 |
| D | NDb |
| E | 27.69 ± 5.10 |
| F | 41.93 ± 3.26 |
A: Korean Cabbage Kimchi, B: Korean Cabbage Kimchi, C: Korean Cabbage Kimchi, D: Nabak Kimchi, E: Dongchimi, F: Korean Cabbage Kimchi
aResults are expressed as the mean ± standard deviation
b ND Not detected
Table 2.
pH and the number of lactic acid bacteria as starter candidates in modified MRS broth containing meat extract
| Experimental groups | 12 h | 24 h | ||
|---|---|---|---|---|
| pH | CFU1 | pH | CFU | |
| Control2 | 4.40 ± 0.01h3 | 8.34 ± 0.01a | 4.00 ± 0.01g | 8.40 ± 0.00a |
| Y2 | 5.13 ± 0.00a | 4.30 ± 0.03gf | 4.39 ± 0.01de | 7.06 ± 0.77b |
| Y8 | 5.01 ± 0.01cd | 4.27 ± 0.01g | 4.40 ± 0.01cde | 7.01 ± 0.66b |
| O8 | 4.98 ± 0.01de | 4.54 ± 0.01cd | 4.41 ± 0.01cb | 7.12 ± 0.00b |
| O55 | 5.04 ± 0.04cb | 4.59 ± 0.02cb | 4.41 ± 0.01cb | 7.09 ± 0.02b |
| H1 | 5.06 ± 0.04b | 4.65 ± 0.05b | 4.38 ± 0.01e | 7.08 ± 0.00b |
| H42 | 5.01 ± 0.00cd | 4.33 ± 0.07gf | 4.42 ± 0.00ab | 7.04 ± 0.03b |
| H41 | 4.96 ± 0.01ef | 4.33 ± 0.04gf | 4.40 ± 0.00cd | 7.11 ± 0.01b |
| H9 | 4.91 ± 0.01g | 4.39 ± 0.10ef | 4.38 ± 0.01e | 7.11 ± 0.00b |
| H46 | 5.01 ± 0.01cd | 4.44 ± 0.01de | 4.43 ± 0.00a | 7.16 ± 0.02b |
| KA20 | 5.01 ± 0.00cd | 4.69 ± 0.02b | 4.41 ± 0.01cb | 7.09 ± 0.03b |
| O52 | 4.92 ± 0.00gf | 4.44 ± 0.03e | 4.30 ± 0.00f | 7.13 ± 0.02b |
1 CFU Lactic acid bacteria; unit: log CFU/mL
2 Control Lactobacillus brevis YG331
3Results are expressed as the mean ± standard deviation
a–hSignifies that different letters within a row are significantly different at p < 0.05, as determined by Duncan’s multiple-range test
In meat fermentation, the LAB derived from the raw materials or the environment was responsible for both a low pH (4.6–5.9) and low lactic acid production resulting from the carbohydrate metabolism. In other words, the starter cultures for the production of fermented sausages should be appropriate in terms of the capacity for rapid growth and acidification [1]. However, since the isolates had overall low rates of acid production, it was difficult to use a single strain to perform fermentation. Therefore, the combined use of commercial starter cultures and the aforementioned isolates allowed for the in situ production of fermented sausages.
Identification of starter candidates
The 11 strains were identified using 16S rRNA gene sequence analysis. The isolated strains were identified as one strain of P. acidilactici, nine strains of L. brevis, and one strain of Weissella halotolerans. The taxonomical similarity for these 11 isolates was found to be 99%. According to previous reports [4, 16, 17], among the strains isolated from kimchi, L. brevis is a high GABA-producing microorganism; however, our studies have shown that P. acidilactici and Weissella halotolerans can also produce GABA.
Identification of the GAD gene
GABA-producing bacteria express the GAD enzyme by using a precursor glutamic acid, with the end product being GABA [12, 18]. The identification process of the GAD gene for the 11 strains isolated from the kimchi samples revealed that a band appeared at 1331 bp. The GAD gene in the L. brevis isolated from kimchi was confirmed with the same line at 1331 bp using the known GABA-producing L. brevis strain KCTC3498 and L. brevis isolate PM3 as positive control groups. A total of nine strains of the genus Lactobacillus including the isolates Y2, Y8, O8, O55, H1, H42, H41, H9, and H46 were selected. We inferred that the sequences in these strains are different from each other because the GAD gene was not identified in P. acidilactici isolate O52 and Weissella halotolerans isolate KA20. Therefore, further study should be conducted.
GABA content produced by LAB was found to be dependent on the conversion of glutamic acid
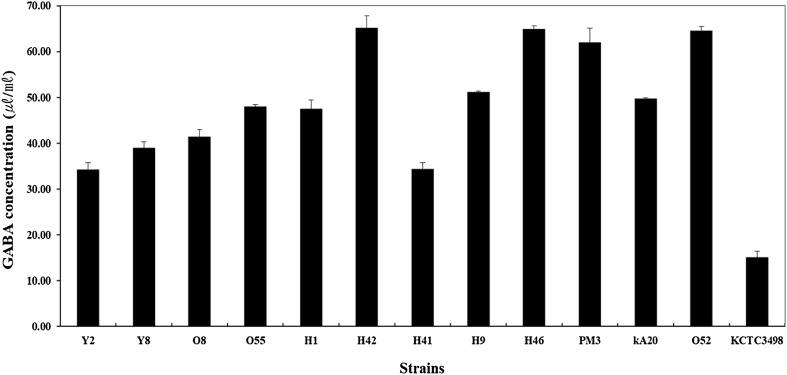
The GABA contents in the 11 isolated strains were quantitatively analyzed using HPLC with the positive control group of two pre-described strains. The positive control strains, isolate PM3, produced a high GABA content of 62.07 ± 0.83 μl/mL, whereas L. brevis KCTC3498 produced 15.09 ± 0.39 μl/mL. The GABA contents of the nine isolated L. brevis that were selected as the GABA-producing strains isolated from the kimchi samples were detected in the range of 34.25–64.91 μl/mL. In addition, the P. acidilactici O52 isolate produced 49.73 ± 0.61 μl/mL of GABA, and the Weissella halotolerans KA20 isolate produced 64.59 ± 3.80 μl/mL GABA. The conversion yields for isolates O8 and H41 were 99.55 and 73.79%, respectively. Finally, a total of 10 strains including isolate Y2 were selected, except for isolate H41 owing to a low conversion yield (Fig. 1).
Fig. 1.
GABA concentrations of the culture filtrates of the isolates grown in MRS broth supplemented with 1% MSG. Results are expressed as the mean ± standard deviation
Determination of BA production by starter culture candidates (in vitro)
The low levels of BA produced were well under the safe limits suggested by Taylor and Brink as follows. i.e., histamine, 50–100 mg/kg; tyramine, 100–800 mg/kg; ß-phenylethylamine, 30 mg/kg; total BA content, 1000 mg/kg. The measurement of the BA production capabilities revealed that isolate KA20 produced the highest level of tyramine at 17.29 ± 4.39 μl/mL among the 11 strains tested. The tyramine contents of the other strains were in the range of 4.02–16.37 μl/mL. As far as other amines were concerned, the contents were found to be lower than 5 μl/mL (data not shown).
Microbial and physicochemical properties of fermented sausages inoculated with starter candidates (in situ)
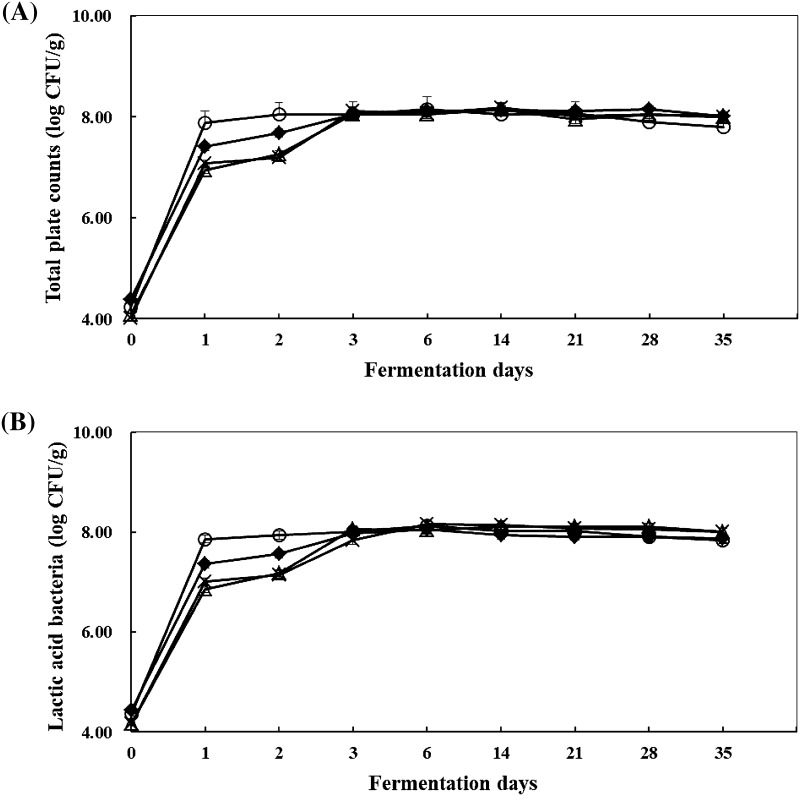
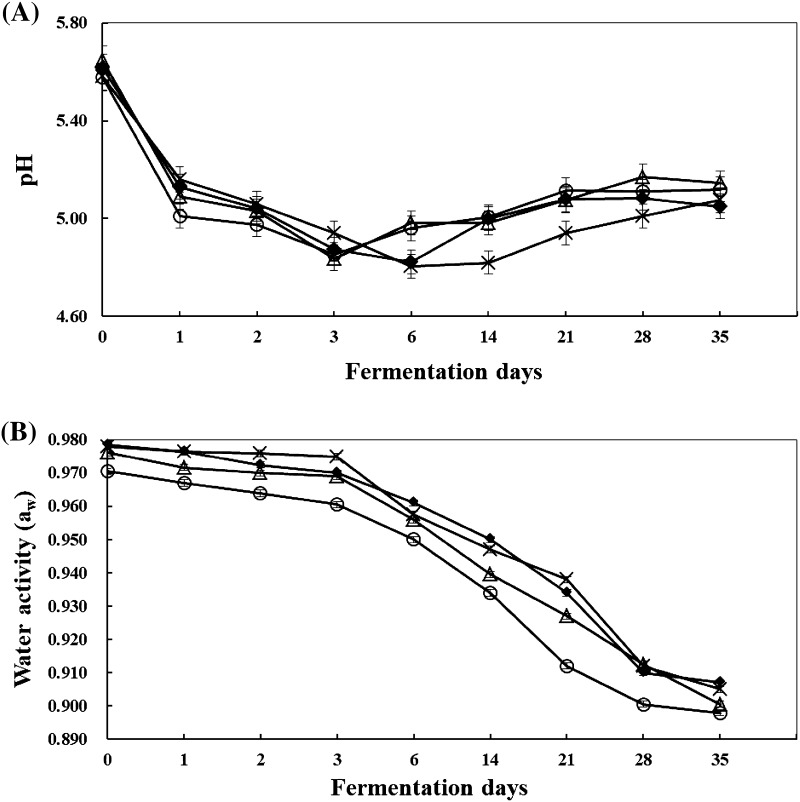
As shown in Fig. 2, the LAB count of fermented sausage inoculated with starter candidates PM3, Y8, and KA20 exhibited rapid growth for 24 h (1 day). However, O52 exhibited slower growth, exceeding 24 h to reach 7 log CFU/g. The concentration of LAB remained relatively stable in all sausages until the end of fermentation to reach a LAB count of 7.84–8.00 log CFU/g at in 35 days. The total viable cell count in all the experimental groups showed a growth pattern similar to that of the LAB count during fermentation. All experimental groups showed similar patterns of pH curves. The pH of all the fermented sausages were in the range of 5.58–5.65 at the initial stage; after six days, the pH reduced to 4.81–4.98 and then increased gradually until the end of fermentation. Water activity steadily decreased until the end of fermentation (Fig. 3).
Fig. 2.
Population of total plate count (A) and lactic acid bacteria (B) during the fermentation of sausage. Data are presented as the average of three independent trials. Bars indicate standard deviation. Symbols closed diamond, inoculated with PM3; open triangle, inoculated with O52; X-sign, inoculated with KA20; and open circle, inoculated with Y8
Fig. 3.
Changes in pH (A) and water activity (B) of during sausages fermentation. Data are presented as the average of three independent trials. Bars indicate standard deviation. Symbols closed diamond, inoculated with PM3; open triangle, inoculated with O52; X-sign, inoculated with KA20; and open circle, inoculated with Y8
BA contents of starter candidates (in situ)
Three isolated LAB were suitable for the production of fermented sausages because their BA production was within the safe levels. The US Food and Drug Administration reported a limit of 750–900 mg/kg total BA content in foods. The highest amount of tyramine was produced by isolate O52, i.e., 22.42 ± 1.10 mg/kg, and the tyramine contents produced by KA20 and Y8 strains were 18.85 ± 2.46 and 16.48 ± 3.30 mg/kg, respectively. Even when accounting for all BA measured, the highest total BA content produced by isolate KA20 was 104.99 ± 1.83 mg/kg, which is far below the limits of the prescribed BA content in foods (data not shown).
Suitability of GABA-producing lactic acid bacterial candidates (in situ)
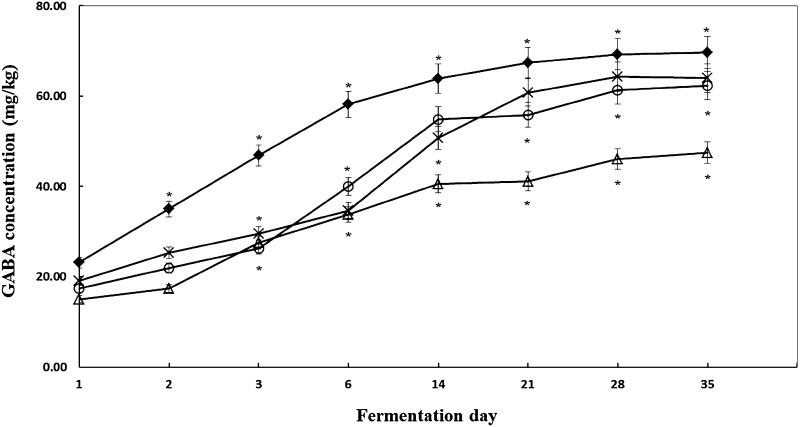
Although the isolates showed high GABA productivity in in vitro test, this was not always the case in in situ test. For example, isolate H46 produced 65 μl/mL of GABA under in vitro conditions; however, the in situ GABA content was detected to be 20.00 μl/mL. The isolates KA20, O52, and Y8 produced relatively high GABA content in the sausage system; however, low rates of acid production made it difficult to use a single strain to perform fermentation without the risk of contamination by pathogenic microorganisms. Therefore, the combined use of commercial starter cultures and the aforementioned isolates allowed for the in situ production of fermented sausages with high GABA productivity. The GABA content in the fermented sausages prepared with isolate PM3 as a positive control strain increased from 23.10 ± 0.74 mg/kg on the first day of fermentation to 69.64 ± 2.20 mg/kg on Day 35. The GABA content in the fermented sausages prepared with isolate KA20 increased from 19.07 ± 1.10 mg/kg on the first day of fermentation to 63.94 ± 3.54 mg/kg on Day 35. The Y8 isolate, which showed a low GABA-producing activity when applied to the fermented sausage, showed an increasing trend from 17.42 ± 2.74 mg/kg on the first day of fermentation to 61.30 ± 2.61 mg/kg on Day 35. The GABA content of sausages fermented in situ by isolates Y8 and KA20 were found to be similar to GABA content obtained from the in situ fermentation of sausages by PM3. However, the GABA content in the fermented sausages manufactured with the O52 isolate increased from 15.55 ± 0.43 mg/kg on the first day of fermentation to 46.02 ± 1.04 mg/kg by Day 35, which was lower than the GABA content produced by the other strains (Fig. 4).
Fig. 4.
Changes in the contents of gamma-aminobutyric acid (GABA) in sausage during fermentation. Data are presented as the average of three independent trials. Bars indicate standard deviation. *p < 0.05 compared with control. Symbols closed diamond, inoculated with PM3; open triangle, inoculated with O52; X-sign, inoculated with KA20; and open circle, inoculated with Y8
The GABA content in flat-fish sikhae has been reported to increase with the extension of the ripening period [19]. This increase in GABA content is due to decreases in the content of glutamic acid (a precursor of GABA) through several enzymatic reactions [20, 21]. In addition, the GABA content in cheonggukjang has been reported to increase by ~ 5.7 times of the initial amount after 72 h of fermentation [22]. Although these experiments were performed with different raw materials obtained from fermented sausages, the trend of the increase in the GABA content is similar to that obtained in this study. Sensory evaluation was performed to assess olfaction, gustation, texture, juiciness, and overall evaluation of fermented sausages produced using a commercial starter (control) and those produced by adding GABA-producing strains. Thus, when each GABA-producing strain was compared with the control group, the Y8 strain showed evaluation results that were similar to those shown by the control group.
In this study, three types of GABA-producing strains were selected after they were evaluated using HPLC. Although one strain (L brevis) was found to possess the GAD gene, two strains (P. acidilactici and Weissella halotolerans) did not possess the GAD gene. Strain sequences, including the GAD sequence, could not be obtained for P. acidilactici and Weissella halotolerans from the USA NCBI GenBank. Nevertheless, the GABA content in the sausages fermented using isolates KA20 and O52 increased as the ripening period progressed. In conclusion, starter candidate Y8 was the optimal strain for sausage fermentation in terms of pH and acidity. Moreover, the Y8 strain produced low amounts of BA and high amounts of GABA in situ. In conclusion, isolate Y8 shows great potential for use as a functional starter culture and is expected to be effective in the production of fermented sausages.
Acknowledgements
This research was supported by the High Value-added Food Technology Development Program (114018-03-3-HD02D) funded by the Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declares that they have no conflict of interest.
References
- 1.Marta H. Josep Ma. M. Bacterial starter cultures for meat fermentation. Food Chem. 1997;59:547–554. doi: 10.1016/S0308-8146(97)00005-8. [DOI] [Google Scholar]
- 2.Frédéric L, Jurgen V, Luc DV. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006;106:270–285. doi: 10.1016/j.ijfoodmicro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 3.Somkuti GA, Renye JA. Molecular analysis of the glutamate decarboxylase locus in Streptococcus thermophilus ST110. J. Ind. Microbiol. Biotechnol. 2012;39:957–963. doi: 10.1007/s10295-012-1114-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Shin BH, Kim YH, Nam SW, Jeon SJ. Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus brevis BH2. Biotechnol. Bioprocess Eng. 2007;12:707–712. doi: 10.1007/BF02931089. [DOI] [Google Scholar]
- 5.Siragusa S, Angelis MD, Cagno RD, Rizzello CG, Coda R, Gobbetti M. Synthesis of γ-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl. Environ. Microbiol. 2007;73:7283–7290. doi: 10.1128/AEM.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama S, Hiramatsu J, Hayakawa K. Production of γ-aminobutyric acid from alcohol distillery lees by Lactobacillus brevis IFO-12005. J. Biosci. Bioeng. 2002;93:95–97. doi: 10.1016/S1389-1723(02)80061-5. [DOI] [PubMed] [Google Scholar]
- 7.Hiraga K, Ueno Y, Sukontasing S, Tanasupawat S, Oda K. Lactobacillus senmaizukei sp. nov., isolated from Japanese pickle. Int. J. Syst. Evol. Microbiol. 2008;58:1625–1629. doi: 10.1099/ijs.0.65677-0. [DOI] [PubMed] [Google Scholar]
- 8.Nomura M, Kimoto H, Someya Y, Furukawa S, Suzuki I. Production of γ-aminobutyric acid by cheese starters during cheese ripening. J. Dairy Sci. 1998;81:1486–1491. doi: 10.3168/jds.S0022-0302(98)75714-5. [DOI] [PubMed] [Google Scholar]
- 9.Yu JJ, Oh SH. γ-aminobutyric acid production and glutamate decarboxylase activity of Lactobacillus sakei OPK2-59 isolated from kimchi. Kor. J. Microbiol. 2011;47:316–322. [Google Scholar]
- 10.Cho SY, Park MJ, Kim KM, Ryu JH, Park HJ. Production of high γ-aminobutyric acid (GABA) sour kimchi using lactic acid bacteria isolated from mukeunjee kimchi. Food Sci. Biotechnol. 2011;20:403–408. doi: 10.1007/s10068-011-0057-y. [DOI] [Google Scholar]
- 11.Codex Alimentarius. Codex standard for kimchi (CODEX STAN 223-2001). In: FAO/WHO Joint Publications: Processed and Quick Frozen Fruits and Vegetables. 5A (2001)
- 12.Higuchi T, Hayashi H, Abe K. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997;179:3362–3364. doi: 10.1128/jb.179.10.3362-3364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbert JB. Selection criteria for lactic acid bacteria to be used as starter cultures for various food commodities. FEMS Microbiol. Rev. 1993;12:253–271. doi: 10.1111/j.1574-6976.1993.tb00022.x. [DOI] [Google Scholar]
- 14.Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int. J. Food Microbiol. 1999;53:33–41. doi: 10.1016/S0168-1605(99)00152-X. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Giglrey B, de Sousa Vieites Baptista JM, Villa TG. Changes in biogenic amines and microbiological analysis in albacore (Thunnus alalunga) muscle during frozen storage. J. Food Prot.®. 1998;61:608–615. doi: 10.4315/0362-028X-61.5.608. [DOI] [PubMed] [Google Scholar]
- 16.Park KB, Oh SH. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour. Technol. 2007;98:312–319. doi: 10.1016/j.biortech.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Seo MJ, Nam YD, Lee SY, Park SL, Yi SH, Lim SI. Expression and characterization of a glutamate decarboxylase from Lactobacillus brevis 877G producing γ-aminobutyric acid. Biosci. Biotechnol. Biochem. 2013;77:853–856. doi: 10.1271/bbb.120785. [DOI] [PubMed] [Google Scholar]
- 18.Park SY, Lee JW, Lim SD. The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from kimchi. Food Sci. Biotechnol. 2014;23:1951–1957. doi: 10.1007/s10068-014-0266-2. [DOI] [Google Scholar]
- 19.Won YG, Yu HH, Chang YH, Hwang HJ. Lactic acid bacterial starter culture with antioxidant and γ-aminobutyric acid biosynthetic activities isolated from flatfish-sikhae fermentation. J. Med. Food. 2015;18:1371–1379. doi: 10.1089/jmf.2015.3458. [DOI] [PubMed] [Google Scholar]
- 20.Jo SJ, Hong CO, Yang SY, Choi KK, Kim HK, Yang H, Lee KW. Changes in contents of γ-aminobutyric acid (GABA) and isoflavones in traditional Korean Doenjang by ripening periods. J Kor. Soc. Food Sci. Nutr. 2011;40:557–564. doi: 10.3746/jkfn.2011.40.4.557. [DOI] [Google Scholar]
- 21.Chang JS, Lee BS, Kim YG. Changes in γ-aminobutyric acid (GABA) and the main constituents by a treatment conditions and of anaerobically treated green tea leaves. Kor. J. Food Sci. Technol. 1992;24:315–319. [Google Scholar]
- 22.Mann SY, Kim EA, Lee GY, Kim RU, Hwang DY, Son HJ, Kim DS. Isolation and identification of GABA-producing microorganism from Chungkookjang. J. Life Sci. 2013;23:102–109. doi: 10.5352/JLS.2013.23.1.102. [DOI] [Google Scholar]