Abstract
Artemisia princeps var. orientalis is a well-known medicinal food, which has been used for the treatment of several diseases including bacterial infection. We examined the antiviral effects of the essential oil from A. princeps var. orientalis and its compounds, borneol, α-thujone and camphor, against murine norovirus-1 (MNV-1) and feline calicivirus-F9 (FCV-F9). The time-of-addition plaque assays were used to determine the ability of essential oil to interfere with viral infection. The maximum activities, following the pretreatment of FCV-F9 and MNV-1, reached 48% inhibition on FCV-F9 and 64% inhibition on MNV-1 at 0.1 and 0.01% of the essential oil, respectively. Neither borneol nor camphor exhibited an antiviral activity, whereas α-thujone, a major compound of the essential oil, showed strong inhibition on FCV-F9 and MNV-1.
Keywords: Murine norovirus, Feline calicivirus, Artemisia princeps var. orientalis, Essential oil, α-Thujone
Introduction
Noroviruses frequently cause acute gastroenteritis outbreaks globally, which leads to high morbidity and a heavy economic burden [1]. Norovirus is transmitted through the fecal–oral route or person-to-person contact. It is highly resistant to harsh conditions of a wide range of temperatures (from freezing to 60 °C) and the limit of infectious doses is approximately less than 20 virions [2]. In adults, norovirus-induced gastroenteritis is acute and self-limiting, but in the elderly and in young children, the illness can last longer [2]. Recent studies of immortalized B cells and stem cell-derived organoids allow in-depth analyses in human norovirus replication [3, 4], to provide an opportunity to develop vaccines and antivirals. Nevertheless, murine norovirus-1 (MNV-1) and feline calicivirus-F9 (FCV-F9) are still used as surrogates for studying norovirus biology [5, 6], when no vaccine or effective antivirals to prevent or control norovirus infection are yet available.
Artemisia species are found mainly in temperate climate regions and have frequently been used for the treatment of diseases such as malaria, hepatitis, and bacterial infections [7, 8]. Artemisia princeps var. orientalis (ssuk in Korea), is an aromatic, edible, and medicinal plant belonging to the Compositae family. The major compounds, borneol (12.1%) and α-thujone (8.7%), were identified in the essential oil from A. princeps var. orientalis [9]. α-Thujone is reported to be a major compound of essential oils from Artemisia, which can be as high as 67% depending on its species [10, 11]. Biological activities of α-thujone have been investigated in recent studies; it has an anti-tumor effect and beneficial effect in the treatment of polycystic ovary syndrome [12, 13]. In the plant, borneol is oxidized to camphor which comprises only 2.9% in the essential oil [9]. Camphor derivatives showed an inhibitory effect on hemagglutinin of influenza virus A and B [14]. However, the antiviral activities of A. princeps var. orientalis essential oil, borneol, α-thujone, and camphor against norovirus surrogates have not been explored. In this study, A. princeps var. orientalis and its compounds were analyzed the antiviral effects on MNV-1 and FCV-F9.
Materials and methods
Viruses and cells
RAW 264.7 cells (mouse leukemic macrophage cell line), Crandell Reese feline kidney (CRFK) cells, and FCV-F9 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). MNV-1 was obtained from Dr. Herbert Virgin at Washington University School of Medicine, USA. RAW 264.7 and CRFK cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco BRL, Karlsruhe, Germany) with 10% heat-inactivated fetal bovine serum (FBS) (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin streptomycin (PS) (Invitrogen, Grand Island, NY, USA) in a 5% CO2 incubator at 37 °C.
Extraction of essential oil
Dried A. princeps var. orientalis (voucher no. DSNPL-0013) which was purchased at Gyeongdong market in Seoul. The aerial parts were crushed for 10 s using a blender. The essential oil was extracted from A. princeps var. orientalis by steam distillation using a Clavenger type apparatus (Hanil Labtech Ltd., Incheon, Korea) for 3 h. The essential oil yield was 1.4%. The major compounds of A. princeps var. orientalis essential oil are in descending order borneol (12.1%), α-thujone (8.7%), T-cadinol (6.7%), and 1, 8-cineole (6.2%) [9]. The essential oil was dried over anhydrous sodium sulfate. Borneol, camphor, and α-thujone were purchased from Sigma–Aldrich.
Cytotoxicity test
RAW 264.7 and CRFK cells were seeded in 96-well tissue culture plates at a density of 1.5 × 105 and 2 × 104 cells per well, respectively. After incubation, 90 µL of DMEM containing 10% FBS-1% PS and 10 µL of the essential oil or its compound was added to the cells in culture and then incubated for 24 h at 37 °C and 5% CO2. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT: Sigma–Aldrich) solution was added into each well and re-incubated at 37 °C, and followed by the addition of dimethyl sulfoxide (DMSO: Sigma–Aldrich) solution to dissolve the formazan crystals. The absorbance value was determined in a microplate reader (SpectraMax M2, Molecular Devices Corp., CA, USA) at 570 nm. The percentage of cell viability = (Abstreatment/Abscontrol) × 100.
Plaque assays
Inhibitory effects of the essential oil or its compounds (borneol, α-thujone, and camphor) were determined by using plaque assays. In order to identify the mechanism of action of the essential oil against MNV-1 or FCV-F9, we tested three modes of time-of-addition [15]. For pretreatment of the virus with the essential oil (or its compound), MNV-1 or FCV-F9 (6 log10 plaque-forming unit (PFU)/mL) was mixed in a ratio of 9:1 with the essential oil (or its compound). After 1 h incubation at room temperature, tenfold dilutions of virus mixture were added to each well for 1 h at 37 °C and 5% CO2 and the virus mixture was removed. And the cells were washed twice with PBS and cells were overlaid with DMEM medium containing 1.5% agarose, 5% FBS, and 0.5% PS. After incubation for 48 h for MNV-1 and 24 h for FCV-F9 at 37 °C in 5% CO2, the cells were stained with 0.5% crystal violet and plaques were counted on each well. In cotreatment, the confluent monolayers of cultured cells were inoculated with 200 μL of virus (2 log10PFU/mL) simultaneously added with the essential oil. After 1 h incubation at 37 °C in 5% CO2, the virus and essential oil were removed, and the next procedures were the same as those used in the pretreatment. In posttreatment, the monolayers were inoculated with virus (2 log10PFU/mL) for 1 h and the inocula were removed. After washing the cells, the essential oil was added to the cells for 1 h at 37 °C in 5% CO2 and then the essential oil was removed. The next procedures were the same as those used in the pretreatment. The untreated control was DMSO which was used as a solvent for the essential oil and its compound. 2-Thiouridine (2TU) was used as a positive control at a concentration of 50 and 200 µM for MNV-1 and FCV-F9, respectively [15]. 2TU showed antiviral activity against MNV-1 and FCV-F9 through binding to the RNA-dependent RNA polymerase of virus [16]. Inhibitory activities were expressed as PFU reduction or relative plaque formation % compared to untreated control.
Statistical analysis
Data were presented as the mean and standard deviation. The significance of differences in the mean was analysed by the Student’s t test (SPSS software, version 13.0, SPSS Inc., Chicago, IL, USA).
Results and discussion
The cytotoxicity of A. princeps var. orientalis essential oil or its compound on CRFK and RAW 264.7 cells was determined by MTT assay. The essential oil at 10−3 dilution-treated CRFK or RAW 264.7 cells showed ≥85% viability. The cell viability of CRFK or RAW 264.7 was ≥90% at 25 mM of α-thujone, 5 mM of borneol, and 25 mM of camphor. Therefore, inhibitory experiments of the essential oil and the individual compounds were performed at concentrations below the cytotoxic levels.
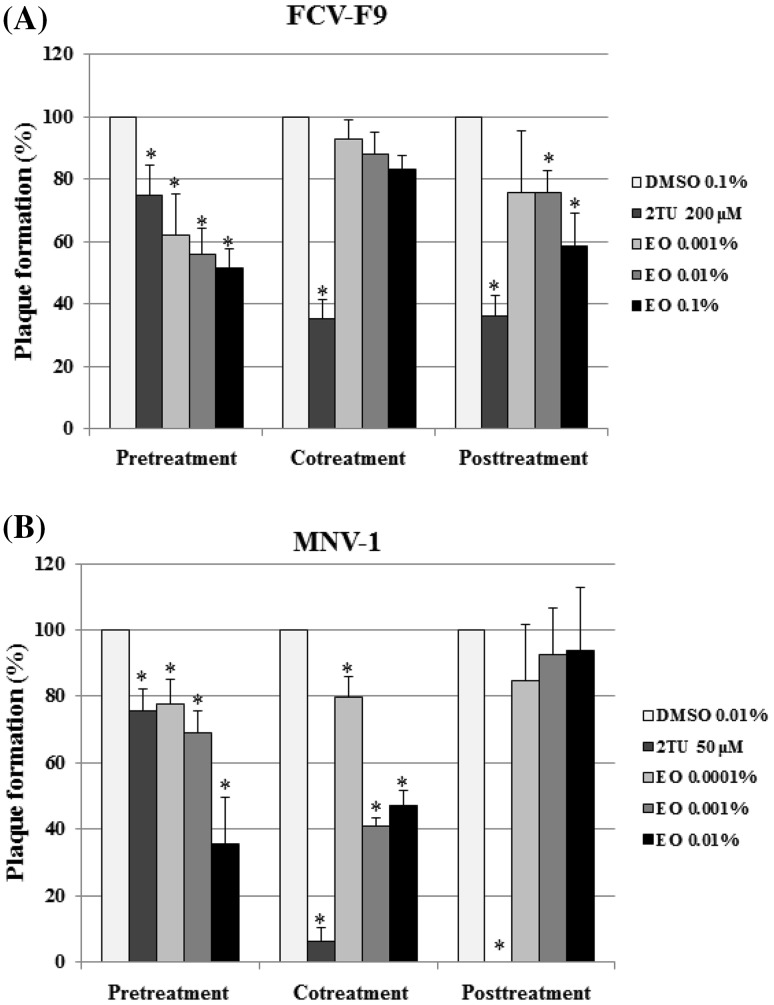
In FCV-F9, moderate inhibition of FCV-F9 was achieved upon pretreatment with 0.1% (v/v) the essential oil in a dose-dependent manner, resulting in 48% inhibition (Fig. 1A), whereas 2TU, used as a postive control, showed 25% inhibition at 200 μM. Cotreatment with the essential oil exhibited very low inhibitory effect against FCV-F9, reaching 17% inhibition at the same concentration. With posttreatment, 41% inhibition was achieved by the essential oil at 0.1% (v/v). In the case of MNV-1, a 64% plaque reduction was obtained after pretreatment of MNV-1 with 0.01% (v/v) essential oil (Fig. 1B), where as 2TU at 50 μM resulted in 24% inhibition. Cotreatment with the essential oil at the same concentration showed a 53% inhibition. However, posttreatment revealed no inhibitory effect at 0.01% (v/v) the essential oil. Our results demonstrated that the essential oil was effective in reducing plaque formation by MNV-1 and FCV-F9 and that the inhibitory effects of the essential oil was consistently high with the pretreatment of the virus in the time-of-addition mode.
Fig. 1.
Inhibitory effects of Artemisia princeps var. orientalis essential oil on (A) FCV-F9 and (B) MNV-1. The essential oil was added at different time points during virus infection. The reduction of relative plaque formation (%) was evaluated using plaque assay oil (EO). DMSO and 2TU were used as the untreated control and positive control, respectively. All measurements were analyzed in triplicate. A single asterisk denotes significant decrease of plaque formation (%) relative to untreated control (p < 0.05)
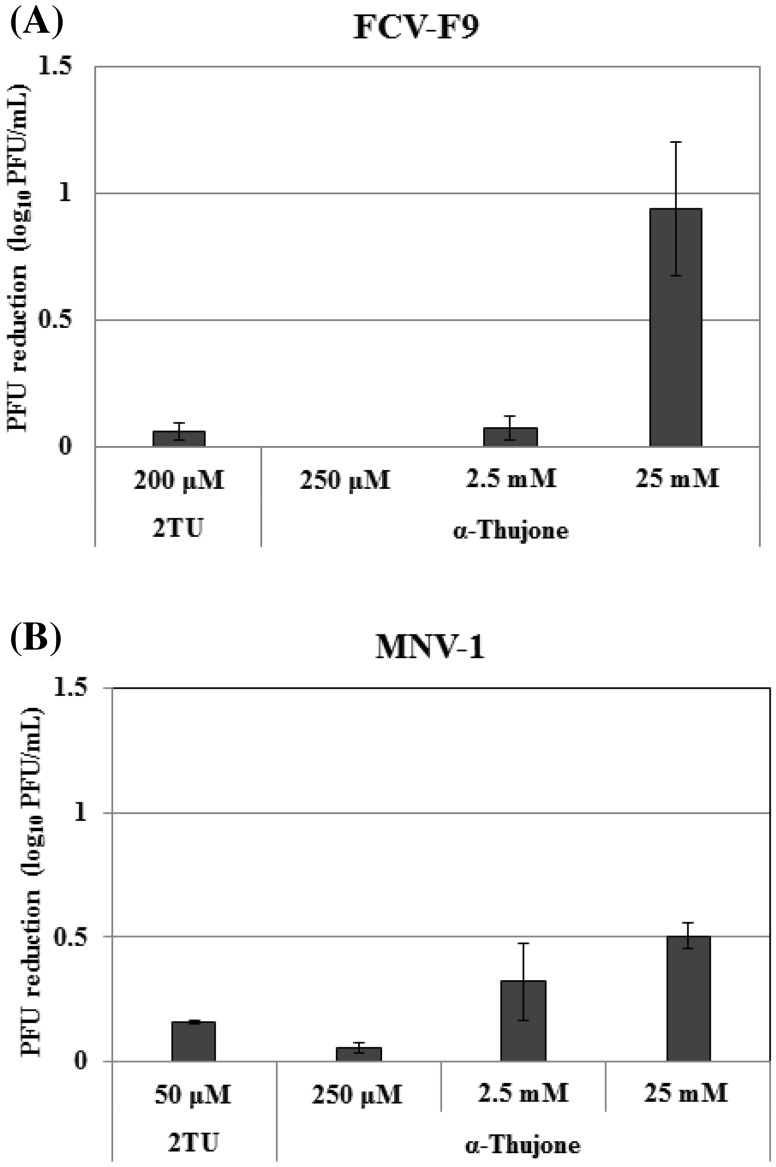
Next, borneol and α-thujone, major compounds of the essential oil, and camphor, an oxidized form of borneol, were examined in the pretreatment of virus mode in which the essential oil showed the maximal antiviral activity. Neither borneol (5 mM) nor camphor (25 mM), exhibited inhibitory effects on FCV-F9 and MNV-1 (Table 1). However, α-thujone showed an inhibitory effect in a dose-dependent manner: 0.94 log10PFU reduction and 0.50 log10PFU reduction were achieved by α-thujone at 25 mM against FCV-F9 and MNV-1, respectively (Fig. 2), whereas 2TU exhibited 0.06 and 0.16 log10PFU reductions on FCV-F9 and MNV-1, respectively. These results were similar to those obtained with oregano oil and its carvacrol which was effective in inhibiting MNV via directly interfere with the virion capsid [17]. Oregano, clove, and zataria essential oils showed strong inhibitory effects on FCV [18]. However, the essential oils from hyssop, marjoram, clove, zataria, and Zanthoxylum schnifolium did not show significant effects on FCV-F9 or MNV-1 infectivity [19–21]. In this study, essential oil was more effective on MNV-1 than FCV-F9, whereas α-thujone caused stronger inhibition against FCV-F9 than MNV-1. Borneol and camphor did not affect these viruses. In this context, only three compounds were tested in this study, suggesting that other compounds in A. princeps var. orientalis essential oil may play a major role in the inhibitory effect. Nevertheless, to determine the antiviral effect of other compounds, minor compounds of the essential oil need be tested in a future study.
Table 1.
Inhibitory effects of borneol and camphor from Artemisia princeps var. orientalis essential oil against norovirus surrogates, FCV-F9 and MNV-1
| Norovirus surrogates | Plaque formation (%) | |||
|---|---|---|---|---|
| DMSO 0.1% (v/v) | 2TU | Borneol 5 mM | Camphor 25 mM | |
| FCV-F9 | 100.00 ± 2.53a | 80.96 ± 8.38b | 102.17 ± 13.57a | 96.32 ± 4.63a |
| MNV-1 | 100.00 ± 1.15a | 73.45 ± 5.16b | 95.12 ± 2.52a | 105.55 ± 1.73a |
Data were expressed as the mean ± SD using triplicates. Plaque formation % for the pretreatment of the virus with borneol or camphor was calculated as relative plaque formation using DMSO untreated control. 2TU (200 μM for FCV-F9 and 50 μM for MNV-1) was used as the positive control
a−b Means followed by different letters in each row are significantly different according to Tukey's test (p < 0.05)
Fig. 2.
Antiviral effect of α-thujone from the essential oil against (A) FCV-F9 (B) MNV-1. The effects of α-thujone against FCV-F9 and MNV-1 were evaluated as PFU reduction (log10 PFU/mL) using plaque assay in the pretreatment of virus with α-thujone at concentration from 250 µM to 25 mM. DMSO and 2TU were used as the untreated and positive control, respectively. All measurements were analyzed in triplicate
Norovirus is more stable at low temperature (4 °C) in which the antiviral activity of inhibitor against the virus may be lower than the room temperature. Elizaquível et al. reported that the antiviral effects of oregano, clove, and zataria essential oils on FCV were more effective at 37 °C than 4 °C [18]. In the present study, antiviral activities of the essential oil and its compounds were tested at room temperature. More studies are needed to elucidate the effect of temperature on viral inhibition of the essential oil and its compounds. A few studies have been reported on the inactivation of essential oil against FCV-F9 or MNV-1, among them antiviral mechanism of essential oil had not been suggested, except oregano oil and carvacrol which caused disruption of MNV-1 capsid.
Essential oils are mixtures of volatile and odoriferous secondary plant metabolites such as monoterpenes and sesquiterpenes. Essential oils are used extensively as food flavoring and they have potential food biopreservatives [17, 18]. The strength of the study lies in the fact that A. princeps var. orientalis, one of the most popular edible and medicinal plants in Korea, can be used to control norovirus mediated foodborne diseases. In conclusion, A. princeps var. orientalis essential oil or its major compound, α-thujone, in the pretreatment of FCV-F9 and MNV-1 showed strong inhibitory effects. Further studies are required to elucidate antiviral mechanisms of the essential oil and α-thujone on FCV-F9 and MNV-1.
Acknowledgements
This work was supported by the Duksung Women’s University Research Grants in 2015. The author thanks Garam Bae, Hyojin Kim, and Mi Oh at Duksung Women’s University for technical assistance.
Compliance with ethical standards
Conflicts of interest
The author declares no conflict of interests.
References
- 1.Bartsch SM, Lopman BA, Ozawa S, Hall AJ, Lee BY. Global economic burden of norovirus gastroenteritis. PLoS One. 2016;11:e0151219. doi: 10.1371/journal.pone.0151219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass RI, Paras UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361(1776–178):5. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, Tibbetts SA, Wallet SM, Karst SM. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346:755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, Kou B, Opekun AR, Burrin D, Graham DY, Ramani S, Atmar RL, Estes MK. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wobus CE, Thackray LB, Virgin HW. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15:668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turi CE, Shipley PR, Murch SJ. North American Artemisia species from the subgenus Tridentatae (Sagebrush): a phytochemical, botanical and pharmacological review. Phytochemistry. 2014;98:9–26. doi: 10.1016/j.phytochem.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG, Lee BH, Baik MY, Park SK, Kim BY, Park SJ, Lee JH, Lee CY, Kim DO. Activated carbon treatment of water extracts of Artemisia princeps Pampanini to retain bioactive phenolic compounds and remove volatiles. Food Sci Biotechnol. 2015;24:1097–1103. doi: 10.1007/s10068-015-0140-x. [DOI] [Google Scholar]
- 9.Chung MS. Changes in the volatile compounds of Artemisia princeps var. orientalis essential oils during storage. Food Sci Biotechnol. 2009;18:481–487. [Google Scholar]
- 10.Abu-Darwish MS, Cabral C, Gonçalves MJ, Cavaleiro C, Cruz MT, Efferth T, Salgueiro L. Artemisia herba-alba essential oil from Buseirah (South Jordan): chemical characterization and assessment of safe antifungal and anti-inflammatory doses. J Ethnopharmacol. 2015;174:153–160. doi: 10.1016/j.jep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Sampietro DA, Lizarraga EF, Ibatayev ZA, Omarova AB, Suleimen YM, Catalán CA. Chemical composition and antimicrobial activity of essential oils from Acantholippia deserticola, Artemisia proceriformis, Achillea micrantha and Libanotis buchtormensis against phytopathogenic bacteria and fungi. Nat Prod Res. 2016;30:1950–1955. doi: 10.1080/14786419.2015.1091453. [DOI] [PubMed] [Google Scholar]
- 12.Küpeli Akkol E, İlhan M, Ayşe Demirel M, Keleş H, Tümen I, Süntar İ. Thuja occidentalis L. and its active compound, α-thujone: promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J Ethnopharmacol. 2015;168:25–30. doi: 10.1016/j.jep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Liu JQ, Zhou ZH, Lv XT, Chen YQ, Sun LQ, Chen FX. Enhancement of CD3AK cell proliferation and killing ability by α-thujone. Int Immunopharmacol. 2016;30:57–61. doi: 10.1016/j.intimp.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Zarubaev VV, Garshinina AV, Tretiak TS, Fedorova VA, Shtro AA, Sokolova AS, Yarovaya OI, Salakhutdinov NF. Broad range of inhibiting action of novel camphor-based compound with anti-hemagglutinin activity against influenza viruses in vitro and in vivo. Antiviral Res. 2015;120:126–133. doi: 10.1016/j.antiviral.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Oh M, Lee JH, Bae SY, Seok JH, Kim S, Chung YB, Han KR, kim KH, Chung MS. Protective effects of red wine and resveratrol for foodborne virus surrogates. Food Control. 2015;47:502–509. doi: 10.1016/j.foodcont.2014.07.056. [DOI] [Google Scholar]
- 16.Alam I, Lee JH, Cho KJ, Han KR, Yang JM, Chung MS, Kim KH. Crystal structures of murine norovirus-1 RNA-dependent RNA polymerase in complex with 2-thiouridine or ribavirin. Virology. 2012;426:143–151. doi: 10.1016/j.virol.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Gilling DH, Kitajima M, Torrey JR, Bright KR. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J Appl Microbiol. 2014;116:1149–1163. doi: 10.1111/jam.12453. [DOI] [PubMed] [Google Scholar]
- 18.Elizaquível P, Azizkhani M, Aznar R, Sánchez G. The effect of essential oils on norovirus surrogates. Food Control. 2013;32:275–278. doi: 10.1016/j.foodcont.2012.11.031. [DOI] [Google Scholar]
- 19.Kovač K, Diez-Valcarce M, Raspor P, Hernández M, Rodríguez-Lázaro D. Natural plant essential oils do not inactivate non-enveloped enteric viruses. Food Environ Virol. 2012;4:209–212. doi: 10.1007/s12560-012-9088-7. [DOI] [PubMed] [Google Scholar]
- 20.Azizkhani M, Elizaquível P, Sánchez G, Selma MV, Aznar R. Comparative efficacy of Zataria multiflora Boiss., Origanum compactum and Eugenia caryophyllus essential oils against E. coli O157:h7, feline calicivirus and endogenous microbiota in commercial baby-leaf salads. Int J Food Microbiol. 2013;166:249–255. doi: 10.1016/j.ijfoodmicro.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Oh M, Chung MS. Effects of oils and essential oils from seeds of Zanthozylum schinifolium against foodborne viral surrogates. Evi Based Complement Alternat Med. 2014 doi: 10.1155/2014/135797. [DOI] [PMC free article] [PubMed] [Google Scholar]