Abstract
The characteristics of cold-water-soluble potato starch (CWSS) produced using alcoholic–alkaline treatment were determined using scanning electron microscopy, rapid visco analysis (RVA), X-ray diffraction, differential scanning calorimetry, storage modulus (G′), loss modulus (G″), and gel images. Scanning electron microscopy revealed a damaged crystalline structure when CWSS was suspended in ethanol. The RVA analysis of native starch showed a peak value of viscosity at high temperatures, whereas the viscosity of CWSS did not show such a peak. The DSC diagram of native starch showed a gelatinization peak temperature; however, no such peak was observed for CWSS. Cold-water solubility tremendously increased in CWSS owing to the loss of crystallinity. The values of G′ and G″ in CWSS decreased with increasing temperature; the opposite was observed for normal potato starch. These results indicate that alcoholic–alkaline treatment affects the starch structure and produces totally different physicochemical properties from native starch; this makes CWSS feasible for use in a variety of food materials.
Keywords: Native starch, Cold-water-soluble starch, Potato starch, Alcoholic–alkaline treatment, Physicochemical property
Introduction
Potato starch is one of the most abundant natural biological polymers and plays a critical role in the alteration of food properties during processing [1]. In addition to being a chemical precursor, it is used in various fields, including food, architecture, paper, textiles, and medicine [2].
However, raw potato starch has limited industrial usage because of its insolubility in water at room temperature; moreover, the density of starch increases greatly after gelatinization, which limits its functionality [3]. Therefore, the need for modified potato starch is evident. Modified potato starch is manufactured via various methods, such as oxidation reaction, esterification, etherification, alkali gelatinization, and cross-linkage [4, 5]. Among these, alkaline treatment with alcohol alters the physicochemical and rheological properties of normal starch and produces cold-water-soluble starch (CWSS), which has a different amylose complex form [6]. Amylopectin from normal potato starch has a double-helix conformation, which does not readily dissolve in cold water [7]. Unlike normal potato starch, which as a double-helix structure, amylopectin and amylose of CWSS have a single-helix structure, which makes them soluble in cold water [8]. During alcoholic–alkaline treatment, starch granules are swollen and hydrated [9]; damaged granules affect the physicochemical or rheological properties, e.g., the viscoelastic property and gel formation due to structural changes [10].
Therefore, in this study, the physicochemical properties of CWSS formed via alcoholic–alkaline treatment, i.e., those related to the particle/gel shape and structural changes, e.g., viscosity, thermostability, cold-water solubility, and dynamic mechanical properties, are investigated.
Materials and methods
Materials
The potato starch used in this study was purchased from Tureban Co. (Koyang, Gyeonggi, Korea). It was stored at 4 °C until used.
Preparation of cold-water-soluble starch
To manufacture CWSS, the method of Chen and Jane [4] was modified. In summary, 10 g of dried potato starch was mixed with 80 mL of 60% ethanol for 10 min; then, 0.66 mL of 3-N NaOH was added once every minute for 15 min, amounting to a total of 10 mL. An additional 40 mL of ethanol (60%) was added and stirred for 10 min. The treatment mixture was neutralized with 10 mL of 3-N HCl, dehydrated using anhydrous ethanol, and centrifuged at 4 °C for 5 min (1500×g) (VS-6000CFN, Vision Scientific Co., Bucheon, Korea). Dehydrated potato starch was dried at 40 °C in a drying oven for 24 h and passed through a 170-mesh sieve.
General properties of potato starch
The general properties of potato starch as a control were analyzed using the AOAC method [11]. Water content was obtained using a dry oven at 105 °C. Crude fat content was measured using Soxtec (ST243, Foss Analytical Co., Louyang, China), and crude protein content was measured using the Kjeldahl apparatus (DNP-1500, Raypa, Seoul, Korea). Crude ash content was measured in a crucible (550–600 °C). All measurements were repeated thrice and the mean values were obtained.
Analysis of starch particles
To analyze the particles of potato starch and CWSS, light microscopy (CX31RTSF, Olympus Corporation, Tokyo, Japan) and scanning electron microscopy (SEM; LEO SUPRA 55, Carl Zeiss, Oberkochen, Germany) were used. The normal-potato-starch particles were dispersed in distilled water, while CWSS was dissolved in ethanol. Both the starch solutions were observed using a polarizing lens under 40× magnification to determine the birefringence.
For SEM, both potato starch and CWSS were dusted onto an aluminum stub covered with double-sided tape; the specimens were coated with platinum. The accelerating voltage for the observation was 3 kV.
Rapid visco analysis
Distilled water was added to 1.5 g potato starch or CWSS to obtain a final mass of 28 g (a 5.3% solution, wet basis). The change in starch viscosity was analyzed using a rapid visco analyzer (RVA-3D, Newport Scientific, NSW, Warriewood, Australia). The temperature was increased at a rate of 12 °C/min from 50 to 95 °C and maintained at 95 °C for 2 min; then, it was decreased at a rate of 12 °C/min to 50 °C.
Water solubility
To measure cold-water solubility, the method of Eastman and Moore [12] was used. One gram of potato starch or CWSS was mixed with 100 mL of distilled water and blended in a Waring Blender (38BL19, Dynamics Corporation of America, New Hartford, CT, USA) at low speed for 15 s and then at a high speed for 2 min. The prepared solution was centrifuged (Combi 514R, Hanil Science Industrial, Gangneung, Korea) at 4 °C for 15 min at 2111×g, and 25 mL of the supernatant was weighed after drying for 4 h in a dry oven at 110 °C. Cold-water solubility was calculated using the following equation:
X-ray diffraction
X-ray diffraction patterns of potato starch and CWSS were obtained by an X-ray diffractometer (D8 Advance, Bruker, Bremen, Germany) with a Ni filter using Cu-Ka radiation at 40 kV and 300 mA in the transmission mode. The X-ray source had a wavelength of 0.154056 nm, and the diffraction patterns were recorded at an infusion angle of 5°–40°.
Measurement of the thermostability of the starch
For comparing the thermostability of potato starch and CWSS, a 75% starch suspension solution was prepared for both; 10 mg of each starch suspension was placed in the aluminum pan of a differential scanning calorimeter (DSC) (DSC 4000, Perkin Elmer, Waltham, MA, USA). The temperature range for the DSC was from 25 to 120 °C at a rate of 12 °C/min. An empty aluminum pan was used as the reference.
Preparation of starch gel
A mixture of native potato starch [6, 10, 14 and 18% (w/w)] was prepared in distilled water and stirred for 15 min at 200 rpm using an agitator (BL606D, Misung Scientific Co, Seoul, Korea) during heating at 95 °C. The prepared starch was sealed with Parafilm® and stored at 4 °C for 24 h to prepare the gel. Mixtures of 1, 3, and 6% (w/w) CWSS were prepared using distilled water at a temperature of 10 °C. These mixtures were also sealed with Parafilm and stored at 4 °C for 1 week to prepare the gel.
Measurement of dynamic viscoelasticity
To measure dynamic viscoelasticity, a dynamic rheometer (Discovery Hybrid Rheometer HR-3, New Castle, PA, USA) was used. Measurements were conducted at a linear viscoelastic range of 2% strain with an angular velocity of 10 rad/s in a temperature range of 30–90 °C. The samples used in this experiment were prepared in a suspension solution with 20% starch content using the plate–plate system (diameter: 4 cm; gap: 500 μm) of the dynamic tester at 25 °C; in addition, the storage modulus (G′) and loss modulus (G″) were measured.
Statistical analysis
One-way ANOVA was performed using the SAS software (version 8.2, SAS Institute, Inc., Cary, NC, USA). Significant differences were verified via Duncan’s multiple range tests at a 95% confidence level.
Results and discussion
Analysis of general properties
The results of the analysis of the general properties of normal potato starch were as follows: water content was 18.29%, crude ash content was 0.20%, crude fat content was 0.02%, and crude protein content was 0.39%. The water content of potato starch typically ranges from 15.4 to 16.4% [13]; however, the sample used in this study showed a higher water content. The crude ash content is normally 0.23%, which is similar to the value calculated in this study [14]. The crude fat percentage of 0.02% obtained in this study was lower than the typical crude fat content of 0.04%, whereas the 0.39% crude protein content was higher than the usual amount, i.e., 0.1% [12, 13].
Shape of starch particles
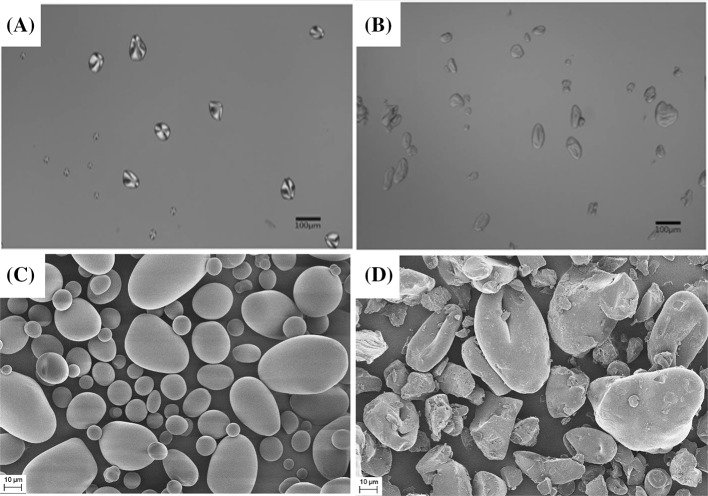
The particles of normal potato starch and CWSS are shown in Fig. 1. Normal potato starch contained large, oval-shaped particles and showed clear birefringence (Fig. 1A), whereas CWSS contained fewer circular particles that were slightly larger and had an intact granular shape. Under a polarized lens, the CWSS did not show birefringence [Fig. 1B]. These results are identical to those of a report stating that treating a normal potato starch granule disrupts the crystalline structure and results in the loss of birefringence [15]. When CWSS was rehydrated with ethanol, the granule shape remained intact; however, when it was rehydrated with water, the granule was no longer intact. This behavior is identical to that of granular CWSS, which does not show birefringence under microscopy but does show swelling of granules when water is added [7].
Fig. 1.
Microscopic images of native-starch and cold-water-soluble starch granules in ethanol and water. (A) Native-starch granules in water observed via a polarizing lens and (B) cold-water-soluble starch granules in ethanol observed via a polarizing lens. SEM micrographs of (C) native starch and (D) cold-water-soluble starch
The SEM results for normal potato starch and CWSS are shown in Fig. 1. Normal potato starch was 10–50 μm in size with oval-shaped granules [Fig. 1C], while CWSS showed 10–60-μm intact granules with different shapes [Fig. 1D]. The major difference between the two types of starch was that the CWSS granules had a dimple in the middle, likely due to starch shrinkage after swelling during treatment [6].
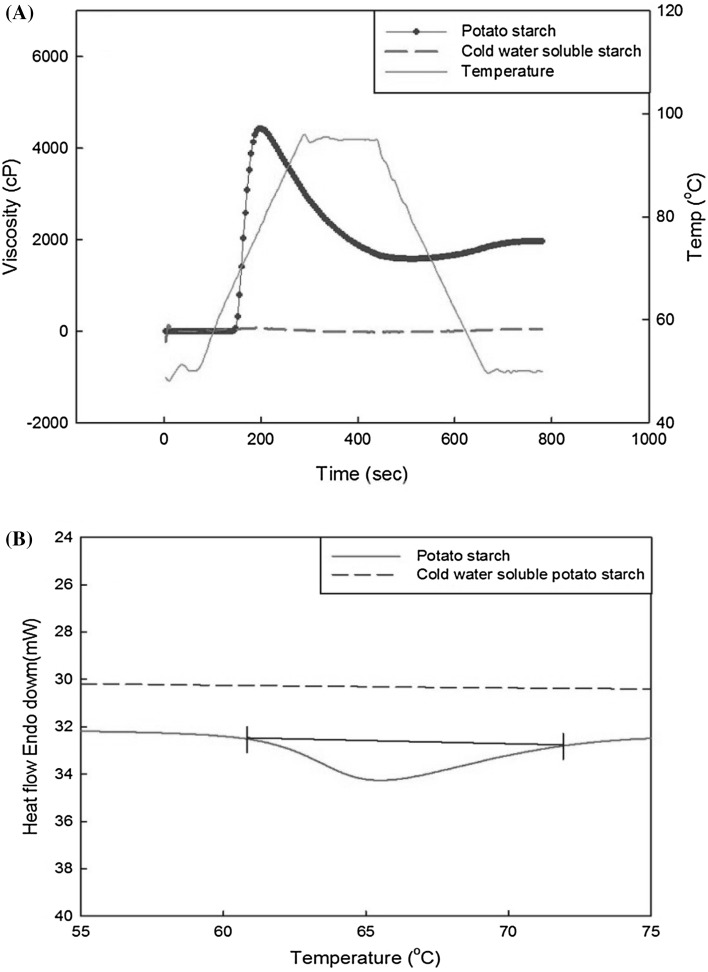
Change in RVA viscosity and thermostability during heating
The changes in the viscosity of potato starch are shown in Fig. 2A. The pasting temperature of normal potato starch was 67.05 °C, the peak viscosity was 4434 cP, trough viscosity was 1580.5 cP, final viscosity was 1971.5 cP, and breakdown occurred at 2853.5 cP. These results were similar to those of a report showing that potato starch has a high breakdown point because its final viscosity is lower than its peak viscosity [16]. Conversely, CWSS did not show any clear gelatinization peak. A previous report similarly showed that the crystalline structure of this starch deteriorated as the gelatinization temperature decreased [4]. CWSS showed a significantly lower viscosity when compared with normal potato starch; this might be a result of the alkali (NaOH) treatment, which may have changed the conformation of the crystalline starch particles [17].
Fig. 2.
Pasting viscosity profiles (A) and differential scanning calorimetry (DSC) diagram (B) of native starch and cold-water-soluble starch during heating
This phenomenon is also evident in thermostability of the two different starches, as shown in the DSC diagram [Fig. 2B]. Potato starch had a single peak at 65.5 °C, while CWSS did not show any peak. Wada et al. [18] reported that the gelatinization-initiating temperature and the enthalpy decreased as the crystalline level of starch was lowered; this might have occurred because the crystalline structure of CWSS was disrupted, as was shown by the change in viscosity during heating.
Cold-water solubility and X-ray diffraction
The cold-water solubilities of potato starch and CWSS are shown in Table 1. The solubility of normal potato starch was low (4.43%), whereas the solubility of CWSS was high (80.01%). Normally, the solubilities of normal starch and CWSS are 4 and 78–93%, respectively [19]. Cold-water solubility is influenced by the amylose content and by the crystalline structure of the starch [20]. Starch particles with low crystallinity generally absorb more water than those with high crystallinity [21]. Thus, the disruption of the crystalline structure of CWSS led to low crystallinity, which allowed the starch chains and the water molecules to interact and greatly increase the solubility.
Table 1.
Cold-water solubility of native potato starch and cold-water-soluble potato starch
| Sample | Cold-water solubility |
|---|---|
| Native potato starch | 4.43 ± 2.9b(1) |
| Cold-water-soluble potato starch | 81.01 ± 0.9a |
Different letters in the same column indicate significant difference based on Duncan’s multiple range test (p < 0.05)
(1) Data are presented as the mean ± standard deviation (n = 3)
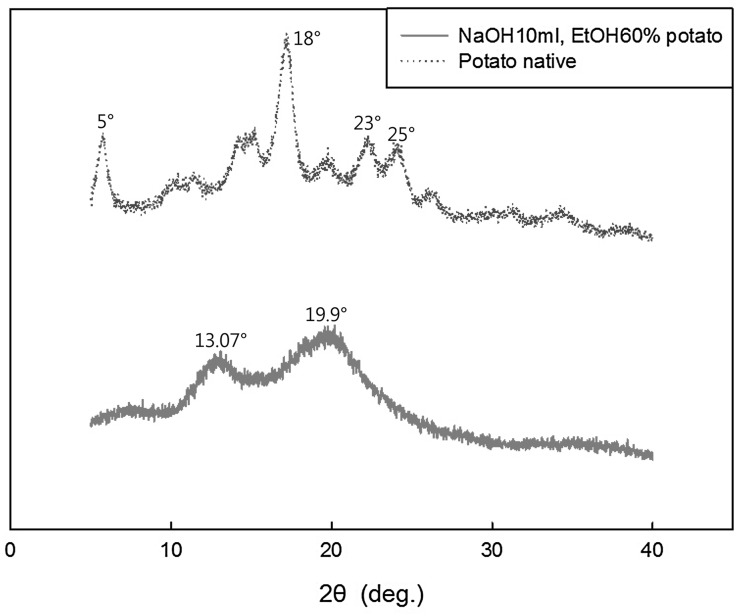
The loss of crystallinity in CWSS can be seen in the X-ray diffraction patterns of normal potato starch and CWSS [Fig. 3]. Normally, potato starch shows B-type peaks at 5°, 18°, and in the range of 23°–25° [22]; the potato starch used in the present study showed a similar trend. However, CWSS did not show this peak trend. Instead, peaks were seen at 13.07° and 19.9°, similar to the V-type peaks at 13.6° and 20.9° [23]; this indicates the presence of more amorphous granular starch in CWSS. The V-amylose complex also has a single helix and is soluble in cold water, which is very different from the properties of normal potato starch [24].
Fig. 3.
X-ray diffraction patterns of native and cold-water-soluble starch
Starch gel analysis
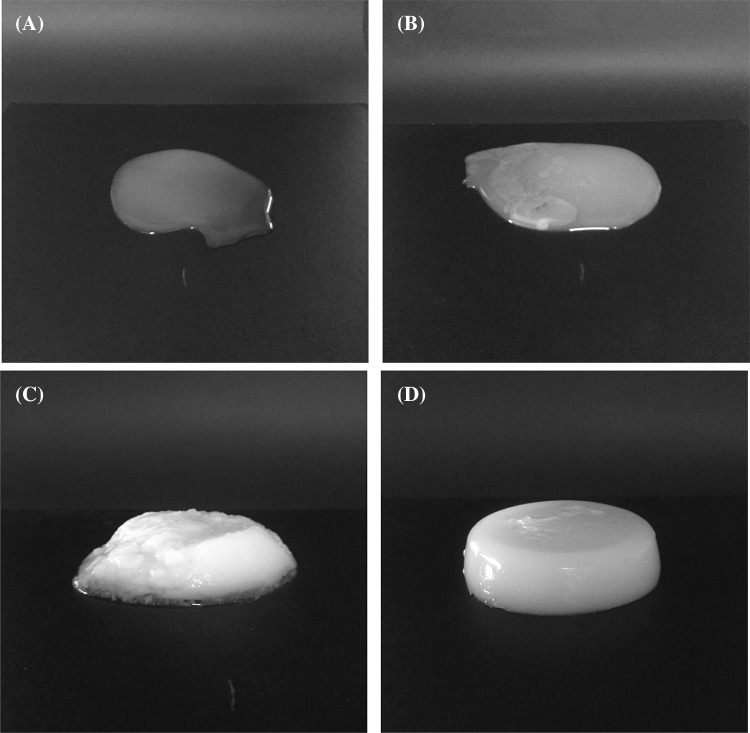
The gel states of normal potato starch and CWSS, which show the distinguishable final gel characteristics, are shown in Fig. 4. A concentration if 6% CWSS showed the maximum soft-gel formation ability. Concentration values higher than 6% showed coagulation and those lower than 6% in CWSS showed no gel formation [Fig. 4A–C]. Normal potato starch at 6% (w/w), however, formed a harder gel compared with CWSS [Fig. 4D]. Usually, gels harden as the starch concentration increases and swollen particles retrograde [24]. Among the components of starch, amylose affects the hardness of the gel and amylopectin affects the elasticity [25]. Alcoholic–alkaline treatment involves negative charges from the OH groups, which separate the double helix, disrupt the crystalline structure, and entangle the amylose and amylopectin [9]; thus, the treatment can affect gel formation.
Fig. 4.
Images of native starch and cold-water-soluble starch gels. (A) Cold-water-soluble starch, 1%; (B) cold-water-soluble starch, 3%; (C) cold-water-soluble starch, 6%; and (D) native starch, 6%
Dynamic viscoelasticity
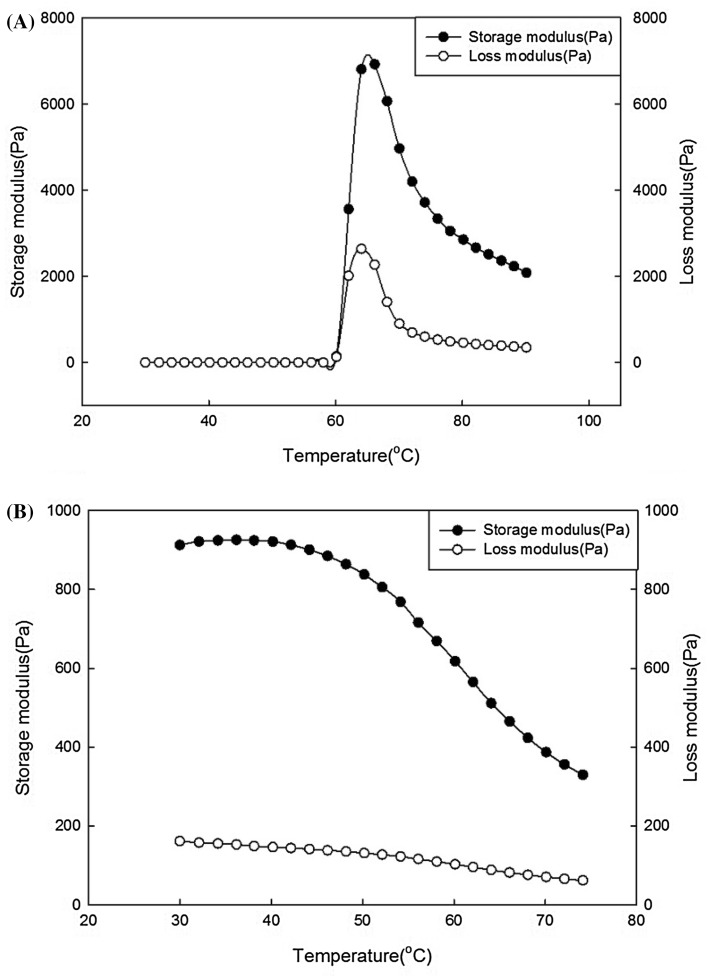
The different dynamic viscoelasticities of normal potato starch and CWSS are shown in Fig. 5. Normal potato starch showed sharp increases in the values of the storage modulus (G′), and loss modulus (G″) at 60–66 °C. In this temperature range, the starch particles underwent a structure-altering endothermic reaction. G′ and G″ increased in this range owing to the strong network formed by the swollen starch particles [26]. G′ and G″ decreased at higher temperatures owing to melting of the inner crystalline regions of the swollen starch particles [27] or destruction of the particle network [28]. Unlike normal potato starch, CWSS had a disrupted double-helix structure and crystalline structure, which increased the mobility of the starch chain as the temperature increased, thereby restricting the interaction of the starch chain [29]; thus, G′ and G″ decreased. Both G′ and G″ of the CWSS were much lower than those of normal potato starch at all temperatures owing to the disruption of the crystalline structure, which provides structural mobility [6].
Fig. 5.
Temperature sweep curve of (A) native starch, and (B) cold-water-soluble starch
Acknowledgements
This research was supported by the High Value-added Food Technology Development Program (314041033HD030), Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Wruzburg OB. Forty years of industrial starch research. Cereal Foods World. 1986;31:897–903. [Google Scholar]
- 2.Tang H, Mitsunaga T, Kawamura Y. Molecular arrangement in blocklets and starch granule architecture. Carbohyd. Polym. 2006;63:555–560. doi: 10.1016/j.carbpol.2005.10.016. [DOI] [Google Scholar]
- 3.Shi AM, Li D, Wang LJ, Li BZ, Adhikari B. Preparation of starch-based nanoparticles through high-pressure homogenization and mini emulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohyd. Polym. 2011;83:1604–1610. doi: 10.1016/j.carbpol.2010.10.011. [DOI] [Google Scholar]
- 4.Chen J, Jane J. Properties of granular cold-water-soluble starches prepared by alcoholic-alkaline treatments. Cereal Chem. 1994;71:623–626. [Google Scholar]
- 5.Jang JK. The study for application of commercial modified starch to frozen and retort foods. J. Korean Soc. Food Sci. Nutr. 1998;27:881–889. [Google Scholar]
- 6.Singh J, Singh N. Studies on the morphological and rheological properties of granular cold water soluble corn and potato starches. Food Hydrocoll. 2003;17:63–72. doi: 10.1016/S0268-005X(02)00036-X. [DOI] [Google Scholar]
- 7.Jane JL, Craig SAS, Seib PA, Hoseney RC. Characterization of granular cold water-soluble starch. Starch/Stärke. 1986;38:258–263. doi: 10.1002/star.19860380803. [DOI] [Google Scholar]
- 8.French AD, Murphy VG. Computer modeling in the study of starch. Cereal Food World. 1977;22:61–70. [Google Scholar]
- 9.Yan H, Zhengbiao GU. Morphology of modified starches prepared by different methods. Food Res. Int. 2010;43:767–772. doi: 10.1016/j.foodres.2009.11.013. [DOI] [Google Scholar]
- 10.Hatcher DW, Bellido GG, Anderson MJ. Flour particle size, starch damage, and alkali reagent: Impact on uniaxial stress relaxation parameters of yellow alkaline noodles. Cereal Chem. 2009;86:361–368. doi: 10.1094/CCHEM-86-3-0361. [DOI] [Google Scholar]
- 11.AOAC. Official Methods of Analysis of AOAC Intl. 18th ed. Method 955.04. Association of Official Analytical Chemists, Gaithersburg. MD, USA Washington DC (2005)
- 12.Eastman JE, Moore CO. Cold water soluble granular starch for gelled food compositions. U.S. Patent 4,465,702 (1984)
- 13.Zaidul ISM, Yamauchi H, Kim SJ. RVA study of mixtures of wheat flour and potato starches with different phosphorus contents. Food Chem. 2007;102:1105–1111. doi: 10.1016/j.foodchem.2006.06.056. [DOI] [Google Scholar]
- 14.Kim HS, Ahn SY. Gelatinization properties of legume, cereal and potato starches. Korean J. Soc. Food Sci. 1994;10:1–6. [Google Scholar]
- 15.Eduard K, Karl B. Process for the preparation of pregelatinized, cold-setting starch. US Patent 3,332,785, 7 July (1967)
- 16.Lee HM, Lee YT. Pasting properties of corn, potato, sweet potato starches and wheat flours with partial rice starch substitution. Food Eng. Prog. 2013;17:238–244. doi: 10.13050/foodengprog.2013.17.3.238. [DOI] [Google Scholar]
- 17.Kaur B, Fazilah A, Karim AA. Alcoholic-alkaline treatment of sago starch and its effect on physicochemical properties. Food Bioprod. Process. 2011;89:463–471. doi: 10.1016/j.fbp.2010.09.003. [DOI] [Google Scholar]
- 18.Wada K, Takahashi K, Shirai K, Kawamura A. Differential thermal analysis applied to examining gelatinization of starches in foods. J. Food Sci. 1979;44:1366–1370. doi: 10.1111/j.1365-2621.1979.tb06440.x. [DOI] [Google Scholar]
- 19.French AD, Murphy VG. Computer modeling in the study of starch. Cereal Food World. 1977;22:61–70. [Google Scholar]
- 20.Jivan MJ, Yarmand M, Madadlou A. Preparation of cold water soluble potato starch and its characterization. J. Food Sci. Tech. 2014;51:601–605. doi: 10.1007/s13197-013-1200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS. Effect of amylose and amylopectin on the texture of mook. Ph.D. Thesis, Seoul National University, Seoul, Korea (1987)
- 22.Kuge T, Kitamura S. Annealing of starch granules; warm water treatment and heat-moisture treatment. J. Jpn. Soc. Starch Sci. 1985;32:65–83. doi: 10.5458/jag1972.32.65. [DOI] [Google Scholar]
- 23.Kaur B, Fazila A, Karim AA. Alcoholic-alkaline treatment of sago starch and its effect on physicochemical properties. Food Bioprod. Process. 2011;89:463–471. doi: 10.1016/j.fbp.2010.09.003. [DOI] [Google Scholar]
- 24.Zhang W, Chen H, Wang J, Wang Y, Xing L. Physicochemical properties of three starches derived from potato, chestnut, and yam as affected by freeze-thaw treatment. Starch/Stärke. 2014;66:353–360. doi: 10.1002/star.201200270. [DOI] [Google Scholar]
- 25.Jung LH, Kim KA. Gel characteristics of starch during steeping of potato. Korean J. Soc. Food Sci. 2001;17:598–603. [Google Scholar]
- 26.Lineback DR. The starch granule organization and properties. Bakers Dig. 1984;58:16–21. [Google Scholar]
- 27.Hsu S, Lu S, Huang C. Viscoelastic changes of rice starch suspensions during gelatinization. J. Food Sci. 2000;65:215–220. doi: 10.1111/j.1365-2621.2000.tb15982.x. [DOI] [Google Scholar]
- 28.Eliasson A. Viscoelastic behaviour during the gelatinization of starch I. Comparison of wheat, maize, potato and waxy barley starches. J. Texture Stud. 1986;17:253–265. doi: 10.1111/j.1745-4603.1986.tb00551.x. [DOI] [Google Scholar]
- 29.Slade L, Levine H, Reid DS. Beyond water activity: recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. Nutr. 1991;30:115–360. doi: 10.1080/10408399109527543. [DOI] [PubMed] [Google Scholar]