Abstract
Recently, amphetamine-like substances derived from the β-phenylethylamine core structure have been detected in dietary supplements. Especially, β-methylphenylethylamine (BMPEA), an amphetamine isomer, has been found in dietary supplements labeled as containing Acacia rigidula. The U. S. Food and Drug Administration determined that BMPEA is not naturally present in food and does not meet the statutory definition of a dietary ingredient. In addition, BMPEA has been classified as a psychotropic drug in South Korea and a doping substance by the World Anti-Doping Agency. The aim of this study was to determine whether dietary supplements contained amphetamine and amphetamine-like substance, including β-phenylethylamine (β-PEA) and BMPEA using LC-PDA and LC–MS/MS. In 10 of 110 samples, illegally added compounds were detected in the following ranges; β-PEA 1.4–122.0 mg/g and BMPEA 4.7–37.6 mg/g. This study will contribute to enhancement of food safety in the South Korea.
Keywords: BMPEA, β-Methylphenylethylamine, Amphetamine, Illegal compound, Acacia rigidula
Introduction
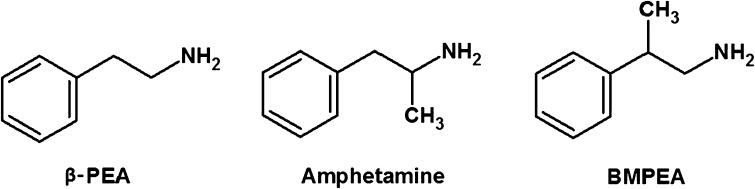
In recent years, the use of dietary supplements for weight control and athletic-performance enhancement has remarkably increased. Dietary supplements can be easily purchased in health related stores, drug stores, gyms, and over the internet. Botanical dietary supplements may be perceived as safe because they are “natural”. Acacia rigidula, also known as blackbrush, is a species of small tree native in south and west Texas, in addition to some parts of Mexico. Recently, β-methylphenylethylamine (BMPEA, 1-amino-2-phenylpropane) has been found in dietary supplements labeled as containing A. rigidula [1, 2]. BMPEA is an organic compound of the phenylethylamine class and a positional isomer of the drug amphetamine (2-amino-1-phenylpropane) (Fig. 1). Although some manufacturers have insisted that BMPEA is an extract of the botanical A. rigidula, the U. S. Food and Drug Administration (FDA) determined that BMPEA is not naturally present in food [3]. BMPEA is not verified as a food additive by either the Korean Ministry of Food and Drug Safety (MFDS) [4, 5] or the FDA [3], because BMPEA does not meet the statutory definition of a dietary ingredient.
Fig. 1.
Structures of β-PEA, amphetamine, and BMPEA
Phenylethylamines act as central nervous system stimulants. Substitution of the ethyl chain carbon, aromatic ring, or terminal amino nitrogen of a phenylethylamine with additional chemical groups imparts various clinical effects to the compound. In dogs and cats, these amphetamine stimulants can increase blood pressure and heart rate, and may cause serious health risks, including fatal stroke, heart attack and sudden death. However, the efficacy and safety of BMPEA in humans have never been reported [2]. In addition, BMPEA is classified as a psychotropic pharmaceutical by the MFDS [6] and a doping substance by the World Anti-Doping Agency (WADA) [7].
On December 24, 2014, the Health Canada recalled a dietary supplement containing the amphetamine-like drug substances, BMPEA, and phenylpropylmethylamine [8]. In April 2015, the FDA issued warning letters to five manufacturers to cease distribution of the products and to correct violations associated with the product [3]. Although some manufacturers have removed BMPEA from dietary supplements, these supplements are still available over the internet.
Therefore, we developed a simultaneous method to detect illegal compounds and monitored botanical and dietary supplement products listing BMPEA as a dietary ingredient. We analyzed the products for the illegal compounds β-phenylethylamine (β-PEA), amphetamine, and BMPEA using liquid chromatography coupled with a photodiode array detector (LC-PDA) or tandem mass spectrometry (LC–MS/MS).
Materials and methods
Chemicals
β-PEA (98% purity) and BMPEA (95% purity) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Toronto Research Chemicals (Canada), respectively. Amphetamine (99.9% purity) was supplied by the MFDS (Cheongju, South Korea). All the standards were dissolved in methanol at 1 mg/mL as stock solutions and stored at 4 °C prior to analysis. All LC-grade solvents were obtained from Merck (Darmstadt, Germany). Potassium phosphate monobasic and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA) as pure (>95%) compounds. Phosphoric acid was obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). The sterile distilled water was purified at 18.2 mΩ with a Thermo Scientific Barnstead Nanopure water purification system (Marietta, OH, USA).
Sample collection and preparation
A total of 110 dietary supplements advertised as weight-loss, fat-burning, energy-enhancement, or athletic-performance enhancement were purchased from the internet websites from 2015 to 2016. All samples were stored at room temperature until analysis.
Five tablets, capsules, or scoops of each sample were prepared as follow: The tablets were pulverized in a mortar and pestle. For the capsules, the five samples were opened and the contents were mixed. For powder samples, five scoops were mixed. After homogenization, 0.5 g of the powdered sample was weighed in a 50 mL centrifuge tube. 50 mL of 70% methanol was added to the sample before sonicating for 20 min. The extract was centrifuged at 13,200 rpm for 5 min at 5 °C. Then, 1 mL of the supernatant was filtered through a 0.45 μm pore polytetrafluoroethylene (PTFE) syringe filter (Teknokroma, Barcelona, Spain). The filtrate was injected into the HPLC system.
LC-PDA analysis
All chromatographic separations were performed using a Nanospace SI-2 HPLC system coupled with a PDA detector from Shiseido (Tokyo, Japan). Gradient elution was performed on a Scherzo SM-C18 column (4.6 mm × 150 mm, 3 μm) from Imtakt (Kyoto, Japan). Mobile phase A consisted of 0.3% phosphoric acid and mobile phase B comprised 10 mM potassium phosphate monobasic: acetonitrile (20:80, v/v). The gradient elution program was as follows: 0–4 min, 10% B; 4–10 min, 15% B; 10–14 min, 20% B; 14–15 min, 100% B; 15–19 min 100% B; 19–20 min, 10% B; 20–25 min, 10% B. The flow rate was 1.0 mL/min, and the column oven temperature was maintained at 40 °C. The injection volume was 10 μL. The eluate was monitored by the PDA detector at 210 nm, and absorption spectra from 200 to 400 nm were recorded.
LC–MS/MS analysis
For qualitative analysis, LC–MS/MS analysis was carried out on a Waters Xevo TQ-S Tandem MS coupled with an ACQUITY UPLC (Milford, MA, USA). Separations were carried out on an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) with 0.1% formic acid in water and 0.1% formic acid in acetonitrile as mobile phases A and B, respectively. The flow rate was 0.3 mL/min, the column temperature was 30 °C, and the injection volume was 2 μL. The gradient profile was as follows: 0–3 min, 5% B; 3–7 min, 10% B; 7–14 min, 80% B; 14–16 min, 80% B; 16–16.1 min 5% B; 16.1–18 min, 5% B. The conditions of mass spectrometry measurement were as follows: positive ionization mode; capillary voltage, 3 kV; cone voltage, 25 V; source temperature, 150 °C; desolvation temperature, 500 °C; desolvation gas flow, 800 L/h.
Determination of selectivity, linearity, LOD, LOQ, accuracy, and precision
The method was validated in terms of its selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision. Selectivity was identified by comparing the chromatograms of a blank solution (mobile phase B), a blank sample and blank samples spiked with a mixture of the 3 compounds. Chromatograms of these 3 solutions were checked for interfering peaks and sufficient separation of the analytes. Linearity was examined with the concentration range of 0.2–50 μg/mL for all studied compounds. Calibration curves for each compound were established with 6 concentrations. The calibration equations and coefficients of determination (r2) were obtained from the calibration curves. The LOD and LOQ were determined experimentally as the concentrations that produced a detector signal that could be clearly distinguished from the baseline noise. The LODs and LOQs were defined as signal-to-noise ratios (S/N) of 3 and 10, respectively. To determine the accuracy of the method, recovery experiments were performed using the spiked samples at a 4 μg/mL of each standard. The experiment was conducted five times. To evaluate precision of the method, percentage of relative standard deviation (% RSD) were calculated in mean values with standard deviations. The concentrations were kept constant at a 4 μg/mL for all studied compounds. The procedure was repeated the following day to determine the inter-assay.
Results and discussion
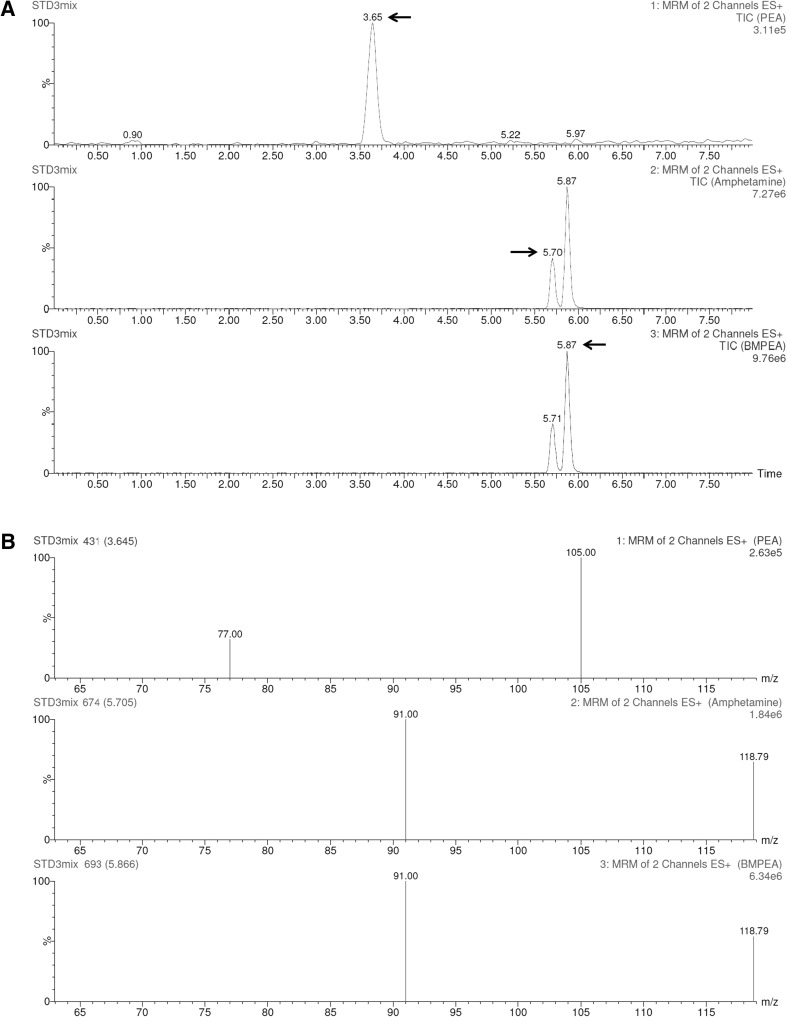
Many kinds of dietary supplements are sold over the internet. Some of these products are illegally advertised as effective for appetite depression or weight loss. Some manufacturers claim that herbal dietary supplements are safe products owing to natural products. In this study, we monitored for the presence of illegally added compounds such as β-PEA, amphetamine, and BMPEA in dietary supplements. In addition to A. rigidula, some of the supplements listed β-PEA or BMPEA as a dietary ingredient. These compounds were assayed using LC-PDA and LC–MS/MS, and a set of multiple reaction monitoring (MRM) transition parameters of LC–MS/MS was established to identify the most optimal conditions for each analyte (Table 1).
Table 1.
Multiple reaction monitoring (MRM) transitions and optimized mass spectrometer parameters
| Analyte | Precursor ion (m/z) | Production (m/z) | Cone voltage (V) | Collision energy (V) |
|---|---|---|---|---|
| β-PEA | 122.0 | 77.0, 105.0 | 30 | 20, 20 |
| Amphetamine | 136.2 | 91.0, 118.8 | 15 | 8, 15 |
| BMPEA | 136.2 | 91.0, 118.8 | 15 | 8, 15 |
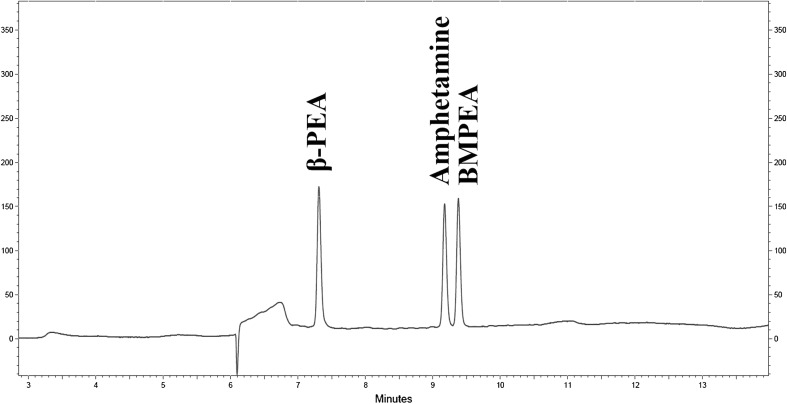
An LC-PDA method was developed to screen for amphetamine and amphetamine-like substances in 110 dietary supplements purchased on the internet. To validate the method, solid-type negative sample were selected. The negative sample had no detectable trace of amphetamine, β-PEA, and BMPEA. A sufficient separation of the standards was achieved by the optimized method (Figs. 2, 3). The blank solution (mobile phase B) and analyte-free matrix showed no interference in the obtained chromatograms. The obtained calibration equations, coefficients of determination (r2), LODs, LOQs, accuracies, and precisions are shown in Table 2. The coefficients of determination (r2) were 1.0000, 0.9999, and 0.9987 for β-PEA, amphetamine and BMPEA, respectively. For the detection of these illegal compounds, the LODs and LOQs were 4.0 and 13.0 μg/mL for β-PEA; and 1.0 and 3.0 μg/mL for amphetamine and BMPEA. The method accuracy was determined by comparing the calculated concentration with the true value spiked into the matrix. As seen in Table 2, the accuracy data were in the acceptable 92.5–96.6% recovery range. The percentages of relative standard deviation (% RSDs) were within 6.6% for all compounds. This LC-PDA method presented herein enables an easy and rapid quantification of the illegal amphetamine and amphetamine-like substances.
Fig. 2.
LC chromatograms of β-PEA, amphetamine, and BMPEA
Fig. 3.
Total ion chromatograms (A) and mass spectra (B) of β-PEA, amphetamine, and BMPEA
Table 2.
Linearity, limits of detection (LOD), limits of quantification (LOQ), accuracy and precision for β-PEA, amphetamine, and BMPEA
| Regression equation | Coefficient of determination (r 2) | LOD (μg/mL) | LOQ (μg/mL) | Accuracy (% Recovery) | Precision (%RSD) | |||
|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Intra-day | Inter-day | |||||
| β- PEA | y = 98,754.6x + 0.11 | 1.0000 | 4.0 | 13.0 | 96.6 ± 0.1a | 93.5 ± 0.2 | 5.0 | 6.6 |
| Amphetamine | y = 1458.4x − 0.01 | 0.9999 | 1.0 | 3.0 | 92.5 ± 0.1 | 93.9 ± 0.1 | 2.8 | 2.4 |
| BMPEA | y = 1275.3x + 0.58 | 0.9987 | 1.0 | 3.0 | 92.9 ± 0.1 | 93.0 ± 0.1 | 3.4 | 2.7 |
aThe values represent the mean ± confidence interval (α = 0.01, n = 15)
The validated LC-PDA method was used to quantify illegal compounds in dietary supplements. β-PEA or BMPEA was identified in 10 of 110 samples. As shown in Table 3, β-PEA was detected at levels of 1.4–122.0 mg/g in 8 samples while BMPEA was found at levels of 4.7–37.6 mg/g in 5 samples. In 3 samples, both β-PEA and BMPEA were detected. We also detected BMPEA in 3 dietary supplements labeled as containing A. rigidula. None of the products contained amphetamine.
Table 3.
Content of β-PEA and BMPEA detected in dietary supplements
| Detected N. |
Concentration | Content | |||
|---|---|---|---|---|---|
| Meana
(mg/g) |
Range (mg/g) |
Contents per unit (mg/unit)b |
Maximum daily dose (mg/day) |
||
| β-PEA | 8 | 42.7 ± 44.4 | 1.4–122.0 | 1.6–97.7 | 1.6–97.7 |
| BMPEA | 5 | 23.9 ± 14.5 | 4.7–37.6 | 5.4–22.7 | 8.8–54.6 |
aThe values represent the mean ± SD
bContent per dosage form, including capsule, tablet, or scoop
Dietary supplements should not be claimed as preventative or therapeutic agents because they are not drug, but are intended to complement the normal diet. Herbal supplements or herbal remedies are frequently adulterated with pharmaceuticals and their analogues. Sometimes illegally synthesized ingredients may be added to dietary supplements or botanical supplements used for their production in amounts exceeding the therapeutic dose, which may pose a threat to public health. Dietary supplements containing A. rigidula have been advertised as fat burners and metabolic activators over the internet. However, A. rigidula has not been previously consumed as a food ingredient. In addition, A. rigidula is reported to contain high levels of toxic amines and alkaloids, including N-methyl-β-phenylethylamine (NMPEA), methamphetamine, dopamine, and N, N-dimethyltyramine [9]. In Europe, a novel food or ingredient is defined as that consumed to a significant degree in the European Community (EC) before May 15, 1997. However, there is no history of the significant consumption of A. rigidula before that date. Under EC regulation 258/97, the Food Standards Agency (FSA) determined that A. rigidula is an unauthorized food and is not permitted to be sold until additional evidence of safety is found [10].
In addition to A. rigidula, β-PEA is widely distributed in a variety of other plant species. It is also found in the mammalian central nervous system, and can be biosynthesized from l-phenylalanine [11, 12]. The structure of β-PEA is similar to that of amphetamine, and its amphetamine-like effects have led to its classification as an endogenous amphetamine [12, 13]. Given these features, β-PEA has been intentionally used for promoting weight loss in dietary supplements. Pawar et al. [1] reported levels of β-PEA in A. rigidula in the range <0.03–1.5 mg/g, and Clement et al. [9] detected levels of 0.9–1.1 mg/g. In this study, the levels of β-PEA detected in the dietary supplements were 1.4–122.0 mg/g, which were clearly higher than those reported in the previous two studies. Thus, considering the low natural abundance of β-PEA in A. rigidula, synthetic β-PEA might have been intentionally added to the dietary supplements.
As shown in Table 3, the calculated maximum daily dose of BMPEA is 8.8–54.6 mg/day, as compared to that (2.9–93.7 mg/day) determined previously by Cohen et al. [2]. Several manufacturers have insisted that BMPEA is an extract of the botanical A. rigidula and claim that it is an all-natural product, despite the FDA [3] determination that BMPEA is not naturally present in food. Furthermore, the substance has not won prior approval for use as a food additive by either the MFDS or the FDA, as it has not been proven safe or effective. Therefore, the inclusion of BMPEA in a dietary supplement is illegal. In South Korea, BMPEA is considered a psychotropic pharmaceutical because it is a positional isomer of amphetamine. Therefore, dietary supplements containing BMPEA, whether natural or synthetic, should not be sold in South Korea.
The continual detection of illegal compounds including BMPEA in dietary supplements has highlighted their threat to public health. Although the FSA, FDA, and Health Canada have already taken some actions, such as prohibiting the sale of dietary supplements containing A. rigidula and recalling the diet pill, GAT JetFUEL Superburn [8, 10]. On the basis of this result, the MFDS immediately announced the harmfulness of BMPEA and dietary supplements containing BMPEA to consumers and requested they avoid dietary supplements labeled with A. rigidula as an ingredient. Further, the MFDS requested that the Korea Communications Commission block the internet sites that sell such products, and informed the Korea Customs Service to prevent customs clearance. This study will contribute to food safety by identifying illegally adulterated dietary supplements and preventing their distribution in the Korean food markets. Further studies are needed to determine the safety and efficacy of these synthetic stimulants in dietary supplements.
Acknowledgement
This study was supported by the Korean Ministry of Food and Drug Safety in 2017 (17161MFDS062).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Pawar RS, Grundel E, Fardin-Kia AR, Rader JI. Determination of selected biogenic amines in Acacia rigidula plant materials and dietary supplements using LS-MS/MS method. J. Pharm. Biomed. Anal. 2014;88:457–466. doi: 10.1016/j.jpba.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Cohen PA, Bloszies C, Yee C, Gerona R. An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements. Drug Test Anal. 2016;8:328–333. doi: 10.1002/dta.1793. [DOI] [PubMed] [Google Scholar]
- 3.FDA (Food and Drug Administration). BMPEA in Dietary Supplements. Available from: http://www.fda.gov/food/dietarysupplements/productsingredients/ucm443790.htm. Accessed 29.06.16.
- 4.MFDS (Ministry of Food and Drug Safety). South Korean Food Additives Code, November 16, 2016. Available from: http://www.mfds.go.kr/eng/eng/index.do?nMenuCode=120&page=2&mode=view&boardSeq=70005. Accessed 02.08.17.
- 5.MFDS (Ministry of Food and Drug Safety). South Korean Food Code, February 3, 2015. Available from: http://www.mfds.go.kr/eng/eng/index.do?nMenuCode=120&page=2&mode=view&boardSeq=69982. Accessed 22.02.17.
- 6.MFDS (Ministry of Food and Drug Safety). South Korean Narcotics Control Act. February 3, 2016. Available from: http://www.mfds.go.kr/eng/eng/index.do?nMenuCode=128&page=1&mode=view&boardSeq=70445. Accessed 22.02.17.
- 7.WADA (World Anti-Doping Agency). The 2016 prohibited list. Available from: http://www.Wada-ama.org/en/resources/science-medicine/prohibited-list. Accessed 22.02.17.
- 8.Health Canada. “Jetfuel Superburn” recalled after Health Canada tests find undeclared drug ingredients. December 24, 2014. Available from: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/43087r-eng.php. Accessed 30. 09.16.
- 9.Clement BA, Goff CM, Forbes DA. Toxic amines and alkaloids from Acacia rigidula. Phytochemistry. 1998;49:1377–1380. doi: 10.1016/S0031-9422(97)01022-4. [DOI] [Google Scholar]
- 10.FSA (Food Standards Agency). Acacia rigidula. March 12, 2014. Available from: http://www.food.gov.uk/sites/default/files/multimedia/pdfs/letters/acacia-rigidula-interested-parties-letter.pdf. Accessed 22.02.17.
- 11.Seigler DS. Phytochemistry of Acacia-sensu lato. Biochem. Syst. Ecol. 2003;31:845–873. doi: 10.1016/S0305-1978(03)00082-6. [DOI] [Google Scholar]
- 12.Berry MD. Mammalian central nervous system trace amines. Pharmacologic amphetamine, physiologic neuromodulators. J. Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 13.Burchett SA, Hicks TP. The mysterious trace amines: protean neuromodulators of synaptic transmission in mammalian brain. Prog. Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]