Abstract
In this study, the antioxidant activities of porcine liver proteins, hydrolyzed using Alcalase®, papain, pepsin, or a microbial suspension of Monascus purpureus (APLH, PaPLH, PePLH, and MPLH, respectively), were investigated. The results indicated that the yield and degree of hydrolysis (DH) of hydrolysates increased with hydrolysis time. The highest yield and peptide content were obtained from APLH, whereas the DH of PaPLH was higher than that of the others. MPLH exhibited the highest 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and reducing power, whereas APLH and PaPLH exhibited the higher ferrous ion-chelating ability than that of the MPLH. The molecular weights of all the hydrolysates were <10 kDa. The PaPLH exhibited the highest contents of total amino acids and hydrophobic amino acids. Fifteen antioxidant fractions obtained from MPLH contained one or more of the following amino acids in their sequences: Tyr, Trp, Ala, Pro, Met, Lys, Asp, Cys, Val, Leu, and His.
Keywords: Alcalase®, Antioxidant activity, Papain, Protein hydrolysates, Monascus purpureus
Introduction
Large quantities of by-products, such as offal, bones, blood, skin, horns, and hoofs, are annually produced by the meat industry. These meat by-products are rich sources of proteins and can serve as potential raw materials for the production of highly valuable products, including bioactive peptides and hydrolysates. Some studies have focused on the generation of bioactive peptides from meat by-products such as porcine liver [1], porcine plasma [2], sheep visceral mass [3], bovine skin, and bovine liver [4, 5]. Porcine liver, a by-product of pig slaughtering, is rich in proteins and various nutrients [1]. In addition to being edible, porcine liver extract has been reported to prevent celiac syndrome, liver cirrhosis, hepatic failure, and gastrointestinal bleeding [1]. Although porcine liver has numerous advantages, it is rarely used in Taiwan. Therefore, increasing the utility and value of porcine liver is a challenge for food producers.
Monascus spp., an edible fungus, has been widely used for producing wines and other fermented foods, particularly in many Asian countries [6]. The enzyme derived from Monascus spp. is considered to be an aspartic proteinase similar to pepsin [7, 8]. It hydrolyzes proteins such as milk casein, bovine serum albumin, soybean protein, and wheat gluten. Thus, it is beneficial for processing foodstuffs [7]. Furthermore, the enzyme has also been widely reported in ancient Chinese literature to have some beneficial effects such as improving food digestion and blood circulation [6]. In Taiwan, it is regarded as a natural food additive source. Monascus spp. has been approved as an edible natural colorant and is permitted for use in foods [9]. The acid proteinase from Monascus anka has been applied to degrade soybean protein and thereafter isolate some functional peptides [10].
Enzymatic hydrolysis of proteins is an efficient method for recovering potent bioactive peptides. Protease specificity and concentration, temperature, pH, protein substrate, and degree of hydrolysis (DH) are the variables affecting enzymatic hydrolysis [11]. Because of their hydroxyl radical scavenging activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging activity, reducing power activity, and chelating effects on metal ions, these water-soluble hydrolysates (amino acids, peptides, and proteins) have been reported to exhibit antioxidant activity [12]. The hydrolysates have been investigated mainly for use in the prevention of lipid oxidation in foods [13, 14]. Antioxidant properties are intensively related to the amino acid composition, structure, and hydrophobicity of the protein hydrolysates [12]. In the Fenton reaction, H2O2 was catalyzed by free metal ions (e.g., Fe2+) to a hydroxyl radical (HO·). Nevertheless, some hydrolysates can donate hydrogen atoms and bind ferrous ions, thus reducing the amount of the ferrous ions, facilitating the transformation of H2O2 to H2O, and eventually achieving the antioxidant function [15]. Through donating protons to the electron-deficient radicals, Sarmadi and Ismail [12] suggested that peptides containing aromatic amino acids can maintain the molecular stabilities via resonance structures and thus enhance the antioxidant properties. Moreover, some hydrophobic amino acids, such as Gly, Ala, Val, Trp, Phe, Ile, Leu, Pro, and Met, showed high affinity toward radical species and unsaturated fatty acids; therefore, they could inhibit lipid peroxidation in a linoleic acid model system [16, 17]. Likewise, protein hydrolysates block the reactions between hydroperoxide and free radicals by inhibiting the oxidized intermediates of lipid peroxidation processes, thus effectively controlling lipid peroxidation [18]. Both Monascus spp. and porcine liver contain functional components; however, the combination of porcine liver proteins hydrolyzed using Monascus spp. is a new area of investigation. Therefore, the objective of this study was to evaluate the antioxidant activity properties of porcine liver hydrolysates, which were obtained using Alcalase®, papain, pepsin, or Monascus purpureus microbial suspension (APLH, PaPLH, PePLH, and MPLH, respectively) after various hydrolysis time (3, 6, and 12 h).
Materials and methods
Preparation of M. purpureus microbial suspension
A M. purpureus CCRC 31499 (Bioresources Collection and Research Center, Food Industry Research and Development Institute, Hsinchu, Taiwan) suspension was prepared using the method of Tseng [19], and the strain was maintained in our laboratory. The strain was cultivated on yeast extract glucose broth medium containing 10% glucose and 0.8% yeast extract at 35 °C for 7 days in a controlled environment. The inoculums were homogenized using a sterile blender at a high speed for 2 min and filtered through a No. 1 filter paper. The suspension, referred to as a crude enzyme extract, was collected and stored at 4 °C for a maximum of 3 days prior to analysis.
Preparation of porcine liver hydrolysates using various proteases
Porcine liver protein hydrolysates (PLH) were prepared according to the method of Hsu [20], with some modifications. Fresh porcine liver (50 g) was mixed with 100 mL of distilled water and then homogenized at 10,000 rpm for 1 min (NISSEI AM-12, Japan). The homogenized liver solution was heated at 95–100 °C for 5 min to inactivate indigenous enzymatic activities for ensuring complete control over the enzymatic reaction. After cooling to 25 °C, the solutions were adjusted to optimal pH and temperature for each enzyme: Alcalase® (pH 8; 50 °C; Sigma Chemical Co., St. Louis, MO, USA), papain (pH 6.5; 37 °C; Merck, Darmstadt, Germany), pepsin (pH 3; 37 °C; Sigma Chemical Co., St. Louis, MO, USA), and microbial suspension from M. purpureus (pH 5; 37 °C). Then, the homogenized liver solutions were digested using enzymes at 1% (v/w) or microbial suspensions at 20% (v/w) for 3, 6, and 12 h. After heating at 95–100 °C for 10 min to inactivate the enzymatic activities, the mixtures were centrifuged at 10,000×g for 10 min at 4 °C (Himac SCR20B, Hitachi, Japan). Filtering the supernatant through a Whatman no. 1 filter paper yielded PLHs, which were used for measuring the DH, lyophilized, and stored at −20 °C until further measurements.
Measurement of yields and DH of hydrolysates
The average yield of hydrolysates was calculated by determining the weight of the freeze-dried PLHs as a percentage of total fresh weight of porcine liver used [21]. The DHs of the PLHs were determined by measuring the increase in free amino groups using a picrylsulfonic acid solution according to the method of Liceaga-Gesualdo and Li-Chan [21] and were calculated as follows:
DH is defined as the ratio between the number of broken peptide bonds (h) and the total number of peptide bonds (h tot) per protein equivalent. The h tot in a meat protein concentrate was assumed to be 7.6 meq/g [22].
Determination of peptide content
The peptide content of the samples was determined according to the method of Church et al. [23]. The method was performed by the addition of 20 μL of lyophilized and hydrolyzed sample to 1.5 mL of ο-phthaldialdehyde (OPA) reagent. The mixture was then incubated at ambient temperature for 2 min, and the absorbance was measured at 340 nm using a spectrophotometer (U-2900, Hitachi, Japan). A standard curve was prepared using the Leu–Gly dipeptide (0–10 mol/mL) and the OPA reagent.
Determination of antioxidant activities
The ferrous ion-chelating activities of the PLHs were determined using the method of Chien et al. [24] and calculated as follows: ferrous ion-chelating activity (%) = (1 − absorbancesample/absorbancecontrol) × 100 at 562 nm. The reducing power was determined by reading absorbance at 700 nm [25]. The DPPH radical scavenging activity was determined using a modified method of Chien et al. [24]. The absorbance of the resulting solution was determined at 517 nm as follows:
Determination of amino acid composition and molecular weight distribution of PLHs
The amino acid compositions of the freeze-dried PLHs were determined using a modified method of You and Wu [26]. Briefly, 0.1 g of PLHs was hydrolyzed with 3 mL of 4 M methanesulfonic acid containing 0.2% 3-(2-aminoethyl) indole under vacuum at 115 °C for 48 h. The total amino acids were analyzed using an Agilent 1100 HPLC system which was equipped with s ZORBAX Eclipse-AAA column (4.6 mm × 150 mm, 3.5 μm) (Agilent Technologies, Santa Clara, CA, USA). The molecular weight distribution of PLHs was determined according to the methods of Aspmo et al. [27] with some modifications. Samples (1 μL) of the hydrolysates were analyzed using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS; Voyager DE-PRO, Applied Biosystems, NY, USA) in the positive ion mode; however, the results were obtained in the linear mode.
Determination of antioxidant peptide sequence
The antioxidant peptide sequence of freeze-dried samples was determined according to the modified method of Petroselli et al. [28]. The samples (3 mg) were dissolved in 1 mL distilled water, and then filtered using Amicon® Ultra 3 K centrifugal filter units (3000 Da MWCO, Millipore, Germany). The peptide fractions were identified through liquid chromatography–tandem mass spectrometry (LC–MS/MS, Thermo LCQ DECA XP MAX system with an electrospray ionization source, ThermoScientific Inc., USA) using a linear gradient of acetonitrile from 5 to 95% containing 1% fluoroacetic acid.
Statistical analysis
The data were analyzed through the analysis of variance using Statistical Analysis Systems (Version 9.1, SAS Institute Inc., Cary, NC, USA) with a 5% level of significance. Means were separated using Scheffe’s test. All analyses were conducted in triplicate and averaged.
Results and discussion
Enzymatic hydrolysis
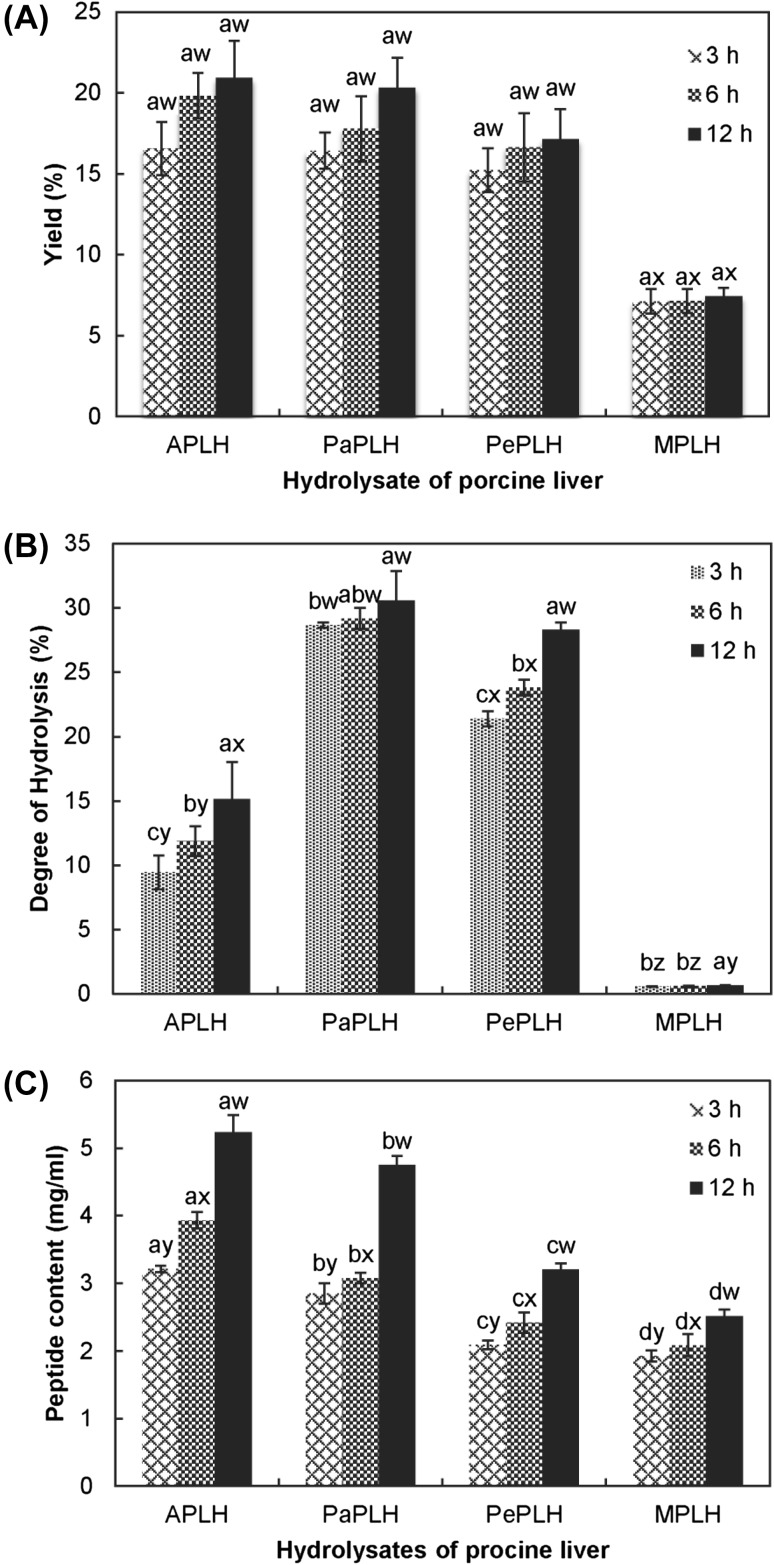
In the present study, M. purpureus microbial suspension, Alcalase®, papain, and pepsin were used for the production of protein hydrolysates from porcine liver. Figure 1(A) depicts the dry weight obtained from each hydrolysate after centrifugation, filtration, and freeze-drying. The hydrolysates APLH and PaPLH exhibited higher yields than that of PePLH and MPLH. Furthermore, the yields of hydrolysates increased with the hydrolysis time. APLH hydrolyzed for 12 h produced the highest yield (20.9%), whereas MPLH hydrolyzed for 3 h exhibited the lowest yield (7.1%). Ravallec-Plé et al. [29] suggested that the amount of hydrolysates produced was a function of processing time.
Fig. 1.
Yield (A), degree of hydrolysis (B), and peptide content (C) of porcine liver hydrolysates. The hydrolysis times are 3, 6, and 12 h. Letters a, b, and c indicate significant differences with the same protease (p < 0.05). Letters w, x, y, and z indicate significant differences with the same hydrolysis time (p < 0.05)
Figure 1(B) illustrates the DH of each hydrolysate. PaPLH had the highest DH, followed by PePLH, APLH, and finally MPLH. PaPLH hydrolyzed for 12 h exhibited the highest DH (30.6%) and MPLH hydrolyzed for 3 h exhibited the lowest DH (0.6%) (p < 0.05). The high DH achieved using papain was probably due to its broad substrate specificity [30], enzyme stability [30], and high proteolytic activity [27, 30]. In contrast, the DH of MPLH was low probably because the enzyme in this M. purpureus microbial suspension was not as pure as that of the other purified, commercially available enzymes. Therefore, lower enzyme activity in this M. purpureus microbial suspension eventually resulted in a significantly low DH (p < 0.05). Compared with the results in this study, a high DH was observed in the study by Lakshman et al. [8], and the high DH was probably also because of the enhancement of enzyme activity after purification of acidic proteinases from M. pilosus.
Moreover, DH increased as the hydrolysis time increased, which was consistent with other studies [8, 20]. Similarly, strong correlations were observed between the DH and hydrolysis time (0.99, 0.93, 0.97, and 0.99, respectively, for APLH, PaPLH, PePLH, and MPLH) during hydrolysis for 3–12 h. DH, defined as the percentage of peptide bonds cleaved by protease, has been applied as an index of DH frequency. Therefore, increasing DH implies generating a large number small peptides and free amino acids [30]. Figure 1(C) depicts the increased peptide content of each hydrolysate as hydrolysis time increases. This uptrend was also observed in the results of DH. Moreover, APLH exhibited the highest peptide content, followed by PaPLH, PeLH, and finally MPLH.
Antioxidant properties of hydrolysates
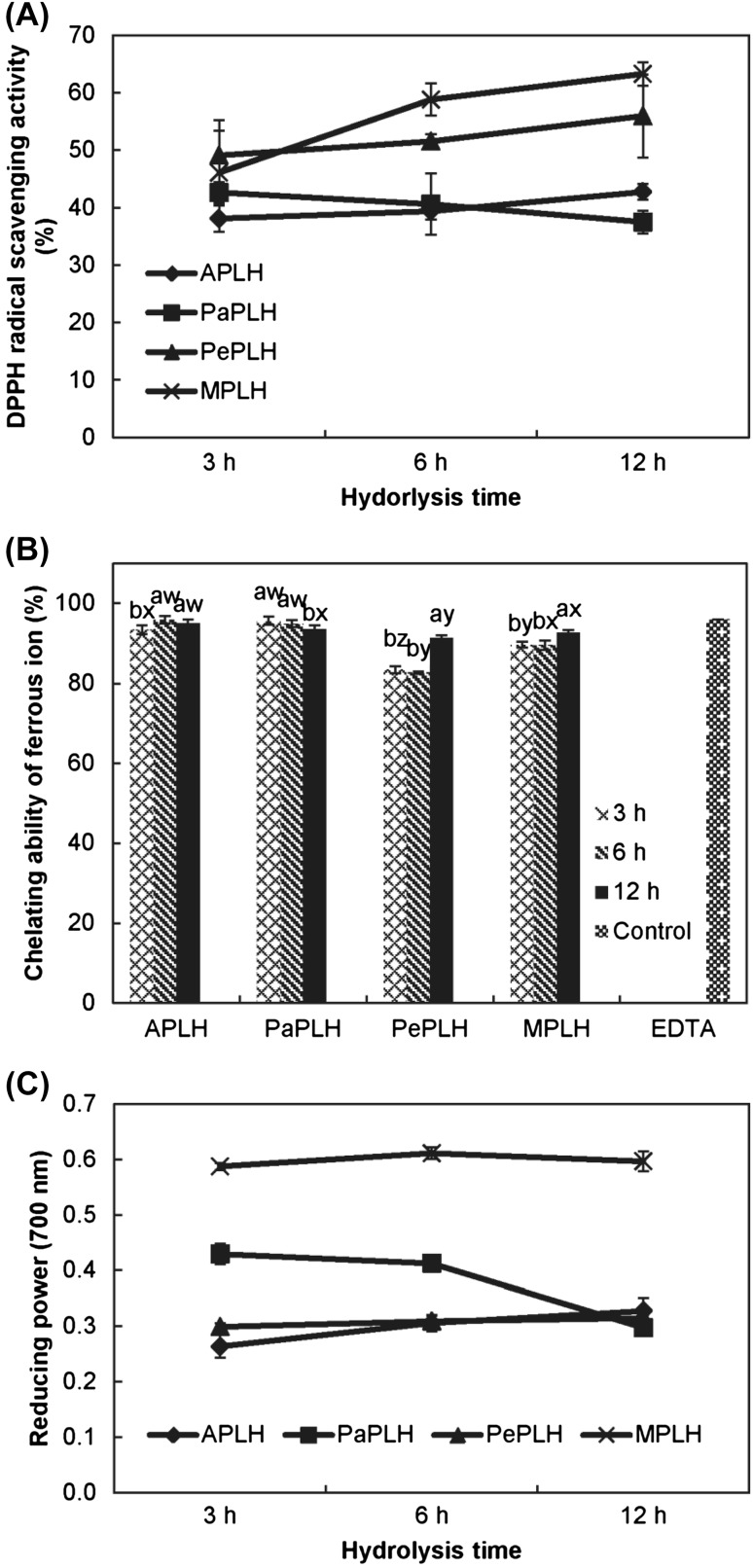
In the present study, MPLH exhibited the highest DPPH radical scavenging activity (63%), followed by PePLH (55%), and finally APLH and PaPLH (42 and 37%, respectively) at 12 h hydrolysis [Fig. 2(A)]. Moreover, APLH and PaPLH exhibited higher ferrous ion-chelating ability (93–96%) than that of PePLH and MPLH [Fig. 2(B)]. Among all the treatments, the 12 h hydrolysates exhibited the most effective (>91%) iron-chelating ability, which was comparable with that of EDTA (95%) at the same concentration of 5 mg/mL. In contrast, MPLH had a significantly higher reducing power than that of the other hydrolysates [Fig. 2(C)] and exhibited approximately 0.6 of the absorbance value after hydrolysis for 12 h. Overall, MPLH exhibited a high DPPH radical scavenging activity and reducing power, whereas PaPLH and APLH exhibited high ferrous ion-chelating ability. Lee et al. [31] indicated that soluble soybean protein, i.e., the water extract from Monascus fermented soybeans, contained cysteine and exhibited improved reducing power, ferrous ions-chelating ability, and scavenging ability against hydroxyl radicals. In our study, the content of cysteine in MPLH was 0.65/100 g dry sample and was higher than that of APLH and PePLH after hydrolysis for 12 h. This might be a reason that MPLH exhibited high DPPH radical scavenging activity and reducing power.
Fig. 2.
DPPH radical scavenging activity (A), chelating ability of ferrous ion (B), and reducing power (C) of porcine liver hydrolysates. The hydrolysis times are 3, 6, and 12 h. Letters a, b, and c indicate significant differences with the same protease (p < 0.05). Letters w, x, y, and z indicate significant differences with the same hydrolysis time (p < 0.05)
Proteases played an important role in influencing the antioxidant activity of hydrolysates because their specificity affects the amount of free amino acid, size and composition of peptides, and amino acid sequence [12]. After enzymatic hydrolysis, the numbers of ionizable groups (NH3+ and COO−) increased as hydrophobicity and the net charge increased. Simultaneously, the molecular size of the polypeptide chain decreased, and a variation of the molecular structure caused the buried hydrophobic interior to become exposed to the aqueous environment [30]. These effects enhanced the antioxidant activity of the hydrolysates. Some enzymes such as papain often cleave nonspecific sites on the hydrolysates [32]. Certain hydrophobic amino acids may not be exposed during hydrolysis because of the nonspecificity of enzymes; therefore, some functionality such as antioxidant activity may be partially lost in this hydrolysate [12, 30].
In terms of hydrolysis time, antioxidant activities (i.e., DPPH radical scavenging activity, chelating ability, and reducing power) of MPLH, PePLH, and APLH increased as the hydrolysis time increased, whereas the antioxidant activities of PaPLH decreased as the hydrolysis time increased. A similar trend was observed in the study by Hmidet et al. [14], in which the DPPH radical scavenging activity and metal chelating activity of cuttlefish hydrolysates were first reported to increase and then decrease during hydrolysis. The hydrolysates were hydrolyzed using crude protease from Bacillus mojavensis A21. The activity of the enzyme was influenced by reaction conditions such as hydrolysis time, pH, and temperature, thereby changing the peptide distribution and chemical properties (such as antioxidant activity) of the produced hydrolysate [30, 32]. In contrast, excessive proteolysis may result in the release of shorter peptides and free amino acids, and the hydrolysates might eventually lose their antioxidant activity [33]. Excessive hydrolysis may result from extending hydrolysis time or high activity of enzymes.
The molecular weight distribution of protein hydrolysates
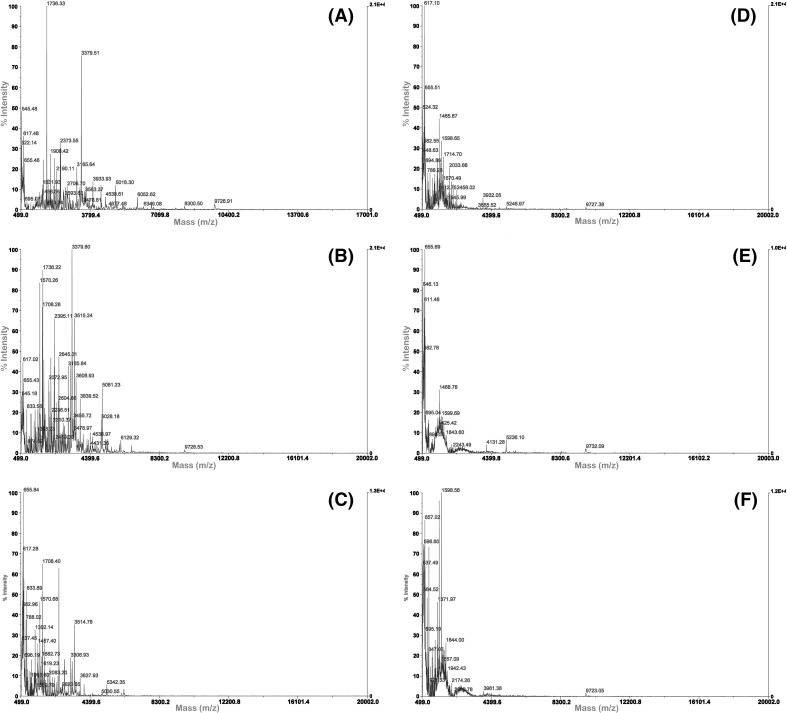
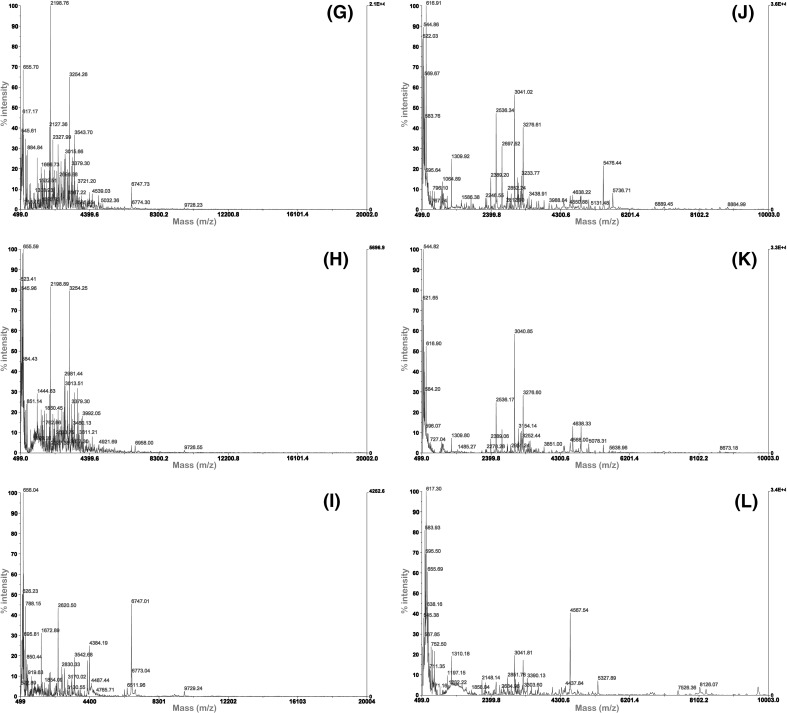
In addition to DH, the type of protease, peptide structure, and performance of protein hydrolysates, including their antioxidant activities, have been reported to be associated with the molecular weight distribution of the hydrolysates [12]. The molecular weight distribution of the porcine liver hydrolysates was determined through MALDI–TOF MS. The spectra revealed that majority of the molecular weights of the hydrolysates primarily ranged from 500 to 6000 Da although the molecular weights of some of the MPLH were as high as 10,000 Da (Fig. 3). Different proteases resulted in different peptide chain lengths and molecular sizes, which considerably affected the antioxidant activities of the hydrolysates [13]. Hsu [20] found that the small-sized peptides (<390 Da) exhibited low antioxidant activity. In this study, PaPLH hydrolyzed for 12 h showed the lowest DPPH radical scavenging activity and reducing power, which may be associated with the low molecular weight of hydrolysate.
Fig. 3.
Molecular weight distribution of porcine liver hydrolysates for different hydrolysis time by MALDI–TOF MS. (A–C) 3, 6, and 12 h APLH, (D–F) 3, 6, and 12 h PaPLH, (G–I) 3, 6, and 12 h PePLH, j–l: 3, 6, and 12 h MPLH
A relationship between the molecular weight distribution of the peptide fractions and hydrolysis time for each specific enzyme was observed. For example, after hydrolysis for 12 h, low molecular weight peptide fractions of the APLH were observed than after hydrolysis for 3 and 6 h [Fig. 3(A–C)]. The majority of molecular weight of APLH peptide fractions ranged from 500 to 4300 Da. A similar result was observed with PaPLH. The increase in low molecular weight peptides because of the extension of hydrolysis time was consistent with the findings in other studies [34], which indicated that low molecular weight peptides (<1.3 kDa) of defatted shrimp processing by-product hydrolysate increased as the hydrolysis time increased. Similarly, an apparent increase in peptides with a molecular weight of 650 Da was also observed in the sardine-processing waste hydrolysates after an extended hydrolysis time [29]. In this study, the size distribution in each hydrolysate was slightly similar, with the exception of one peak corresponding to an apparent molecular weight of 6747 Da in PePLH after 12 h of hydrolysis [Fig. 3(I)].
Amino acid composition of hydrolysates
The amino acid compositions of PLHs obtained after 12 h of hydrolysis are presented in Table 1. The total contents of amino acids of APLH, PaPLH, PePLH, and MPLH were 41.8, 70.2, 60.0, and 58.3/100 g dry sample, respectively. Some differences in amino acid contents among the four hydrolysates are associated with the hydrolysis conditions such as enzyme type, pH, and temperature [30]. The observed higher amino acid content of PaPLH could result from the high DH from papain [Fig. 1(B)]; therefore, a large amount of free amino acids is produced during the hydrolysis process [12].
Table 1.
Amino acid composition of porcine liver protein hydrolysates (g amino acids/100 g dry sample)
| Amino acid | APLHd | PaPLHd | PePLHd | MPLHd |
|---|---|---|---|---|
| Asp | 3.06 | 6.70 | 5.40 | 5.63 |
| Glu | 9.68 | 13.51 | 13.16 | 10.84 |
| Cys | 0.39 | 0.83 | 0.58 | 0.65 |
| Ser | 0.86 | 2.32 | 0.98 | 1.65 |
| His | 1.37 | 1.83 | 1.83 | 1.44 |
| Gly | 5.42 | 4.76 | 3.83 | 4.73 |
| Thr | 1.26 | 2.96 | 1.44 | 2.25 |
| Arg | 2.23 | 4.90 | 2.41 | 4.48 |
| Ala | 3.65 | 5.38 | 5.95 | 4.62 |
| Tyr | 0.57 | 1.53 | 2.01 | 1.08 |
| Val | 1.95 | 3.37 | 3.40 | 2.93 |
| Met | 0.67 | 1.18 | 1.16 | 1.40 |
| Trp | 0.22 | 0.65 | 0.45 | 0.44 |
| Phe | 1.59 | 2.80 | 2.59 | 2.32 |
| Ile | 1.31 | 3.34 | 2.96 | 2.66 |
| Leu | 2.37 | 5.32 | 4.69 | 4.48 |
| Lys | 2.95 | 6.23 | 4.59 | 4.60 |
| Pro | 2.25 | 2.60 | 2.60 | 2.13 |
| TAAa | 41.8 | 70.2 | 60.0 | 58.3 |
| THAAb | 19.4 | 29.4 | 27.6 | 25.7 |
| TAAAc | 3.75 | 6.81 | 6.88 | 5.28 |
a TAA Total amino acids
b THAA Total hydrophobic amino acids include Gly, Ala, Val, Met,Trp, Phe, Ile, Leu, Pro
c TAAA Total aroma amino acids include Tyr, His, Trp, and Phe
dFour PLHs hydrolysis at 12 h
The contents of the total hydrophobic amino acids in APLH, PaPLH, PePLH, and MPLH were 19.4, 29.4, 27.6, and 25.7/100 g dry sample, respectively. Moreover, the content of the total aromatic amino acids in APLH, PaPLH, PePLH, and MPLH was 3.75, 6.81, 6.88, and 5.28/100 g dry sample, respectively. Hydrophobic amino acids, including Gly, Ala, Val, Trp, Phe, Ile, Leu, Pro, and Met, were reported to contribute to the solubility of the peptide in lipids and to facilitate stronger interactions with radical species, thereby interfering with the propagation cycle of lipid peroxidation [12]. In contrast, aromatic amino acids (Tyr, His, Trp, and Phe) can donate protons to electron-deficient radicals and stabilize them; moreover, they can maintain the molecular stability through a resonance structure [12, 14]. In this study, PaPLH exhibited a high content of hydrophobic amino acids and aromatic amino acids, and it exhibited increased ferrous ion-chelating ability; however, MPLH exhibited an increased DPPH radical scavenging capacity and reducing power without a high content of hydrophobic amino acids and aromatic amino acids. The presence of the hydrophobic and aromatic amino acids may promote the antioxidant activity of protein hydrolysates; however, the correct positioning of amino acids in peptide sequences is also a critical factor affecting the antioxidant activity of peptides [12]. Hence, high DPPH radical scavenging capacity and reducing power of MPLH may result from the correct positioning of amino acids in the peptide sequence.
Characterization of antioxidant peptides
MPLH was further selected to determine its peptide sequence because of its high antioxidant activity. According to some research, the low molecular weight fraction (<3 kDa) exhibited the highest antioxidant activity [2]. Therefore, fractions with molecular weights <3 kDa fraction of MPLH were filtered and analyzed. The <3 kDa peptides derived from MPLH were obtained and resolved through LC–MS/MS into 15 fractions. The amino acid sequences and molecular masses of the 15 fractions are presented in Table 2. The molecule weights of some fractions were >3 kDa because the fractions were rough filtered by an Amicon® Ultra 3 K centrifugal filter. The 15 fractions of MPLH contained Val, Leu, and Ala at their N-termini and Trp, Tyr, His, Pro, Gly, Phe, Met, and Cys in the sequence. Park et al. [35] noted that peptides containing Leu at their N-terminal positions exhibited the highest antioxidant activity and promoted interaction between peptides and fatty acids. Sarmadi and Ismail [12] also reported that the peptide containing Val at the N-terminal or Tyr, Trp, Ala, Pro, Met, Lys, Asp, and Cys in the sequences can exhibit antioxidant activity. Moreover, His at the N-terminal acted as a metal ion chelator and at C-terminal played a free radical scavenger in the sequences.
Table 2.
Antioxidant peptides of porcine liver protein hydrolysates separated by electron spray ionization-mass spectrometry
| Sequences | Observeda | Mr(expt)b |
|---|---|---|
| TPPIPVIPDDHP | 649.8 | 1297.7 |
| SDGAGAVIIASEDAVKK | 816.3 | 1630.6 |
| VIMGITFLAAGTSVPDCMASLIVARQGLGDMAVSNTIGSNVFDILV | 1572.1 | 4713.3 |
| DVNLAGTVRMMQAFLPDMKRRRSG | 933.6 | 2797.7 |
| PKSPAPAAAAPAVQ | 426.5 | 1276.4 |
| TAFLFLGVLVSIIMLSPGVESQLYKLPWVCEEGTGSTVIL | 1436.9 | 4307.8 |
| EKDNTSSFEGFIL | 744.3 | 1486.6 |
| ASFSEAEGFSKAPAPI | 536.3 | 1605.9 |
| HWFTYNETMTVESVTQAVSNLALQFGEEDADPGAMSRPFGV | 1516.3 | 4545.9 |
| PPSVTVDDLKNLFTEAGCSVKAFKFFQKDRKMALIQLG | 1419.6 | 4255.9 |
| PLSPSSPTESPTFS | 479.1 | 1434.3 |
| MGGNMEIIVVLNITATNRLLQDEETGLPMFKSEGCEVILVSV | 1526.7 | 4577.2 |
| LEGVQRCGLKRKSEELDNHSSAMQIVDELSILPAMLQTNMGN | 1557.3 | 4668.9 |
| RQAPVGMSDVWLTML | 578.8 | 1733.4 |
| LELTEVKESLKKALAGGVTLGLAIEPRAGTSSPQSPVF | 1298.9 | 3893.6 |
aObserved is an experimental m/z value
bMr(expt) is an experimental m/z value transformed to a relative molecular mass
In this study, we observed that the peptide sequences showed Leu, His, and Val at the N-terminal. All the 15 fractions contained one or plural Tyr, Trp, Ala, Pro, Met, Lys, Asp, and Cys in the sequences. It is assumed that amino acids in peptide sequences influence the antioxidant activity. Some amino acids incorporated in small peptides can improve their antioxidant properties [12]. Certainly, the 15 fractions from MPLH exhibited higher DPPH radical scavenging activity, ferrous ion-chelating ability, and reducing power when they contained those specific amino acids.
In conclusion, the yield and DH of porcine liver hydrolysates increased with hydrolysis time. Samples that were hydrolyzed using a M. purpureus microbial suspension exhibited increased DPPH scavenging activity and reducing power even at a comparatively lower yield. Methods to increase its yield should be addressed in future studies. The MALDI–TOF MS spectral analysis results indicated that molecular weight decreased as the hydrolysis time increased and the molecular weights of all hydrolysates were <10 kDa. PaPLH exhibited the highest total amino acid content and hydrophobic amino acid content among the tested hydrolysates. The 15 antioxidant fractions obtained from MPLH contained one or more of the amino acids Tyr, Trp, Ala, Pro, Met, Lys, Asp, Cys, Val, Leu, and His in their sequences. Enzymatic hydrolysis using M. purpureus can be applied for obtaining antioxidant hydrolysates from porcine liver with potential applications for preventing lipid oxidation in food products.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Shimizu M, Tanabe S, Morimatsu F, Nagao K, Yanagita T, Kato N, Nishimura T. Consumption of pork-liver protein hydrolysate reduces body fat in otsuka long-evans tokushima fatty rats by suppressing hepatic lipogenesis. Biosci. Biotech. Bioch. 2006;70(1):112–118. doi: 10.1271/bbb.70.112. [DOI] [PubMed] [Google Scholar]
- 2.Liu Q, Kong B, Xiong YL, Xia X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118(2):403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- 3.Bhaskar N, Modi VK, Govindaraju K, Radha C, Lalitha RG. Utilization of meat industry by products: Protein hydrolysate from sheep visceral mass. Bioresource Technol. 2007;98:388–394. doi: 10.1016/j.biortech.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Kim SK, Kim YT, Byun HG, Park PJ, Ito H. Purification and characterization of antioxidative peptides from bovine skin. J. Biochem. Mol. Biol. 2001;34(3):219–224. [Google Scholar]
- 5.Bernardini RD, Harnedy P, Bolton D, Kerry J, O’Neill E, Mullen AM, Hayes M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011;124(4):1296–1307. doi: 10.1016/j.foodchem.2010.07.004. [DOI] [Google Scholar]
- 6.Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D. Constituents of red yeast rice, a traditional Chinese food and medicine. J. Agr. Food Chem. 2000;48:5220–5225. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- 7.Kuba M, Tana C, Tawata S, Yasuda M. Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Process Biochem. 2005;40:2191–2196. doi: 10.1016/j.procbio.2004.08.010. [DOI] [Google Scholar]
- 8.Lakshman PLN, Tachibana S, Toyama H, Taira T, Suganuma T, Suntornsuk W, Yasuda M. Application of an acid proteinase from Monascus purpureus to reduce antigenicity of bovine milk whey protein. J. Ind. Microbiol. Biot. 2011;38(9):1485–1492. doi: 10.1007/s10295-010-0933-0. [DOI] [PubMed] [Google Scholar]
- 9.FDA (Food and Drug Administration, Taiwan). Sanitary standard for edible natural colorants (FDA Food No. 8246254). https://consumer.fda.gov.tw/Law/Detail.aspx?nodeID=518&lawid=139&k=%u5929%u7136%u98DF%u7528%u8272%u7D Accessed May 31, 2017.
- 10.Yasuda M, Sakaguchi M. Degradation of soybean protein by an acid proteinase from Monascus anka. Food Sci. Technol. Int. 1998;4(1):6–8. doi: 10.3136/fsti9596t9798.4.6. [DOI] [Google Scholar]
- 11.Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi) Food Chem. 2007;103(4):1385–1394. doi: 10.1016/j.foodchem.2006.10.055. [DOI] [Google Scholar]
- 12.Sarmadi BH, Ismail A. Antioxidative peptides from food proteins. A review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 13.You L, Zhao M, Cui C, Zhao H, Yang B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2009;10(2):235–240. doi: 10.1016/j.ifset.2008.08.007. [DOI] [Google Scholar]
- 14.Hmidet N, Balti R, Nasri R, Sila A, Bougatef A, Nasri M. Improvement of functional properties and antioxidant activities of cuttlefish (Sepia officinalis) muscle proteins hydrolyzed with Bacillus mojavensis A21 proteases. Food Res. Int. 2011;44:2703–2711. doi: 10.1016/j.foodres.2011.05.023. [DOI] [Google Scholar]
- 15.Yu L, Sun J, Liu S, Bi J, Zhang C, Yang Q. Ultrasonic-assisted enzymolysis to improve the antioxidant activities of peanut (Arachin conarachin L.) antioxidant hydrolysate. Int. J. Mol. Sci. 2012;13:9051–9068. doi: 10.3390/ijms13079051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendis E, Rajapakse N, Byun HG, Kim SK. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005;77(17):2166–2178. doi: 10.1016/j.lfs.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005;38(2):175–182. doi: 10.1016/j.foodres.2004.10.002. [DOI] [Google Scholar]
- 18.Zhu KX, Su CY, Guo XN, Peng W, Zhou HM. Influence of ultrasound during wheat gluten hydrolysis on the antioxidant activities of the resulting hydrolysate. Int. J. Food Sci. Tech. 2011;46(5):1053–1059. doi: 10.1111/j.1365-2621.2011.02585.x. [DOI] [Google Scholar]
- 19.Tseng YY. The utilization of Monascus purpureus as a curing agent in meat products. Ph.D. dissertation, National Chung-Hsing University, Taichung, Taiwan (1999)
- 20.Hsu KC. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 2010;122:42–48. doi: 10.1016/j.foodchem.2010.02.013. [DOI] [Google Scholar]
- 21.Liceaga-Gesualdo AM, Li-Chan ECY. Functional properties of fish protein hydrolysate from herring (Clupea harengus) J. Food Sci. 1999;64(6):1000–1004. doi: 10.1111/j.1365-2621.1999.tb12268.x. [DOI] [Google Scholar]
- 22.Adler-Nissen J. Enzymic hydrolysis of food proteins. U.S.A: Elsevier Applied Science Publishers, N.Y; 1986. [Google Scholar]
- 23.Church FC, Swaisgood HE, Porter DH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983;66:1219–1227. doi: 10.3168/jds.S0022-0302(83)81926-2. [DOI] [Google Scholar]
- 24.Chien PJ, Sheu F, Huang WT, Su MS. Effect of molecular weight of chitosans on their antioxidative activities in apple juice. Food Chem. 2007;102:1192–1198. doi: 10.1016/j.foodchem.2006.07.007. [DOI] [Google Scholar]
- 25.Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 26.You SJ, Wu J. Angiotensin-I converting enzyme inhibitory and antioxidant activities of egg protein hydrolysates produced with gastrointestinal and nongastrointestinal enzymes. J. Food Sci. 2011;76(6):C801–C807. doi: 10.1111/j.1750-3841.2011.02228.x. [DOI] [PubMed] [Google Scholar]
- 27.Aspmo SI, Horna SJ. Eijsink VGH. Enzymatic hydrolysis of Atlantic cod (Gadus morhua L.) viscera. Process Biochem. 2005;40:1957–1966. doi: 10.1016/j.procbio.2004.07.011. [DOI] [Google Scholar]
- 28.Petroselli G, Mandal MK, Chen LC, Ruiz GT, Wolcan E, Hiraoka K, Nonami H, Erra-Balsells R. Mass spectrometry of rhenium complexes: a comparative study by using LDI-MS, MALDI-MS. PESI-MS and ESI-MS. J. Mass Spectrom. 2012;47(3):312–321. doi: 10.1002/jms.2965. [DOI] [PubMed] [Google Scholar]
- 29.Ravallec-Plé R, Charlot C, Pires C, Braga V, Batista I, Wormhoudt AV, Gal YL, Fouchereau-Péron M. The presence of bioactive peptides in hydrolysates prepared from processing waste of sardine (Sardina pilchardus) J. Sci. Food Agr. 2001;81:1120–1125. doi: 10.1002/jsfa.921. [DOI] [Google Scholar]
- 30.Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Cr. Rev. Food Sci. 2000;40(1):43–81. doi: 10.1080/10408690091189266. [DOI] [PubMed] [Google Scholar]
- 31.Lee YL, Yang JH, Mau JL. Antioxidant properties of water extracts from Monascus fermented soybeans. Food Chem. 2008;106(3):1128–1137. doi: 10.1016/j.foodchem.2007.07.047. [DOI] [Google Scholar]
- 32.Nagodawithana TW, Nelles L. Trivedi NB. In: Pasupuleti VK, Demain AL, editors. Protein Hydrolysates in Biotechnology. Dordrecht Heidelberg London: Springer Verlag; 2010. [Google Scholar]
- 33.Jin T, Wu Y. Effects of production factors on the antioxidant activity of protein hydrolysates from little hairtail (Trichiurus haumela) of East China Sea. J. Food Agric. Environ. 2013;11(1):95–98. [Google Scholar]
- 34.Huang GR, Zhao J, Jiang JX. Effect of defatting and enzyme type on antioxidative activity of shrimp processing byproducts hydrolysate. Food Sci. Biotechnol. 2011;20(3):651–657. doi: 10.1007/s10068-011-0092-8. [DOI] [Google Scholar]
- 35.Park PJ, Jung WK, Nam KS, Shahidi F, Kim SK. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001;78(6):651–656. doi: 10.1007/s11746-001-0321-0. [DOI] [Google Scholar]